Abstract
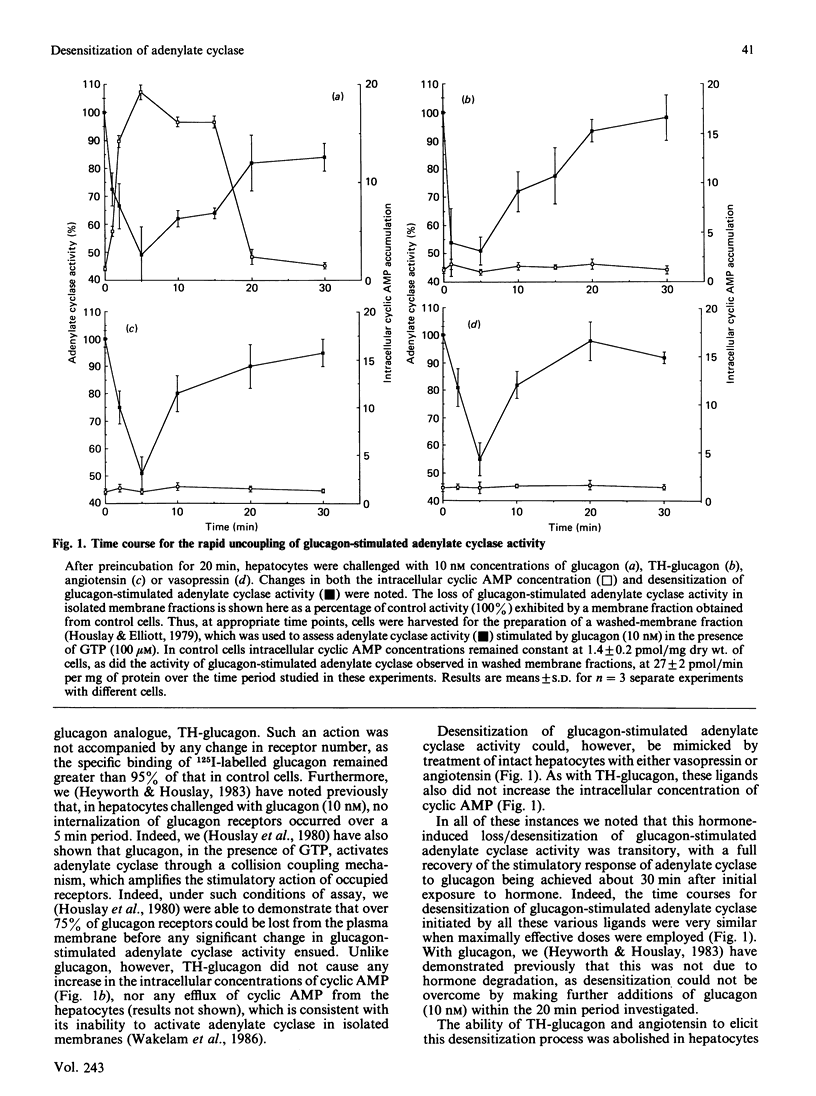
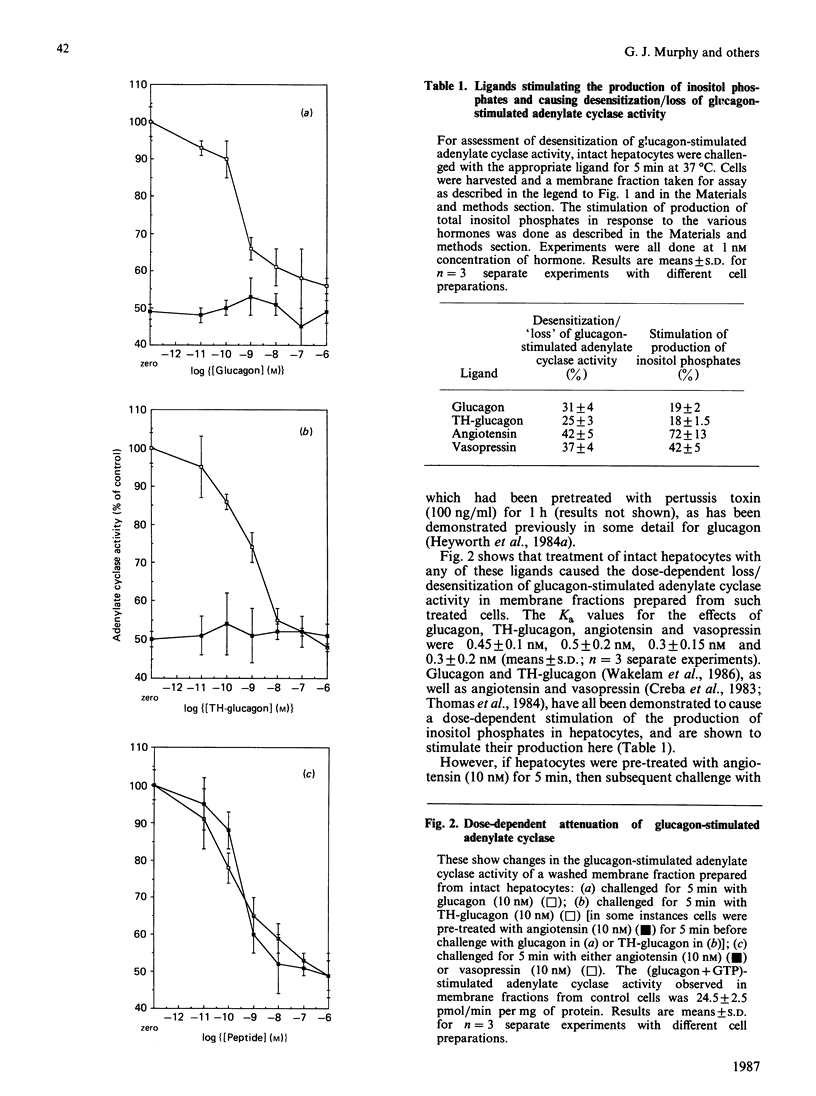
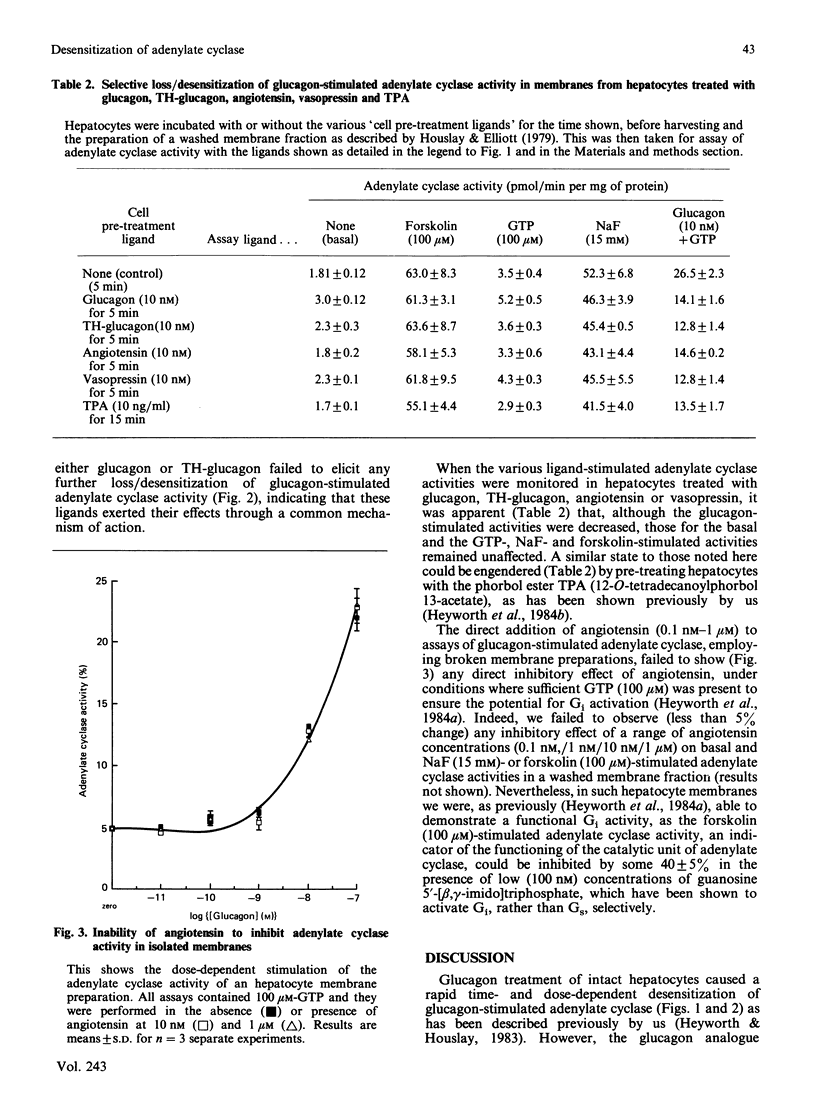
Treatment of intact hepatocytes with glucagon, TH-glucagon [( 1-N-alpha-trinitrophenylhistidine, 12-homoarginine]glucagon), angiotensin or vasopressin led to a rapid time- and dose-dependent loss of the glucagon-stimulated response of the adenylate cyclase activity seen in membrane fractions isolated from these cells. Intracellular cyclic AMP concentrations were only elevated with glucagon. All ligands were capable of causing both desensitization/loss of glucagon-stimulated adenylate cyclase activity and stimulation of inositol phospholipid metabolism in the intact hepatocytes. Maximally effective doses of angiotensin precluded any further inhibition/desensitizing action when either glucagon or TH-glucagon was subsequently added to these intact cells, as has been shown previously for the phorbol ester TPA (12-O-tetradecanoylphorbol 13-acetate) [Heyworth, Wilson, Gawler & Houslay (1985) FEBS Lett. 187, 196-200]. Treatment of intact hepatocytes with these various ligands caused a selective loss of the glucagon-stimulated adenylate cyclase activity in a washed membrane fraction and did not alter the basal, GTP-, NaF- and forskolin-stimulated responses. Angiotensin failed to inhibit glucagon-stimulated adenylate cyclase activity when added directly to a washed membrane fraction from control cells. Glucagon GR2 receptor-stimulated adenylate cyclase is suggested to undergo desensitization/uncoupling through a cyclic AMP-independent process, which involves the stimulation of inositol phospholipid metabolism by glucagon acting through GR1 receptors. This action can be mimicked by other hormones which act on the liver to stimulate inositol phospholipid metabolism. As the phorbol ester TPA also mimics this process, it is proposed that protein kinase C activation plays a pivotal role in the molecular mechanism of desensitization of glucagon-stimulated adenylate cyclase. The site of the lesion in desensitization is shown to be at the level of coupling between the glucagon receptor and the stimulatory guanine nucleotide regulatory protein Gs, and it is suggested that one or both of these components may provide a target for phosphorylation by protein kinase C.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. D., Buxton I. L., Brunton L. L. Enhancement of adenylate cyclase activity in S49 lymphoma cells by phorbol esters. Putative effect of C kinase on alpha s-GTP-catalytic subunit interaction. J Biol Chem. 1985 Mar 10;260(5):2625–2628. [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Exton J. H. Studies on the hepatic calcium-mobilizing activity of aluminum fluoride and glucagon. Modulation by cAMP and phorbol myristate acetate. J Biol Chem. 1986 Aug 25;261(24):11056–11063. [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Exton J. H. Stimulation of 1,2-diacylglycerol accumulation in hepatocytes by vasopressin, epinephrine, and angiotensin II. J Biol Chem. 1985 Nov 15;260(26):14201–14207. [PubMed] [Google Scholar]
- Bregman M. D., Trivedi D., Hruby V. J. Glucagon amino groups. Evaluation of modifications leading to antagonism and agonism. J Biol Chem. 1980 Dec 25;255(24):11725–11731. [PubMed] [Google Scholar]
- Corvera S., Huerta-Bahena J., Pelton J. T., Hruby V. J., Trivedi D., García-Sáinz J. A. Metabolic effects and cyclic AMP levels produced by glucagon, (1-N alpha-Trinitrophenylhistidine,12-homoarginine)glucagon and forskolin in isolated rat hepatocytes. Biochim Biophys Acta. 1984 Aug 17;804(4):434–441. doi: 10.1016/0167-4889(84)90071-5. [DOI] [PubMed] [Google Scholar]
- Cote T. E., Epand R. M. Nalpha-trinitrophenyl glucagon: an inhibitor of glucagon-stimulated cyclic AMP production and its effects on glycogenolysis. Biochim Biophys Acta. 1979 Jan 18;582(2):295–306. doi: 10.1016/0304-4165(79)90392-1. [DOI] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix C. J., Cooke B. A. Effect of lutropin and cycloheximide on lutropin receptors and cyclic AMP production in Leydig tumour cells in vitro. Biochem J. 1981 Jun 15;196(3):713–719. doi: 10.1042/bj1960713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix C. J., Habberfield A. D., Cooke B. A. Adenosine potentiates lutropin-stimulated cyclic AMP production and inhibits lutropin-induced desensitization of adenylate cyclase in rat Leydig tumour cells. Biochem J. 1985 Aug 15;230(1):211–216. doi: 10.1042/bj2300211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sáinz J. A., Mendlovic F., Martínez-Olmedo M. A. Effects of phorbol esters on alpha 1-adrenergic-mediated and glucagon-mediated actions in isolated rat hepatocytes. Biochem J. 1985 May 15;228(1):277–280. doi: 10.1042/bj2280277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden T. K. Agonist-induced desensitization of the beta-adrenergic receptor-linked adenylate cyclase. Pharmacol Rev. 1983 Mar;35(1):5–32. [PubMed] [Google Scholar]
- Heyworth C. M., Hanski E., Houslay M. D. Islet-activating protein blocks glucagon desensitization in intact hepatocytes. Biochem J. 1984 Aug 15;222(1):189–194. doi: 10.1042/bj2220189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Houslay M. D. Challenge of hepatocytes by glucagon triggers a rapid modulation of adenylate cyclase activity in isolated membranes. Biochem J. 1983 Jul 15;214(1):93–98. doi: 10.1042/bj2140093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Wilson S. P., Gawler D. J., Houslay M. D. The phorbol ester TPA prevents the expression of both glucagon desensitisation and the glucagon-mediated block of insulin stimulation of the peripheral plasma membrane cyclic AMP phosphodiesterase in rat hepatocytes. FEBS Lett. 1985 Aug 5;187(2):196–200. doi: 10.1016/0014-5793(85)81241-2. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Dipple I., Elliott K. R. Guanosine 5'-triphosphate and guanosine 5'-[beta gamma-imido]triphosphate effect a collision coupling mechanism between the glucagon receptor and catalytic unit of adenylate cyclase. Biochem J. 1980 Mar 15;186(3):649–658. doi: 10.1042/bj1860649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D., Elliott K. R. Cholera toxin mediated activation of adenylate cyclase in intact rat hepatocytes. FEBS Lett. 1979 Aug 15;104(2):359–363. doi: 10.1016/0014-5793(79)80852-2. [DOI] [PubMed] [Google Scholar]
- Houslay M. D. Insulin, glucagon and the receptor-mediated control of cyclic AMP concentrations in liver. Twenty-second Colworth medal lecture. Biochem Soc Trans. 1986 Apr;14(2):183–193. doi: 10.1042/bst0140183. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Metcalfe J. C., Warren G. B., Hesketh T. R., Smith G. A. The glucagon receptor of rat liver plasma membrane can couple to adenylate cyclase without activating it. Biochim Biophys Acta. 1976 Jun 17;436(2):489–494. doi: 10.1016/0005-2736(76)90210-8. [DOI] [PubMed] [Google Scholar]
- Irvine F., Pyne N. J., Houslay M. D. The phorbol ester TPA inhibits cyclic AMP phosphodiesterase activity in intact hepatocytes. FEBS Lett. 1986 Nov 24;208(2):455–459. doi: 10.1016/0014-5793(86)81068-7. [DOI] [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Kelleher D. J., Pessin J. E., Ruoho A. E., Johnson G. L. Phorbol ester induces desensitization of adenylate cyclase and phosphorylation of the beta-adrenergic receptor in turkey erythrocytes. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4316–4320. doi: 10.1073/pnas.81.14.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss Z., Mhina V. Rat liver plasma membranes contain a lipid-dependent protein kinase activity. FEBS Lett. 1982 Nov 1;148(1):131–134. doi: 10.1016/0014-5793(82)81258-1. [DOI] [PubMed] [Google Scholar]
- Lowitt S., Farese R. V., Sabir M. A., Root A. W. Rat Leydig cell phospholipid content is increased by luteinizing hormone and 8-bromo-cyclic AMP. Endocrinology. 1982 Oct;111(4):1415–1417. doi: 10.1210/endo-111-4-1415. [DOI] [PubMed] [Google Scholar]
- Mauger J. P., Claret M. Mobilization of intracellular calcium by glucagon and cyclic AMP analogues in isolated rat hepatocytes. FEBS Lett. 1986 Jan 20;195(1-2):106–110. doi: 10.1016/0014-5793(86)80140-5. [DOI] [PubMed] [Google Scholar]
- Milligan G., Klee W. A. The inhibitory guanine nucleotide-binding protein (Ni) purified from bovine brain is a high affinity GTPase. J Biol Chem. 1985 Feb 25;260(4):2057–2063. [PubMed] [Google Scholar]
- Molcho J., Zakut H., Naor Z. Gonadotropin-releasing hormone stimulates phosphatidylinositol labeling and prostaglandin E production in Leydig cells. Endocrinology. 1984 Mar;114(3):1048–1050. doi: 10.1210/endo-114-3-1048. [DOI] [PubMed] [Google Scholar]
- Morgan N. G., Blackmore P. F., Exton J. H. Age-related changes in the control of hepatic cyclic AMP levels by alpha 1- and beta 2-adrenergic receptors in male rats. J Biol Chem. 1983 Apr 25;258(8):5103–5109. [PubMed] [Google Scholar]
- Mukhopadhyay A. K., Schumacher M. Inhibition of hCG-stimulated adenylate cyclase in purified mouse Leydig cells by the phorbol ester PMA. FEBS Lett. 1985 Jul 22;187(1):56–60. doi: 10.1016/0014-5793(85)81213-8. [DOI] [PubMed] [Google Scholar]
- Nambi P., Peters J. R., Sibley D. R., Lefkowitz R. J. Desensitization of the turkey erythrocyte beta-adrenergic receptor in a cell-free system. Evidence that multiple protein kinases can phosphorylate and desensitize the receptor. J Biol Chem. 1985 Feb 25;260(4):2165–2171. [PubMed] [Google Scholar]
- Plas C., Nunez J. Glycogenolytic response to glucagon of cultured fetal hepatocytes. Refractoriness following prior exposure to glucagon. J Biol Chem. 1975 Jul 25;250(14):5304–5311. [PubMed] [Google Scholar]
- Pobiner B. F., Hewlett E. L., Garrison J. C. Role of Ni in coupling angiotensin receptors to inhibition of adenylate cyclase in hepatocytes. J Biol Chem. 1985 Dec 25;260(30):16200–16209. [PubMed] [Google Scholar]
- Quilliam L. A., Dobson P. R., Brown B. L. Modulation of cyclic AMP accumulation in GH3 cells by a phorbol ester and thyroliberin. Biochem Biophys Res Commun. 1985 Jun 28;129(3):898–903. doi: 10.1016/0006-291x(85)91976-x. [DOI] [PubMed] [Google Scholar]
- Rebois R. V., Patel J. Phorbol ester causes desensitization of gonadotropin-responsive adenylate cyclase in a murine Leydig tumor cell line. J Biol Chem. 1985 Jul 5;260(13):8026–8031. [PubMed] [Google Scholar]
- Ribeiro J. A., Sebastião A. M. Adenosine receptors and calcium: basis for proposing a third (A3) adenosine receptor. Prog Neurobiol. 1986;26(3):179–209. doi: 10.1016/0301-0082(86)90015-8. [DOI] [PubMed] [Google Scholar]
- Santos A., Blazquez E. Direct evidence of a glucagon-dependent regulation of the concentration of glucagon receptors in the liver. Eur J Biochem. 1982 Jan;121(3):671–677. doi: 10.1111/j.1432-1033.1982.tb05838.x. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Nambi P., Peters J. R., Lefkowitz R. J. Phorbol diesters promote beta-adrenergic receptor phosphorylation and adenylate cyclase desensitization in duck erythrocytes. Biochem Biophys Res Commun. 1984 Jun 29;121(3):973–979. doi: 10.1016/0006-291x(84)90772-1. [DOI] [PubMed] [Google Scholar]
- Sistare F. D., Picking R. A., Haynes R. C., Jr Sensitivity of the response of cytosolic calcium in Quin-2-loaded rat hepatocytes to glucagon, adenine nucleosides, and adenine nucleotides. J Biol Chem. 1985 Oct 15;260(23):12744–12747. [PubMed] [Google Scholar]
- Smith S. A., Elliott K. R., Pogson C. I. Differential effects of tryptophan on glucose synthesis in rats and guinea pigs. Biochem J. 1978 Dec 15;176(3):817–825. doi: 10.1042/bj1760817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Alexander J., Williamson J. R. Relationship between inositol polyphosphate production and the increase of cytosolic free Ca2+ induced by vasopressin in isolated hepatocytes. J Biol Chem. 1984 May 10;259(9):5574–5584. [PubMed] [Google Scholar]
- Uhing R. J., Jiang H., Prpic V., Exton J. H. Regulation of a liver plasma membrane phosphoinositide phosphodiesterase by guanine nucleotides and calcium. FEBS Lett. 1985 Sep 2;188(2):317–320. doi: 10.1016/0014-5793(85)80394-x. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Murphy G. J., Hruby V. J., Houslay M. D. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature. 1986 Sep 4;323(6083):68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- Wallace A. V., Heyworth C. M., Houslay M. D. N6-(Phenylisopropyl)adenosine prevents glucagon both blocking insulin's activation of the plasma-membrane cyclic AMP phosphodiesterase and uncoupling hormonal stimulation of adenylate cyclase activity in hepatocytes. Biochem J. 1984 Aug 15;222(1):177–182. doi: 10.1042/bj2220177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetton A. D., Needham L., Dodd N. J., Heyworth C. M., Houslay M. D. Forskolin and ethanol both perturb the structure of liver plasma membranes and activate adenylate cyclase activity. Biochem Pharmacol. 1983 May 15;32(10):1601–1608. doi: 10.1016/0006-2952(83)90334-9. [DOI] [PubMed] [Google Scholar]
- Wilson P. D., Dixon B. S., Dillingham M. A., Garcia-Sainz J. A., Anderson R. J. Pertussis toxin prevents homologous desensitization of adenylate cyclase in cultured renal epithelial cells. J Biol Chem. 1986 Feb 5;261(4):1503–1506. [PubMed] [Google Scholar]


