Abstract
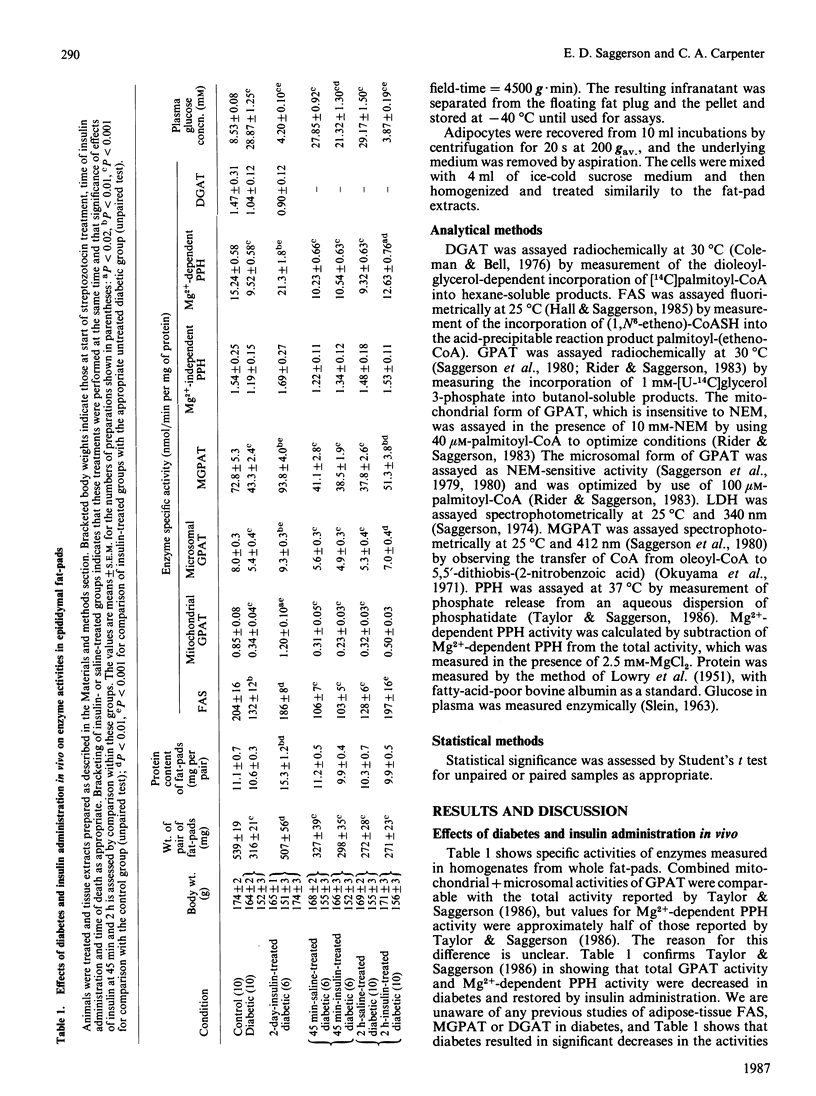
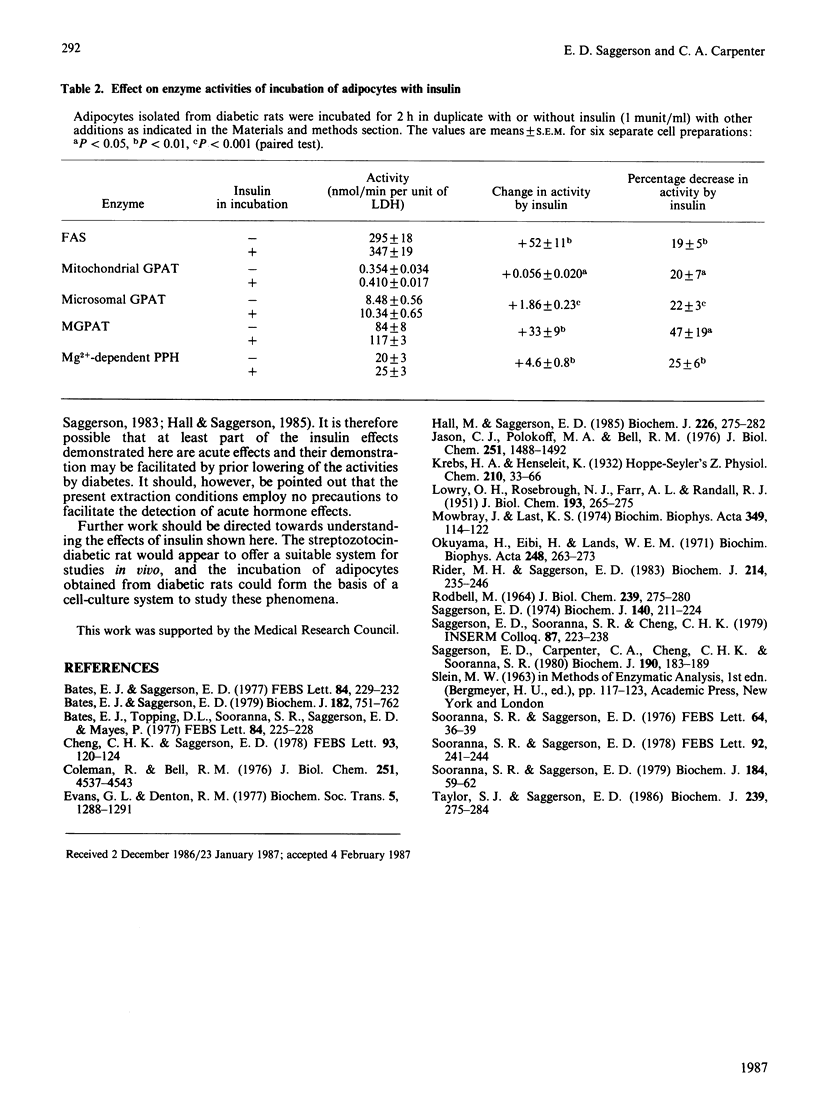
At 2 days after administration of streptozotocin (100 mg/kg), activities in rat epididymal fat-pads of the following enzymes were significantly decreased: fatty acyl-CoA synthetase (FAS), mitochondrial and microsomal forms of glycerolphosphate acyltransferase (GPAT), monoacylglycerolphosphate acyltransferase (MGPAT) and Mg2+-dependent phosphatidate phosphohydrolase (PPH). There were no significant changes in diacylglycerol acyltransferase or Mg2+-independent PPH. Insulin administration to diabetic rats over 2 days restored activities of FAS, both forms of GPAT, MGPAT and Mg2+-dependent PPH. Significant restoration of all five activities was also seen 2 h after a single administration of insulin, but was not observed 45 min after insulin treatment. Insulin significantly increased all five enzyme activities when adipocytes from diabetic rats were incubated for 2 h with a mixture of glucose, lactate, pyruvate and amino acids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates E. J., Saggerson D. A selective decrease in mitochondrial glycerol phosphate acyltransferase activity in livers from streptozotocin-diabetic rats. FEBS Lett. 1977 Dec 15;84(2):229–232. doi: 10.1016/0014-5793(77)80694-7. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Saggerson E. D. A study of the glycerol phosphate acyltransferase and dihydroxyacetone phosphate acyltransferase activities in rat liver mitochondrial and microsomal fractions. Relative distribution in parenchymal and non-parenchymal cells, effects of N-ethylmaleimide, palmitoyl-coenzyme A concentration, starvation, adrenalectomy and anti-insulin serum treatment. Biochem J. 1979 Sep 15;182(3):751–762. doi: 10.1042/bj1820751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E. J., Topping D. L., Sooranna S. P., Saggerson D., Mayes P. A. Acute effects of insulin on glycerol phosphate acyl transferase activity, ketogenesis and serum free fatty acid concentration in perfused rat liver. FEBS Lett. 1977 Dec 15;84(2):225–228. doi: 10.1016/0014-5793(77)80693-5. [DOI] [PubMed] [Google Scholar]
- Cheng C. H., Saggerson E. D. Rapid antagonistic actions of noradrealine and insulin on rat adipocyte phosphatidate phosphohydrolase activity. FEBS Lett. 1978 Sep 1;93(1):120–124. doi: 10.1016/0014-5793(78)80818-7. [DOI] [PubMed] [Google Scholar]
- Coleman R., Bell R. M. Triacylglycerol synthesis in isolated fat cells. Studies on the microsomal diacylglycerol acyltransferase activity using ethanol-dispersed diacylglycerols. J Biol Chem. 1976 Aug 10;251(15):4537–4543. [PubMed] [Google Scholar]
- Evans G. L., Denton R. M. Regulation of fatty acid synthesis and esterification in rat epididymal adipose tissue: effects of insulin, palmitate and 2-bromopalmitate [proceedings]. Biochem Soc Trans. 1977;5(5):1288–1291. doi: 10.1042/bst0051288. [DOI] [PubMed] [Google Scholar]
- Hall M., Saggerson E. D. Reversible inactivation by noradrenaline of long-chain fatty acyl-CoA synthetase in rat adipocytes. Biochem J. 1985 Feb 15;226(1):275–282. doi: 10.1042/bj2260275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jason C. J., Polokoff M. A., Bell R. M. Triacylglycerol synthesis in isolated fat cells. An effect of insulin on microsomal fatty acid coenzyme A ligase activity. J Biol Chem. 1976 Mar 10;251(5):1488–1492. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mowbray J., Last K. S. Evidence against the incorporation into protein of amino acids directly from the membrane transport system in rat heart. Biochim Biophys Acta. 1974 Apr 27;349(1):114–122. doi: 10.1016/0005-2787(74)90014-8. [DOI] [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. II. KINETICS AND FEEDBACK CONTROL. J Biol Chem. 1964 Jan;239:275–284. [PubMed] [Google Scholar]
- Okuyama H., Eibl H., Lands W. E. Acyl coenzyme A:2-acyl-sn-glycerol-3-phosphate acyltransferase activity in rat liver microsomes. Biochim Biophys Acta. 1971 Nov 5;248(2):263–273. doi: 10.1016/0005-2760(71)90014-2. [DOI] [PubMed] [Google Scholar]
- Rider M. H., Saggerson E. D. Regulation by noradrenaline of the mitochondrial and microsomal forms of glycerol phosphate acyltransferase in rat adipocytes. Biochem J. 1983 Jul 15;214(1):235–246. doi: 10.1042/bj2140235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A., Cheng C. H., Sooranna S. R. Subcellular distribution and some properties of N-ethylmaleimide-sensitive and-insensitive forms of glycerol phosphate acyltransferase in rat adipocytes. Biochem J. 1980 Jul 15;190(1):183–189. doi: 10.1042/bj1900183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. Lipogenesis in rat and guinea-pig isolated epididymal fat-cells. Biochem J. 1974 May;140(2):211–224. doi: 10.1042/bj1400211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooranna S. R., Saggerson E. D. A stable decrease in long chain fatty acyl CoA synthetase activity after treatment of rat adipocytes with adrenaline. FEBS Lett. 1978 Aug 15;92(2):241–244. doi: 10.1016/0014-5793(78)80762-5. [DOI] [PubMed] [Google Scholar]
- Sooranna S. R., Saggerson E. D. Inactivation of rat adipocyte pyruvate dehydrogenase by palmitate. Protection against this effect by insulin in the presence of glucose. Biochem J. 1979 Oct 15;184(1):59–62. doi: 10.1042/bj1840059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooranna S. R., Saggerson E. D. Interactions of insulin and adrenaline with glycerol phosphate acylation processes in fat-cells from rat. FEBS Lett. 1976 Apr 15;64(1):36–39. doi: 10.1016/0014-5793(76)80242-6. [DOI] [PubMed] [Google Scholar]
- Taylor S. J., Saggerson E. D. Adipose-tissue Mg2+-dependent phosphatidate phosphohydrolase. Control of activity and subcellular distribution in vitro and in vivo. Biochem J. 1986 Oct 15;239(2):275–284. doi: 10.1042/bj2390275. [DOI] [PMC free article] [PubMed] [Google Scholar]


