Abstract
Akv1-99, a variant of Akv murine leukemia virus, induces B-cell lymphomas with nearly 100% incidence and a mean latency period of 12 months after injection into newborn NMRI mice. PCR amplification and sequence analyses of DNA flanking integrated proviruses revealed proviral insertion into the N-ras/unr (upstream of N-ras) locus in 2 out of 13 B-cell lymphomas, both of which appeared clonal by Southern blotting analysis. These two tumors showed increased expression levels of N-ras by Northern blotting, as did a third tumor shown by reverse transcriptase PCR to have a nonclonal provirus integration located in the same area. However, no significant changes in expression were observed when using a specific probe for the unr gene. All proviruses were integrated in the same transcriptional orientation as unr and N-ras genes. By promoter insertion, the two Akv1-99 proviruses integrated between exon −1 and exon 1 of N-ras gave rise to two different spliced products, whereas the provirus integrated into unr used only an exon skipping pattern. The absence of mutations of the N-ras codons 12, 13, 18, and 61 suggests that activation of the proto-oncogene is exclusively due to overexpression by retroviral promoter insertion, and furthermore, Northern blot analyses indicate that the expression of unr is unaffected by N-ras overexpression even in the case where the unr gene itself is the target of proviral insertion. Thus, altogether our findings indicate that overexpression of N-ras plays a role in development of murine leukemia virus-induced B-cell lymphomas, leaving the expression of the tightly linked unr gene unaltered.
Akv is an ecotropic nonacutely transforming murine leukemia virus (MLV) derived from the AKR mouse strain. Akv, as well as an Akv mutant (Akv1-99) with only one copy of the 99-bp transcriptional enhancer, induces B-cell lymphomas with latency periods of about 12 months when inoculated into newborn NMRI mice (20). As for other nonacutely transforming retroviruses lacking internal oncogenes, one of the major steps in induction of disease involves provirus integration near a cellular proto-oncogene of an appropriate target cell that results in aberrant expression of the gene (17, 28). During the last few decades, analyses of provirus integration sites in DNA from MLV-induced tumors have identified far more than 100 genes or loci which are thought to play critical roles in the progress of disease (reviewed in reference 24).
The ras family genes Kirsten (K-ras) and Harvey (H-ras) have previously been reported to be targeted by MLVs in a myeloid and a thymic tumor cell line, respectively, inducing disruption of the normal gene properties (10, 12, 27), while two cases of murine B-cell lymphoma have shown MLV provirus insertion in N-ras (11, 26). These proto-oncogenes, which participate in normal cellular growth signaling, have also been reported to be activated by single point mutations (1). Thus, N-ras mutations can be found in a variety of human tumor types (2), including hematological malignancies such as acute myeloid and lymphoblastic leukemias (16, 21). The clear involvement of N-ras in disease has led to extensive investigations concerning regulatory elements (23), locus organization (13), and transcriptional regulation (15) associated with the discovery of a 5′ closely linked gene, unr (upstream of N-ras) (7, 14, 22). The ubiquitous expression of N-ras and unr, besides the same transcriptional orientation of the genes, has motivated several studies regarding possible regulation of the N-ras expression by unr or vice versa (3, 4, 19). However, no mechanism of expression modulation between N-ras and unr has been clearly detected. unr contains internal repeats with homology to cold shock domains (8), and despite previously reported analyses concerning structure (4, 13), nucleic acid-binding properties (9), and genomic organization (3), the function of the gene remains unclear. Homozygous unr−/− mice have not been obtained, though, which suggests an embryonic lethal effect of the deletion (3).
During our search for critical genes involved in development of Akv1-99-induced B-cell lymphomas, we discovered three cases of provirus integrations into the N-ras/unr locus. All three tumors showed increased N-ras expression, while the expression of unr was unaltered. The three integrated proviruses had the same orientation of transcription as N-ras, as did two previously reported cases of MLV insertions at this site in lymphomas (11, 26), and all five integrations were located in a narrow area of about 800 bp. This region covers the very 3′ end of unr and the noncoding exon (exon −1) and first intron of N-ras and has been shown previously to be of importance for regulation of N-ras expression (15). Analogous regions have been shown elsewhere to be susceptible to integration of proviruses oriented cotranscriptionally in the targeted K-ras and H-ras genes (12, 27). Therefore, this area in general appears to be particularly susceptible to retroviral insertion in MLV-induced lymphomas, suggesting this region to be of importance for the induction-progression of disease development. Altogether, our findings indicate that overexpression of N-ras plays a role in development of MLV-induced B-cell lymphomas, leaving the expression of the tightly linked unr gene unaltered.
Amplification and sequence analyses of DNA flanking integrated proviruses.
In order to identify genes involved in tumor development, we isolated proviral flanking sequences from tumors induced by the B-lymphomagenic virus Akv1-99. A simple two-step PCR method, which has previously been described as an efficient technique for isolation and sequencing of provirus-host junctions (25, 26), was employed. By this, a total of 30 proviral flanking sequences were obtained from 13 independent Akv1-99-induced lymphomas, all of which had been widely analyzed earlier in regard to morphology and molecular characterization (20). Following amplification and sequencing of proviral flanks, database search comparisons were performed, revealing that 12 of 30 isolated sequences were homologous to known sequences. Moreover, two of these sequences, amplified from tumors 3 and 11, showed homology to the locus N-ras/unr, thus suggesting this locus to be a preferred target in MLV-induced B-cell lymphoma development.
Clonal proviral integrations into the N-ras/unr locus.
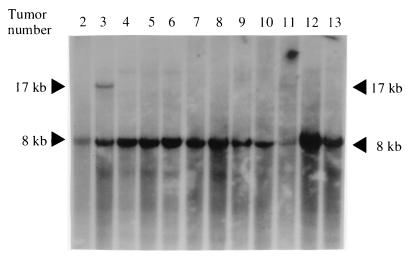
The two perfect homologies to the N-ras/unr locus sequence were obtained from DNA from tumors 3 and 11. Both proviral integrations were verified by PCR using N-ras and provirus-specific primers (data not shown), and results confirmed that the sites of integration were in the very 5′ end of the N-ras locus, namely, 835 and 85 bp upstream of exon 1, respectively, and that the transcriptional orientations of the two proviruses were the same as those of the N-ras and unr genes (Fig. 1). To investigate the clonality of the integrated proviruses (in the N-ras/unr locus), Southern blotting was performed as described previously (26) on HindIII-digested tumor DNAs (Fig. 2), with an N-ras/unr probe (Fig. 1, probe A), and in both cases a rearranged fragment of the expected size (17 kb) was observed, indicating that the two proviruses were clonally integrated. In addition, the absence of rearrangements in the other tumor DNAs indicated that no additional clonal Akv1-99 provirus integrations in the N-ras/unr locus were present in the remaining 10 lymphoma samples analyzed (Fig. 2).
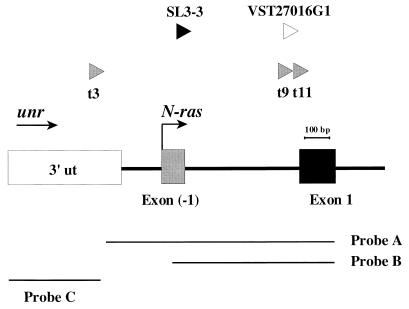
FIG. 1.
Diagram showing the locations and orientations of the three Akv1-99 insertions detected in tumor 3 (before nucleotide 335), tumor 9 (before nucleotide 1062), and tumor 11 (before nucleotide 1085) in this study (gray arrowheads) as well as previously reported insertions, SL3-3 (before nucleotide 668) and VST27016G1 (before nucleotide 1084) (11, 25) (black and white arrowheads, respectively). All five proviruses are inserted within an area of about 800 bp. Arrows indicate orientations of transcription of unr and N-ras. Shown below are the probes made by PCR that were used for Southern and Northern hybridizations. Probe A (915 bp) covers the very last untranslated 3′ region of unr and the first two N-ras exons (positions 382 to 1297); probe B (646 bp) comprises the first two N-ras exons (positions 651 to 1297); probe C (369 bp) contains the last 3′ exon of unr (positions 1 to 369). Positions indicated correspond to the GenBank sequence with accession no. L19607. ut, untranslated.
FIG. 2.
Southern blot analysis of HindIII-digested tumor DNAs from Akv1-99 tumors hybridized with a mouse N-ras probe (probe A), shown in Fig. 1. A rearranged fragment of about 17 kb in tumors 3 and 11 can be detected, while no arrangements are seen in the remaining tumors. Arrowheads indicate positions of the germ line (8 kb) and rearranged fragments (17 kb).
Expression levels of the mouse N-ras and unr genes.
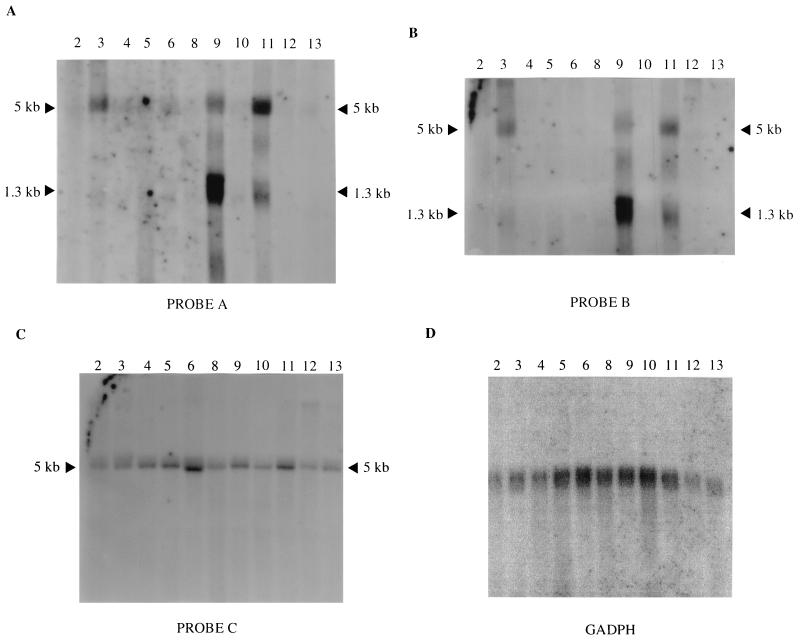
To examine if the integrated proviruses affected the expression of N-ras, Northern blot analyses were performed (Fig. 3) using the three different probes shown in Fig. 1. Previous reports have shown three species of N-ras mRNA of 5.0, 2.4, and 1.3 kb, respectively, where the 2.4-kb transcript signal has been reported to be weak, and the difference in sizes reflects usage of different poly(A) sites all downstream of the coding region (5, 18, 19). In agreement with these observations, two predominant transcripts of 5.0 and 1.3 kb were observed. Analyses using probe A, which covers the first two N-ras exons and the very last untranslated 3′ region of unr, showed overexpressed N-ras transcripts in three tumors, namely, tumor 3, tumor 9, and tumor 11 (Fig. 3A). In all three cases, the 5.0-kb transcript was observed to be overexpressed compared to the remaining tumors and using glyceraldehyde-3-phosphate dehydrogenase as an internal control (Fig. 3D). In addition, the 1.3-kb transcript was overexpressed in both tumor 9 and tumor 11, but the ratio between the intensities of the 5.0- and 1.3-kb transcripts appeared to be inversely proportional in the two tumors (Fig. 3A).
FIG. 3.
Northern blot hybridizations of RNA (23 μg) extracted from 11 independent tumors induced by Akv1-99. Hybridization was performed using either probe A (A), probe B (B), or probe C (C) (probes are shown in Fig. 1). Arrowheads indicate N-ras mRNA sizes. Tumor numbers are given on top of each lane. Hybridization with glyceraldehyde-3-phosphate dehydrogenase was used as an internal control (D).
Rehybridization of the filter with probe B, covering only N-ras exons, presented a similar pattern of increased expression for N-ras in the three tumors (Fig. 3B). In contrast, however, a probe containing the last 3′ exon of unr (probe C) showed no major differences in expression levels of unr in the 11 examined tumors (Fig. 3C), indicating that the expression of the unr gene was not affected by the integrated proviruses, nor by overexpression of N-ras. The overexpression of N-ras observed for tumor 9 was clarified by later experiments (see below).
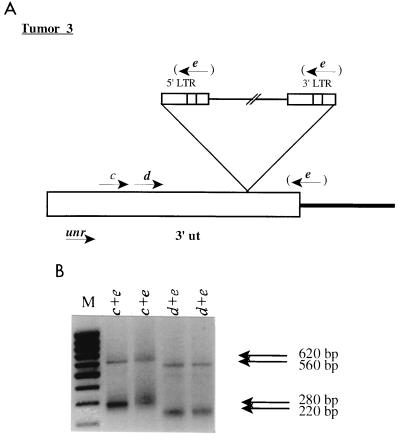
To this point, we have identified a clonal integration into the unr gene in tumor 3 which did not affect expression of the target gene but caused an altered expression of the downstream N-ras gene. This led us to investigate the character of the unr transcripts in tumor 3. PCR analyses of tumor cDNA were performed using unr-specific primers (primer c or d [Fig. 4]) and a primer matching the oligo(dT) primer tag (primer e [Fig. 4]), amplifying the very 3′ region of possible unr-containing transcripts. Two products for each primer combination were obtained, and sequencing analyses demonstrated that the smaller fragments (280 and 220 bp) corresponded to the normal unr transcript, while the larger ones (620 and 560 bp) were consistent with transcripts ending in the integrated proviruses using the normal viral poly(A) signal. Two resulting products are in accordance with one allele containing a provirus while the other lacks that integration. However, the difference in size is not sufficient under our conditions of Northern blot analysis (Fig. 3C) to definitely establish whether the observed 5.0-kb band comprises both transcripts.
FIG. 4.
RT-PCR analyses of tumor 3. (A) Representation of the provirus integrated into the unr gene. Primer c or d (positions 217 to 242 or 269 to 290, respectively; positions correspond to the GenBank sequence with the accession no. L19607), together with primer e, was used for the amplification of possible unr transcripts. Primer e hybridizes to the 5′ end of a poly(T) primer [5′-GGGTCTAGAGCTCGAGTCAC(T)16V-3′ (V = A/G/C)] which was used for reverse transcription. The figure is not drawn to scale. ut, untranslated; LTR, long terminal repeat. (B) PCR amplifications of the reverse-transcribed cDNA. Amplifications were done twice on the same preparation of cDNA. Primers used are indicated on top of each lane. Sizes of observed fragments are indicated at the right. M, molecular size markers (1,000, 900, 800, 700, 600, 500, 400, 300, and 200 bp, top to bottom, respectively).
Taken together, these Northern blotting experiments revealed overexpression of N-ras in lymphomas 3, 9, and 11, while unr expression levels seemed to be unaffected by the integrated provirus.
Point mutation and splicing analyses of the N-ras/unr locus.
Point mutations and increased expression have been reported previously to induce disruption in the normal function of N-ras (1). In order to investigate possible mutagenesis in significant codons, including codons 12, 13, 18, and 61, mutations of which in the human gene are well known to be responsible for the activation of the N-ras oncogene (1, 6), reverse transcriptase PCR (RT-PCR) and cDNA sequence analyses for all tumor samples were performed. These analyses, which comprised codons 1 to 66 of the N-ras protein, revealed no alterations (data not shown), indicating that, in tumors 3, 9, and 11, the proto-oncogene is activated solely by overexpression. The analyses of the sequences of the amplified cDNA in tumor 9 showed that also in this case a provirus had inserted into N-ras, and it was located at the downstream end of intron 1 (Fig. 1), thus being consistent with the increased expression level of N-ras observed for this tumor (see above).
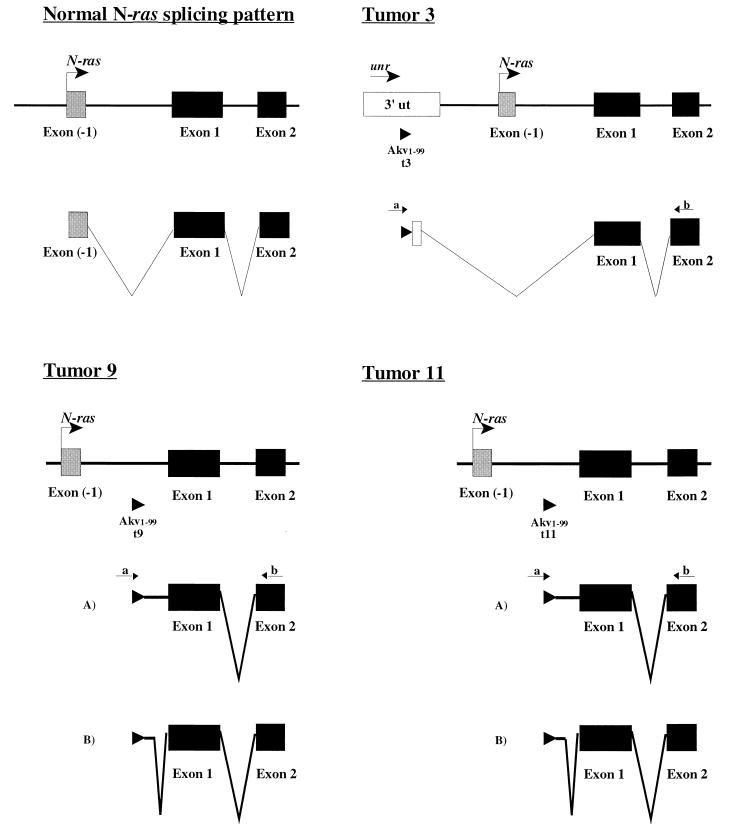
Furthermore, the RT-PCR analyses revealed that two different patterns of splicing were followed in tumors 9 and 11, while a single pathway was observed in tumor 3 (Fig. 5). In all cases, splicing was carried out using cryptic splice donor sites. RT-PCR of tumor 3 using a provirus-specific primer (primer a [Fig. 5]) and a primer located at the 5′ end of N-ras exon 2 (primer b [Fig. 5]) followed by sequence analyses showed skipping of the last 90 nucleotides of the last unr exon as well as of the N-ras noncoding exon −1. Two different mRNAs were observed when analyzing tumor 9 using the same primer set; in one case, all the intron fragment located between the provirus and the first coding exon (exon 1) was present, while in a second case the majority of that intron was spliced out. A similar pattern was observed in tumor 11, in which the same functional, cryptic splice donor site located in the first intron was used, together with the normal splice acceptor site at exon 1 of N-ras (Fig. 5). The role of these different splice products, e.g., whether they affect the differential usage of the poly(A) sites, cannot be determined.
FIG. 5.
Splicing of N-ras. In tumor 3, exon −1 is skipped during RNA processing (cryptic splice donor and normal exon 1 splice acceptor sites, located at positions 357 and 1170, respectively; positions correspond to the GenBank sequence with accession no. L19607). In tumors 9 and 11, two mRNAs are generated. Splice product A contains the intron fragment from the provirus to exon 1, and splice product B contains a minor intron 1 fragment (cryptic splice donor and normal exon 1 splice acceptor sites, at positions 1097 and 1170, respectively; positions correspond to the GenBank sequence with accession no. L19607). Provirus-specific primer a (5′-TCCGAATCGTGGTCTCGCTGATCCTTGG-3′) and N-ras primer b (positions 428 to 405; GenBank accession no. X13664) were used in all cases. Arrowheads indicate the locations of the proviruses and proviral sequences. ut, untranslated.
Conclusions.
Herein, we have amplified and analyzed flanking sequences of integrated proviruses in lymphomas induced by the Akv1-99 MLV and identified three integrations in the N-ras/unr locus. All three proviruses were shown to be located in a region previously reported to contain a provirus integration in an SL3-3 MLV-induced tumor (26) and in an AKXD mouse tumor (11). Expression analyses showed an increase of N-ras RNA levels in the three tumors containing a proviral insertion in this locus, despite the fact that only two of them seemed to be clonal as assessed by Southern blotting analysis. The tumors found to harbor the integration were classified earlier as B-cell tumors (tumors 3 and 11) and mixed T-cell–B-cell tumors (tumor 9) (20), while the other, previously described proviral N-ras integrations were classified as B-cell tumors (11, 26; unpublished results). This altogether suggests that N-ras is targeted by MLV primarily in B-cell tumors.
Sequencing analyses of DNA from all tumors showed no point mutations in any of the N-ras codons critical for human oncogenic activation by point mutations, indicating that activation is exclusively a consequence of the retroviral integration. Thus, in summary, we have shown that N-ras is activated by promoter insertion in 3 out of 13 independent Akv1-99-induced tumors, suggesting a tight relationship between MLV-induced B-cell lymphoma development and N-ras proviral integration. Moreover, we have also shown that unr expression seems unaffected by the activation of N-ras.
Acknowledgments
This project was supported by the Danish Cancer Research Fund, the Novo Nordic Foundation, the Danish Cancer Society, the Karen Elise Jensen Foundation, the Danish Natural Sciences and Medical Research Councils, DNA Technology A/S, and Universidad Autónoma de Madrid.
REFERENCES
- 1.Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 2.Bos J L. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. . (Erratum, 50:1352, 1990.) [PubMed] [Google Scholar]
- 3.Boussadia O, Amiot F, Cases S, Triqueneaux G, Jacquemin-Sablon H, Dautry F. Transcription of unr (upstream of N-ras) down-modulates N-ras expression in vivo. FEBS Lett. 1997;420:20–24. doi: 10.1016/s0014-5793(97)01479-8. [DOI] [PubMed] [Google Scholar]
- 4.Boussadia O, Jacquemin-Sablon H, Dautry F. Exon skipping in the expression of the gene immediately upstream of N-ras (unr/NRU) Biochim Biophys Acta. 1993;1172:64–72. doi: 10.1016/0167-4781(93)90270-n. [DOI] [PubMed] [Google Scholar]
- 5.Chang H Y, Guerrero I, Lake R, Pellicer A, D'Eustachio P. Mouse N-ras genes: organization of the functional locus and of a truncated cDNA-like pseudogene. Oncogene Res. 1987;1:129–136. [PubMed] [Google Scholar]
- 6.Demunter A, Ahmadian M R, Libbrecht L, Stas M, Baens M, Scheffzek K, Degreef H, De Wolf-Peeters C, van Den Oord J J. A novel N-ras mutation in malignant melanoma is associated with excellent prognosis. Cancer Res. 2001;61:4916–4922. [PubMed] [Google Scholar]
- 7.Doniger J, DiPaolo J A. Coordinate N-ras mRNA up-regulation with mutational activation in tumorigenic guinea pig cells. Nucleic Acids Res. 1988;16:969–980. doi: 10.1093/nar/16.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doniger J, Landsman D, Gonda M A, Wistow G. The product of unr, the highly conserved gene upstream of N-ras, contains multiple repeats similar to the cold-shock domain (CSD), a putative DNA-binding motif. New Biol. 1992;4:389–395. [PubMed] [Google Scholar]
- 9.Ferrer N, Garcia-Espana A, Jeffers M, Pellicer A. The unr gene: evolutionary considerations and nucleic acid-binding properties of its long isoform product. DNA Cell Biol. 1999;18:209–218. doi: 10.1089/104454999315420. [DOI] [PubMed] [Google Scholar]
- 10.George D L, Glick B, Trusko S, Freeman N. Enhanced c-Ki-ras expression associated with Friend virus integration in a bone marrow-derived mouse cell line. Proc Natl Acad Sci USA. 1986;83:1651–1655. doi: 10.1073/pnas.83.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen G M, Skapura D, Justice M J. Genetic profile of insertion mutations in mouse leukemias and lymphomas. Genome Res. 2000;10:237–243. doi: 10.1101/gr.10.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ihle J N, Smith-White B, Sisson B, Parker D, Blair D G, Schultz A, Kozak C, Lunsford R D, Askew D, Weinstein Y, Isfort R J. Activation of the c-H-ras proto-oncogene by retrovirus insertion and chromosomal rearrangement in a Moloney leukemia virus-induced T-cell leukemia. J Virol. 1989;63:2959–2966. doi: 10.1128/jvi.63.7.2959-2966.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacquemin-Sablon H, Dautry F. Organization of the unr/N-ras locus: characterization of the promoter region of the human unr gene. Nucleic Acids Res. 1992;20:6355–6361. doi: 10.1093/nar/20.23.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeffers M, Paciucci R, Pellicer A. Characterization of unr; a gene closely linked to N-ras. Nucleic Acids Res. 1990;18:4891–4899. [PMC free article] [PubMed] [Google Scholar]
- 15.Jeffers M, Pellicer A. Multiple intragenic elements regulate the expression of the murine N-ras gene. Oncogene. 1992;7:2115–2123. [PubMed] [Google Scholar]
- 16.Kiyoi H, Naoe T, Nakano Y, Yokota S, Minami S, Miyawaki S, Asou N, Kuriyama K, Jinnai I, Shimazaki C, Akiyama H, Saito K, Oh H, Motoji T, Omoto E, Saito H, Ohno R, Ueda R. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–3080. [PubMed] [Google Scholar]
- 17.Kung H J, Boerkoel C, Carter T H. Retroviral mutagenesis of cellular oncogenes: a review with insights into the mechanisms of insertional activation. Curr Top Microbiol Immunol. 1991;171:1–25. doi: 10.1007/978-3-642-76524-7_1. [DOI] [PubMed] [Google Scholar]
- 18.Leon J, Guerrero I, Pellicer A. Differential expression of the ras gene family in mice. Mol Cell Biol. 1987;7:1535–1540. doi: 10.1128/mcb.7.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Fernandez L A, Lopez-Alanon D M, del Mazo J. Different developmental pattern of N-ras and unr gene expression in mouse gametogenic and somatic tissues. Biochim Biophys Acta. 1995;1263:10–16. doi: 10.1016/0167-4781(95)00065-o. [DOI] [PubMed] [Google Scholar]
- 20.Lovmand J, Sorensen A B, Schmidt J, Ostergaard M, Luz A, Pedersen F S. B-cell lymphoma induction by Akv murine leukemia viruses harboring one or both copies of the tandem repeat in the U3 enhancer. J Virol. 1998;72:5745–5756. doi: 10.1128/jvi.72.7.5745-5756.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakao M, Janssen J W, Seriu T, Bartram C R. Rapid and reliable detection of N-ras mutations in acute lymphoblastic leukemia by melting curve analysis using LightCycler technology. Leukemia. 2000;14:312–315. doi: 10.1038/sj.leu.2401645. [DOI] [PubMed] [Google Scholar]
- 22.Nicolaiew N, Triqueneaux G, Dautry F. Organization of the human N-ras locus: characterization of a gene located immediately upstream of N-ras. Oncogene. 1991;6:721–730. [PubMed] [Google Scholar]
- 23.Paciucci R, Pellicer A. Dissection of the mouse N-ras gene upstream regulatory sequences and identification of the promoter and a negative regulatory element. Mol Cell Biol. 1991;11:1334–1343. doi: 10.1128/mcb.11.3.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg N, Jolicoeur P. Retroviral pathogenesis. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 475–586. [PubMed] [Google Scholar]
- 25.Sørensen A B, Duch M, Jørgensen P, Pedersen F S. Amplification and sequence analysis of DNA flanking integrated proviruses by a simple two-step polymerase chain reaction method. J Virol. 1993;67:7118–7124. doi: 10.1128/jvi.67.12.7118-7124.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sørensen A B, Duch M, Amtoft H W, Jørgensen P, Pedersen F S. Sequence tags of provirus integration sites in DNAs of tumors induced by the murine retrovirus SL3–3. J Virol. 1996;70:4063–4070. doi: 10.1128/jvi.70.6.4063-4070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trusko S P, Hoffman E K, George D L. Transcriptional activation of cKi-ras proto-oncogene resulting from retroviral promoter insertion. Nucleic Acids Res. 1989;17:9259–9265. doi: 10.1093/nar/17.22.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsichlis P N, Lazo P A. Virus-host interactions and the pathogenesis of murine and human oncogenic retroviruses. Curr Top Microbiol Immunol. 1991;171:95–171. doi: 10.1007/978-3-642-76524-7_5. [DOI] [PubMed] [Google Scholar]