Abstract
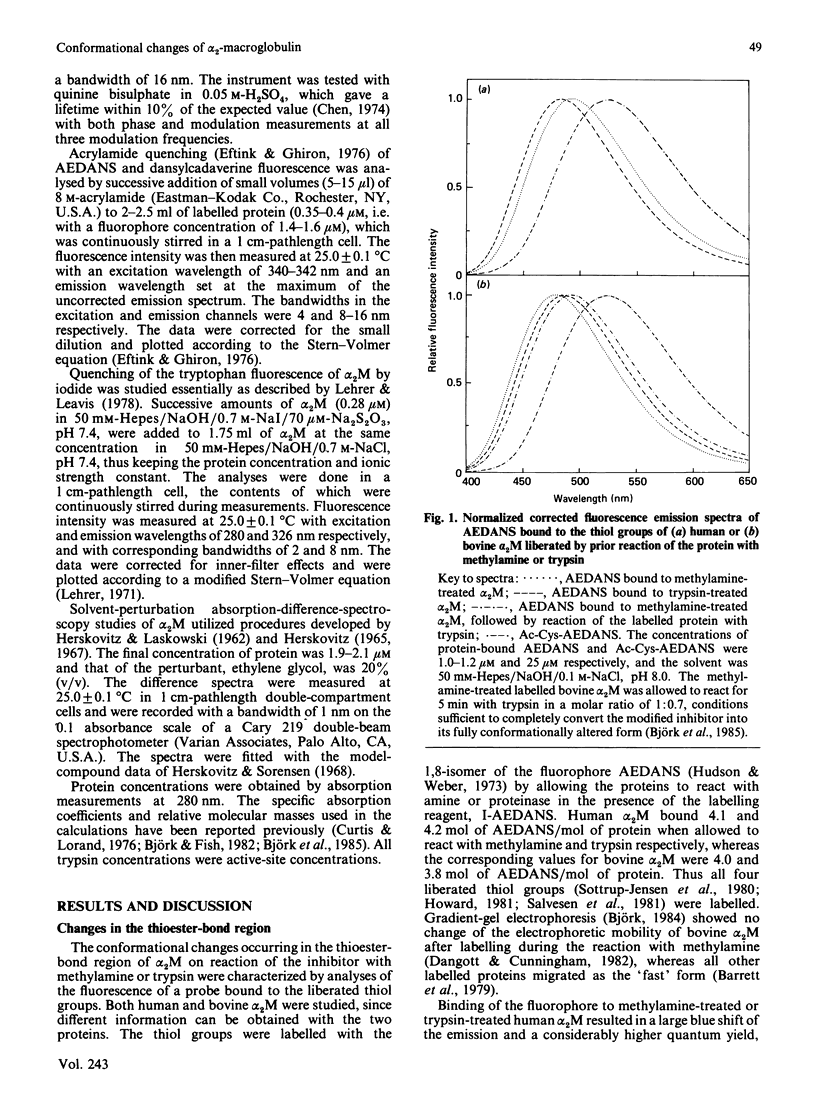
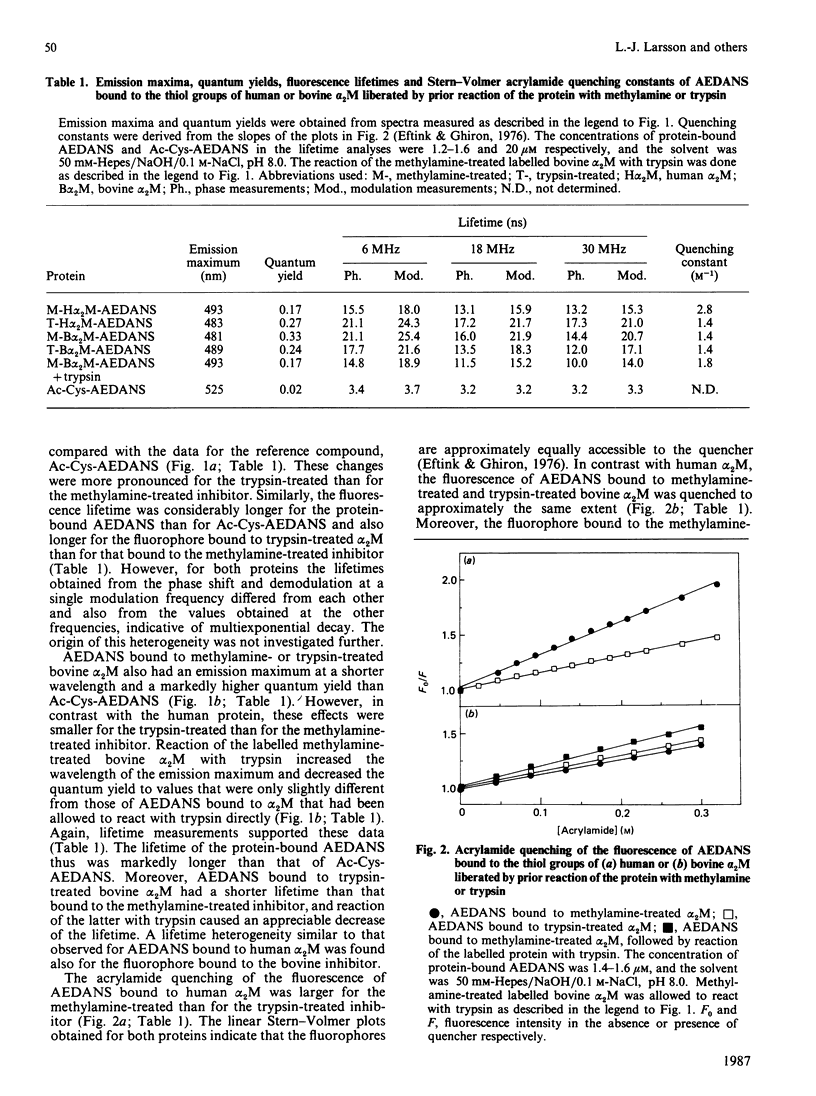
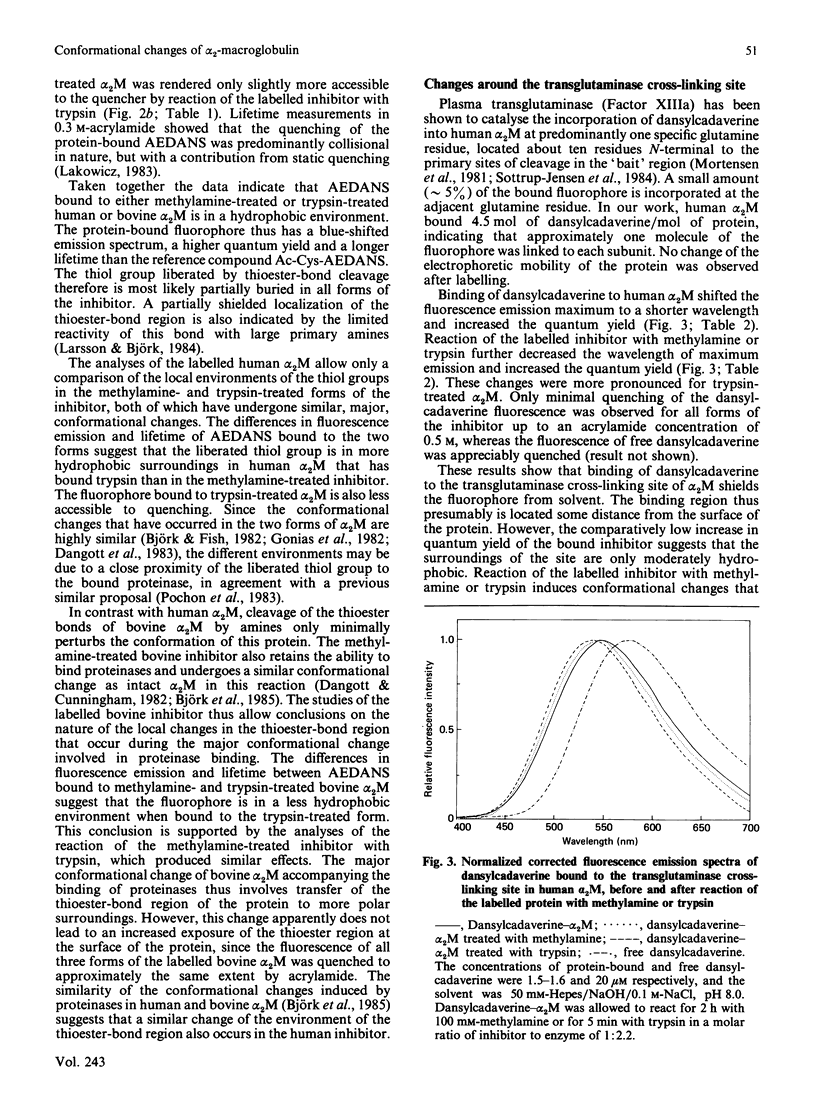
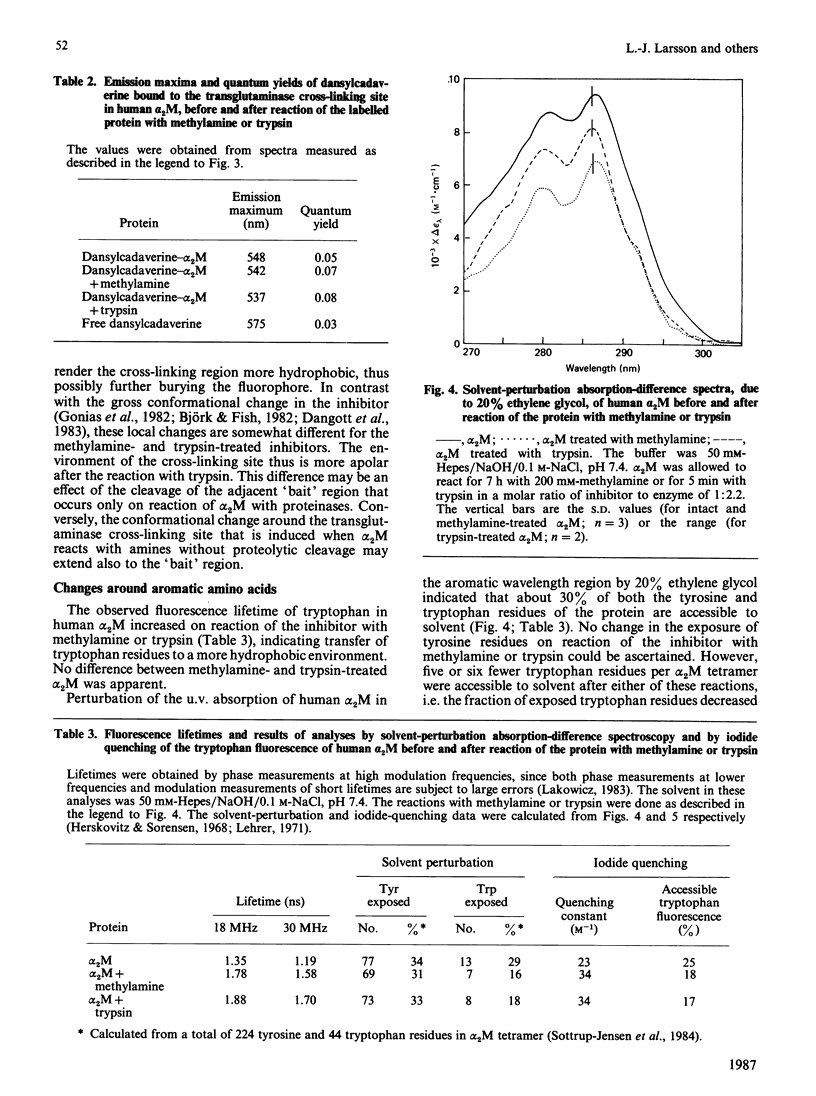
The conformational changes around the thioester-bond region of human or bovine alpha 2M (alpha 2-macroglobulin) on reaction with methylamine or trypsin were studied with the probe AEDANS [N-(acetylaminoethyl)-8-naphthylamine-1-sulphonic acid], bound to the liberated thiol groups. The binding affected the fluorescence emission and lifetime of the probe in a manner indicating that the thioester-bond region is partially buried in all forms of the inhibitor. In human alpha 2M these effects were greater for the trypsin-treated than for the methylamine-treated inhibitor, which both have undergone similar, major, conformational changes. This difference may thus be due to a close proximity of the thioester region to the bound proteinase. Reaction of trypsin with thiol-labelled methylamine-treated bovine alpha 2M, which retains a near-native conformation and inhibitory activity, indicated that the major conformational change accompanying the binding of proteinases involves transfer of the thioester-bond region to a more polar environment without increasing the exposure of this region at the surface of the protein. Labelling of the transglutaminase cross-linking site of human alpha 2M with dansylcadaverine [N-(5-aminopentyl)-5-dimethylaminonaphthalene-1-sulphonamide] suggested that this site is in moderately hydrophobic surroundings. Reaction of the labelled inhibitor with methylamine or trypsin produced fluorescence changes consistent with further burial of the cross-linking site. These changes were more pronounced for trypsin-treated than for methylamine-treated alpha 2M, presumably an effect of the cleavage of the adjacent 'bait' region. Solvent perturbation of the u.v. absorption and iodide quenching of the tryptophan fluorescence of human alpha 2M showed that one or two tryptophan residues in each alpha 2M monomer are buried on reaction with methylamine or trypsin, with no discernible change in the exposure of tyrosine residues. Together, these results indicate an extensive conformational change of alpha 2M on reaction with amines or proteinases and are consistent with several aspects of a recently proposed model of alpha 2M structure [Feldman, Gonias & Pizzo (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 5700-5704].
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk I. Binding of proteinases to human alpha 2-macroglobulin with its thioester bonds cleaved by methylamine in the presence of a thiol-group-cyanylating reagent. Biochem J. 1985 Oct 15;231(2):451–457. doi: 10.1042/bj2310451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk I., Fish W. W. Evidence for similar conformational changes in alpha 2-macroglobulin on reaction with primary amines or proteolytic enzymes. Biochem J. 1982 Nov 1;207(2):347–356. doi: 10.1042/bj2070347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk I., Larsson L. J., Lindblom T., Raub E. Stoichiometry of reactions of alpha 2-macroglobulin with trypsin and chymotrypsin. Biochem J. 1984 Jan 1;217(1):303–308. doi: 10.1042/bj2170303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk I., Lindblom T., Lindahl P. Changes of the proteinase binding properties and conformation of bovine alpha 2-macroglobulin on cleavage of the thio ester bonds by methylamine. Biochemistry. 1985 May 21;24(11):2653–2660. doi: 10.1021/bi00332a010. [DOI] [PubMed] [Google Scholar]
- Björk I. Non-productive activation of the proteinase binding sites of alpha 2-macroglobulin on reaction of the inhibitor with matrix-linked trypsin. Biochem Biophys Res Commun. 1984 Jan 30;118(2):691–695. doi: 10.1016/0006-291x(84)91358-5. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Dansyl labeled proteins: determination of extinction coefficienc and number of bound residues with radioactive dansyl chloride. Anal Biochem. 1968 Oct 24;25(1):412–416. doi: 10.1016/0003-2697(68)90116-4. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Fluorescence lifetime reference standards for the range 0.189 to 115 nanoseconds. Anal Biochem. 1974 Feb;57(2):593–604. doi: 10.1016/0003-2697(74)90115-8. [DOI] [PubMed] [Google Scholar]
- Curtis C. G., Lorand L. Fibrin-stabilizing factor (factor XIII). Methods Enzymol. 1976;45:177–191. doi: 10.1016/s0076-6879(76)45018-8. [DOI] [PubMed] [Google Scholar]
- Dangott L. J., Cunningham L. W. Residual alpha 2-macroglobulin in fetal calf serum and properties of its complex with thrombin. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1243–1251. doi: 10.1016/s0006-291x(82)80131-9. [DOI] [PubMed] [Google Scholar]
- Dangott L. J., Puett D., Cunningham L. W. Conformational changes induced in human alpha 2-macroglobulin by protease and nucleophilic modification. Biochemistry. 1983 Jul 19;22(15):3647–3653. doi: 10.1021/bi00284a017. [DOI] [PubMed] [Google Scholar]
- Davies P. J., Davies D. R., Levitzki A., Maxfield F. R., Milhaud P., Willingham M. C., Pastan I. H. Transglutaminase is essential in receptor-mediated endocytosis of alpha 2-macroglobulin and polypeptide hormones. Nature. 1980 Jan 10;283(5743):162–167. doi: 10.1038/283162a0. [DOI] [PubMed] [Google Scholar]
- Davies P. J., Murtaugh M. P. Transglutaminase and receptor-mediated endocytosis in macrophages and cultured fibroblasts. Mol Cell Biochem. 1984;58(1-2):69–77. doi: 10.1007/BF00240606. [DOI] [PubMed] [Google Scholar]
- Eccleston E. D., Howard J. B. Reaction of methylamine with human alpha 2-macroglobulin. Mechanism of inactivation. J Biol Chem. 1985 Aug 25;260(18):10169–10176. [PubMed] [Google Scholar]
- Feldman S. R., Gonias S. L., Pizzo S. V. Model of alpha 2-macroglobulin structure and function. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5700–5704. doi: 10.1073/pnas.82.17.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganrot P. O. The combining ratio between trypsin and serum alpha-2-macroglobulin. Acta Chem Scand. 1966;20(8):2299–2300. doi: 10.3891/acta.chem.scand.20-2299. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Pizzo S. V. Characterization of functional human alpha 2-macroglobulin half-molecules isolated by limited reduction with dithiothreitol. Biochemistry. 1983 Feb 1;22(3):536–546. doi: 10.1021/bi00272a003. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Reynolds J. A., Pizzo S. V. Physical properties of human alpha 2-macroglobulin following reaction with methylamine and trypsin. Biochim Biophys Acta. 1982 Aug 10;705(3):306–314. doi: 10.1016/0167-4838(82)90252-7. [DOI] [PubMed] [Google Scholar]
- HERSKOVITS T. T. CONFORMATION OF PROTEINS AND POLYPEPTIDES. I. EXTENSION OF THE SOLVENT PERTURBATION TECHNIQUE OF DIFFERENCE SPECTROSCOPY TO THE STUDY OF PROTEINS AND POLYPEPTIDES IN ORGANIC SOLVENTS. J Biol Chem. 1965 Feb;240:628–638. [PubMed] [Google Scholar]
- HERSKOVITS T. T., LASKOWSKI M., Jr Location of chromophoric residues in proteins by solvent perturbation. I. Tyrosyls in serum albumins. J Biol Chem. 1962 Aug;237:2481–2492. [PubMed] [Google Scholar]
- Hall P. K., Roberts R. C. Physical and chemical properties of human plasma alpha2-macroglobulin. Biochem J. 1978 Jul 1;173(1):27–38. doi: 10.1042/bj1730027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel P. C. Human alpha2-macroglobulin. Methods Enzymol. 1976;45:639–652. doi: 10.1016/s0076-6879(76)45055-3. [DOI] [PubMed] [Google Scholar]
- Harpel P. C. Studies on human plasma alpha 2-macroglobulin-enzyme interactions. Evidence for proteolytic modification of the subunit chain structure. J Exp Med. 1973 Sep 1;138(3):508–521. doi: 10.1084/jem.138.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits T. T., Sorensen M. Studies of the location of tyrosyl and tryptophyl residues in proteins. I. Solvent perturbation data of model compounds. Biochemistry. 1968 Jul;7(7):2523–2532. doi: 10.1021/bi00847a012. [DOI] [PubMed] [Google Scholar]
- Howard J. B. Reactive site in human alpha 2-macroglobulin: circumstantial evidence for a thiolester. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2235–2239. doi: 10.1073/pnas.78.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson E. N., Weber G. Synthesis and characterization of two fluorescent sulfhydryl reagents. Biochemistry. 1973 Oct 9;12(21):4154–4161. doi: 10.1021/bi00745a019. [DOI] [PubMed] [Google Scholar]
- Jones J. M., Creeth J. M., Kekwick R. A. Thio reduction of human 2 -macroglobulin. The subunit structure. Biochem J. 1972 Mar;127(1):187–197. doi: 10.1042/bj1270187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Cherek H., Balter A. Correction of timing errors in photomultiplier tubes used in phase-modulation fluorometry. J Biochem Biophys Methods. 1981 Sep;5(3):131–146. doi: 10.1016/0165-022x(81)90012-9. [DOI] [PubMed] [Google Scholar]
- Larsson L. J., Björk I. Kinetics of appearance of sulfhydryl groups in alpha 2-macroglobulin on reaction of the inhibitor with amines. Biochemistry. 1984 Jun 5;23(12):2802–2807. doi: 10.1021/bi00307a041. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S., Leavis P. C. Solute quenching of protein fluorescence. Methods Enzymol. 1978;49:222–236. doi: 10.1016/s0076-6879(78)49012-3. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S. Solute perturbation of protein fluorescence. The quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry. 1971 Aug 17;10(17):3254–3263. doi: 10.1021/bi00793a015. [DOI] [PubMed] [Google Scholar]
- Mortensen S. B., Sottrup-Jensen L., Hansen H. F., Rider D., Petersen T. E., Magnusson S. Sequence location of a putative transglutaminase crosslinking site in human alpha 2-macroglobulin. FEBS Lett. 1981 Jul 6;129(2):314–317. doi: 10.1016/0014-5793(81)80191-3. [DOI] [PubMed] [Google Scholar]
- Pochon F., Favaudon V., Bieth J. Localization of the proteinase-induced thiol groups in alpha 2-macroglobulin. Biochem Biophys Res Commun. 1983 Mar 29;111(3):964–969. doi: 10.1016/0006-291x(83)91394-3. [DOI] [PubMed] [Google Scholar]
- Pochon F., Favaudon V., Tourbez-Perrin M., Bieth J. Localization of the two protease binding sites in human alpha 2-macroglobulin. J Biol Chem. 1981 Jan 25;256(2):547–550. [PubMed] [Google Scholar]
- Salvesen G. S., Barrett A. J. Covalent binding of proteinases in their reaction with alpha 2-macroglobulin. Biochem J. 1980 Jun 1;187(3):695–701. doi: 10.1042/bj1870695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen G. S., Sayers C. A., Barrett A. J. Further characterization of the covalent linking reaction of alpha 2-macroglobulin. Biochem J. 1981 May 1;195(2):453–461. doi: 10.1042/bj1950453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm H. J., Schramm W. Computer averaging of single molecules of alpha 2-macroglobulin and the alpha 2-macroglobulin/trypsin complex. Hoppe Seylers Z Physiol Chem. 1982 Aug;363(8):803–812. doi: 10.1515/bchm2.1982.363.2.803. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Lønblad P. B., Stepanik T. M., Petersen T. E., Magnusson S., Jörnvall H. Primary structure of the 'bait' region for proteinases in alpha 2-macroglobulin. Nature of the complex. FEBS Lett. 1981 May 18;127(2):167–173. doi: 10.1016/0014-5793(81)80197-4. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Petersen T. E., Magnusson S. A thiol-ester in alpha 2-macroglobulin cleaved during proteinase complex formation. FEBS Lett. 1980 Dec 1;121(2):275–279. doi: 10.1016/0014-5793(80)80361-9. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Petersen T. E., Magnusson S. Trypsin-induced activation of the thiol esters in alpha 2-macroglobulin generates a short-lived intermediate ('nascent' alpha 2-M) that can react rapidly to incorporate not only methylamine or putrescine but also proteins lacking proteinase activity. FEBS Lett. 1981 Jun 1;128(1):123–126. doi: 10.1016/0014-5793(81)81096-4. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Stepanik T. M., Kristensen T., Wierzbicki D. M., Jones C. M., Lønblad P. B., Magnusson S., Petersen T. E. Primary structure of human alpha 2-macroglobulin. V. The complete structure. J Biol Chem. 1984 Jul 10;259(13):8318–8327. [PubMed] [Google Scholar]
- Strickland D. K., Bhattacharya P., Olson S. T. Kinetics of the conformational alterations associated with nucleophilic modification of alpha 2-macroglobulin. Biochemistry. 1984 Jul 3;23(14):3115–3124. doi: 10.1021/bi00309a002. [DOI] [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Structural characterization of human alpha2-macroglobulin subunits. J Biol Chem. 1979 Jun 10;254(11):4452–4456. [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van Den Berghe H. Demonstration of an alpha2-macroglobulin receptor in human fibroblasts, absent in tumor-derived cell lines. J Biol Chem. 1979 Jun 25;254(12):5155–5160. [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van den Berghe H. Functional modifications of alpha 2-macroglobulin by primary amines. I. Characterization of alpha 2 M after derivatization by methylamine and by factor XIII. J Biol Chem. 1981 Sep 10;256(17):9016–9022. [PubMed] [Google Scholar]
- Wu K., Wang D., Feinman R. D. Inhibition of proteases by alpha 2-macroglobulin. The role of lysyl amino groups of trypsin in covalent complex formation. J Biol Chem. 1981 Oct 25;256(20):10409–10414. [PubMed] [Google Scholar]
- van Leuven F. Human alpha 2 macroglobulin. Mol Cell Biochem. 1984;58(1-2):121–128. doi: 10.1007/BF00240611. [DOI] [PubMed] [Google Scholar]


