Abstract
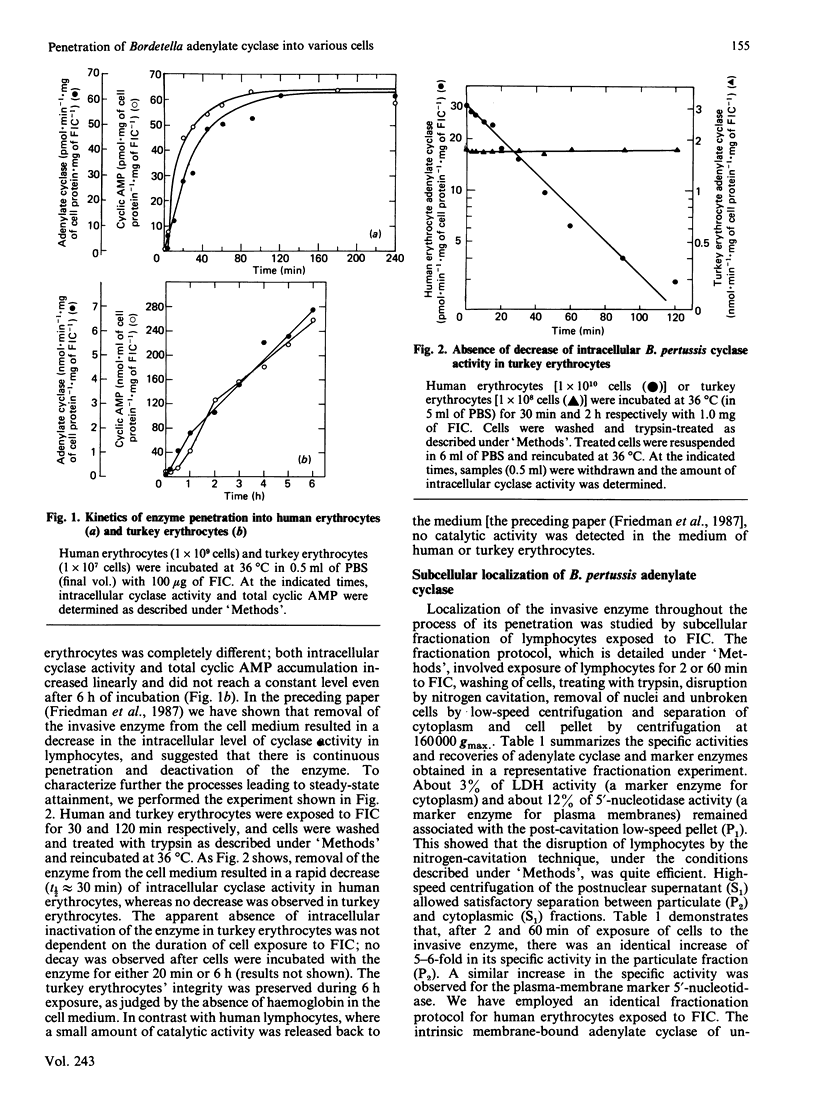
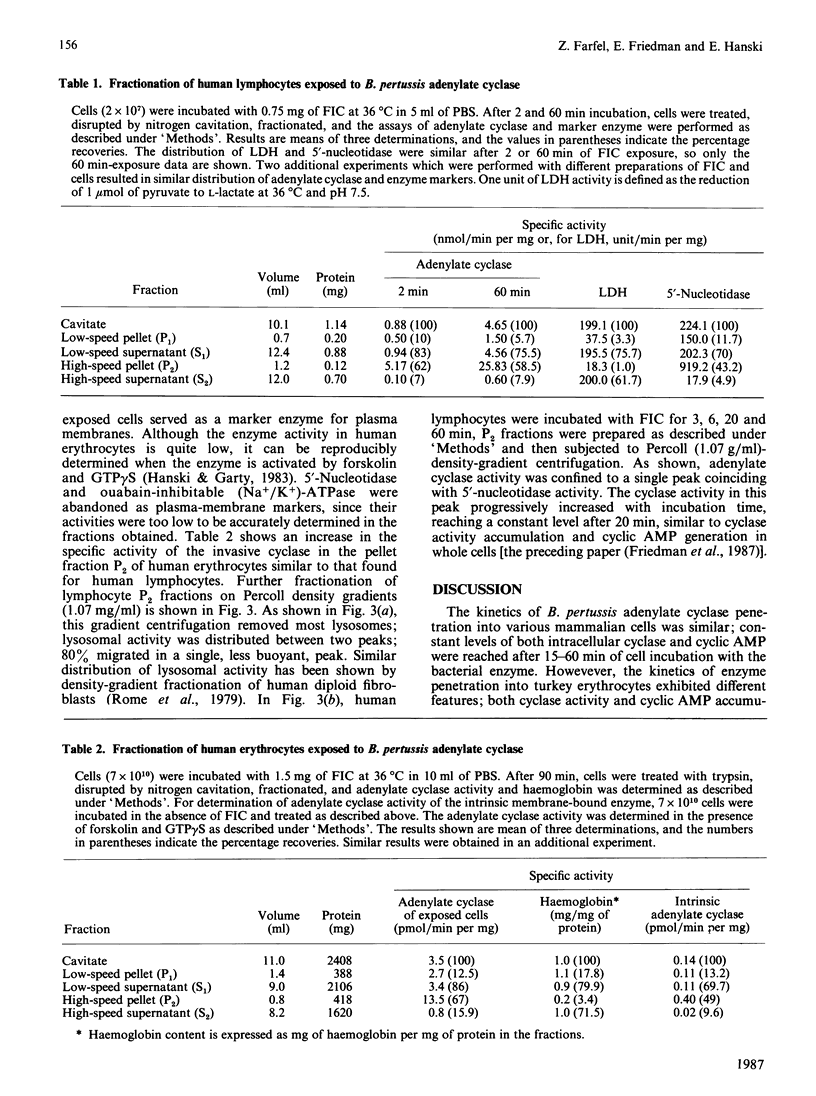
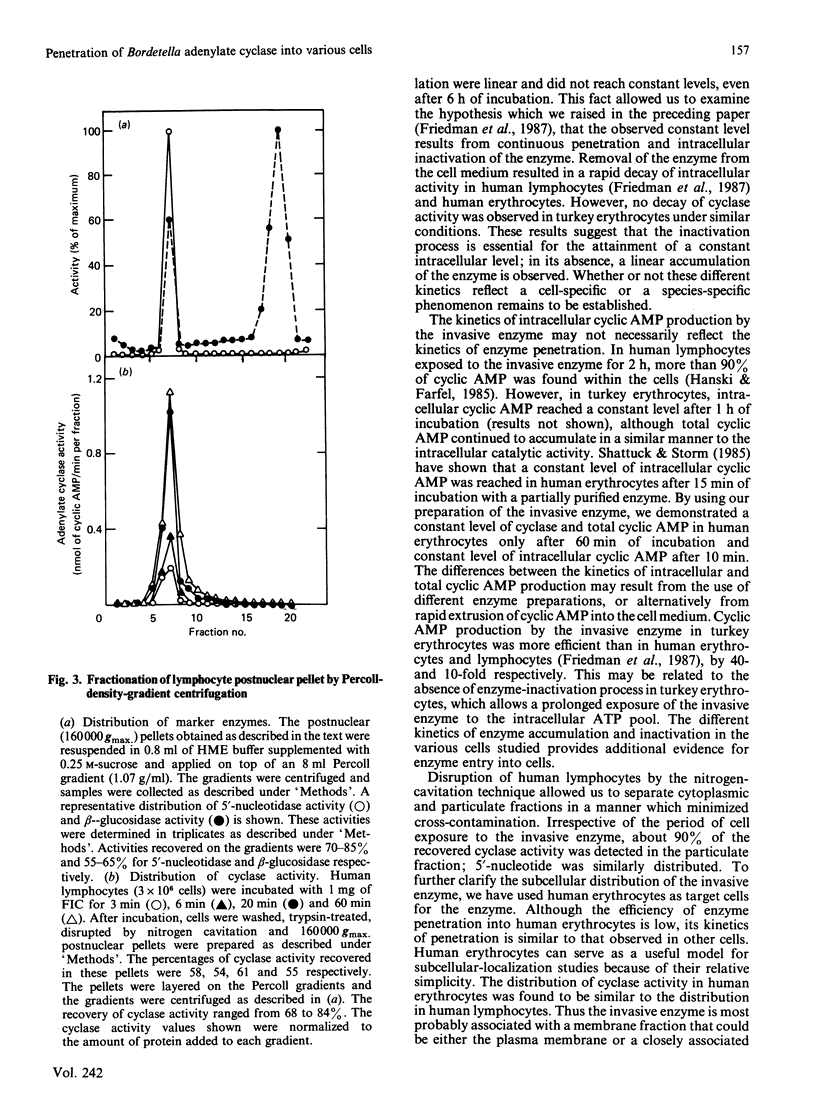
The penetration of Bordetella pertussis adenylate cyclase into various mammalian cells exhibits similar kinetics; the accumulation of both intracellular cyclase activity and cyclic AMP is rapid, reaching constant levels after 15-60 min of incubation. The kinetics of enzyme penetration into turkey erythrocytes is different; cyclase activity and cyclic AMP accumulate linearly and do not reach constant levels even after 6 h of incubation. In the preceding paper [Friedman, Farfel & Hanski (1987) Biochem. J. 243, 145-151] we have suggested that the constant level of intracellular cyclase activity reflects a steady state formed by continuous penetration and intracellular inactivation of the enzyme. In contrast with other mammalian cells, no inactivation of cyclase is observed in turkey erythrocytes. These results further support the notion that there is continuous penetration and deactivation of the invasive enzyme in mammalian cells. A 5-6-fold increase in specific activity of the invasive cyclase is detected in a pellet fraction of human lymphocytes in which a similar increase in specific activity of the plasma-membrane marker 5'-nucleotidase is observed. A similar increase in the invasive-cyclase specific activity is detected in a membrane fraction of human erythrocytes. Cyclase activity in a membrane-enriched fraction of human lymphocytes reached a constant level after 20 min of cell exposure to the enzyme. Similar time courses were observed for accumulation of cyclase activity and cyclic AMP in whole lymphocytes [Friedman, Farfel & Hanski (1987) Biochem, J. 243, 145-151]. We suggest therefore that cyclic AMP generation by the invasive enzyme as well as the intracellular inactivation process occur while it is associated with a membrane fraction identical, or closely associated, with the plasma membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila J. L., Casanova M. A., Avila A., Bretaña A. Acid and neutral hydrolases in Trypanosoma cruzi. Characterization and assay. J Protozool. 1979 May;26(2):304–311. doi: 10.1111/j.1550-7408.1979.tb02786.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Slungaard A. S., Graf E., Panter S. S., Eaton J. W. Bordetella adenylate cyclase toxin: entry of bacterial adenylate cyclase into mammalian cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:183–187. [PubMed] [Google Scholar]
- Friedman E., Farfel Z., Hanski E. The invasive adenylate cyclase of Bordetella pertussis. Properties and penetration kinetics. Biochem J. 1987 Apr 1;243(1):145–151. doi: 10.1042/bj2430145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Farfel Z. Bordetella pertussis invasive adenylate cyclase. Partial resolution and properties of its cellular penetration. J Biol Chem. 1985 May 10;260(9):5526–5532. [PubMed] [Google Scholar]
- Hanski E., Garty N. B. Activation of adenylate cyclase by sperm membranes. The role of guanine nucleotide binding proteins. FEBS Lett. 1983 Oct 17;162(2):447–452. doi: 10.1016/0014-5793(83)80805-9. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Chimeric toxins. Pharmacol Ther. 1981;15(3):355–381. doi: 10.1016/0163-7258(81)90050-4. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Allietta M. M., Neufeld E. F. Two species of lysosomal organelles in cultured human fibroblasts. Cell. 1979 May;17(1):143–153. doi: 10.1016/0092-8674(79)90302-7. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Maguire M. E., Sturgill T. W., Biltonen R. L., Gilman A. G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J Biol Chem. 1977 Aug 25;252(16):5761–5775. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Shattuck R. L., Storm D. R. Calmodulin inhibits entry of Bordetella pertussis adenylate cyclase into animal cells. Biochemistry. 1985 Nov 5;24(23):6323–6328. doi: 10.1021/bi00344a001. [DOI] [PubMed] [Google Scholar]
- Tsuzuki J., Wu H. C. Receptors for a cytotoxic lectin, abrin and their role in cell intoxication. Biochim Biophys Acta. 1982 Jul 22;720(4):390–399. doi: 10.1016/0167-4889(82)90117-3. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984 Aug;150(2):219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]


