Abstract
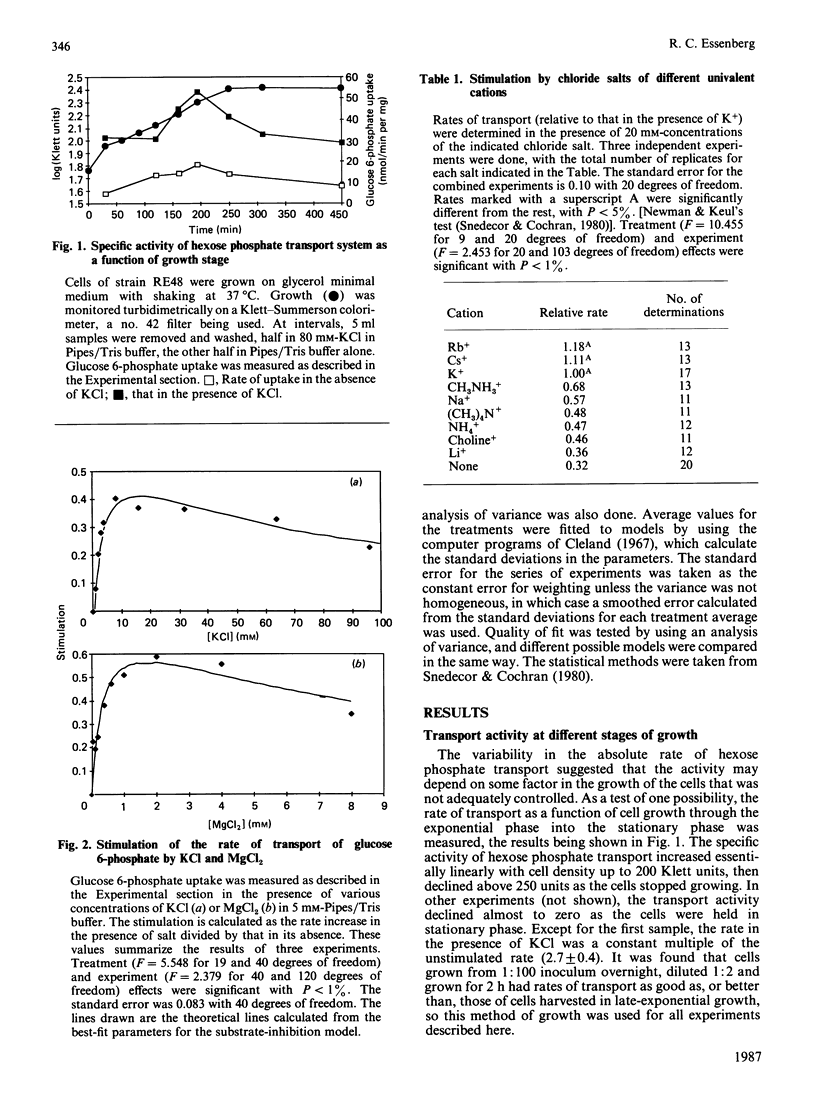
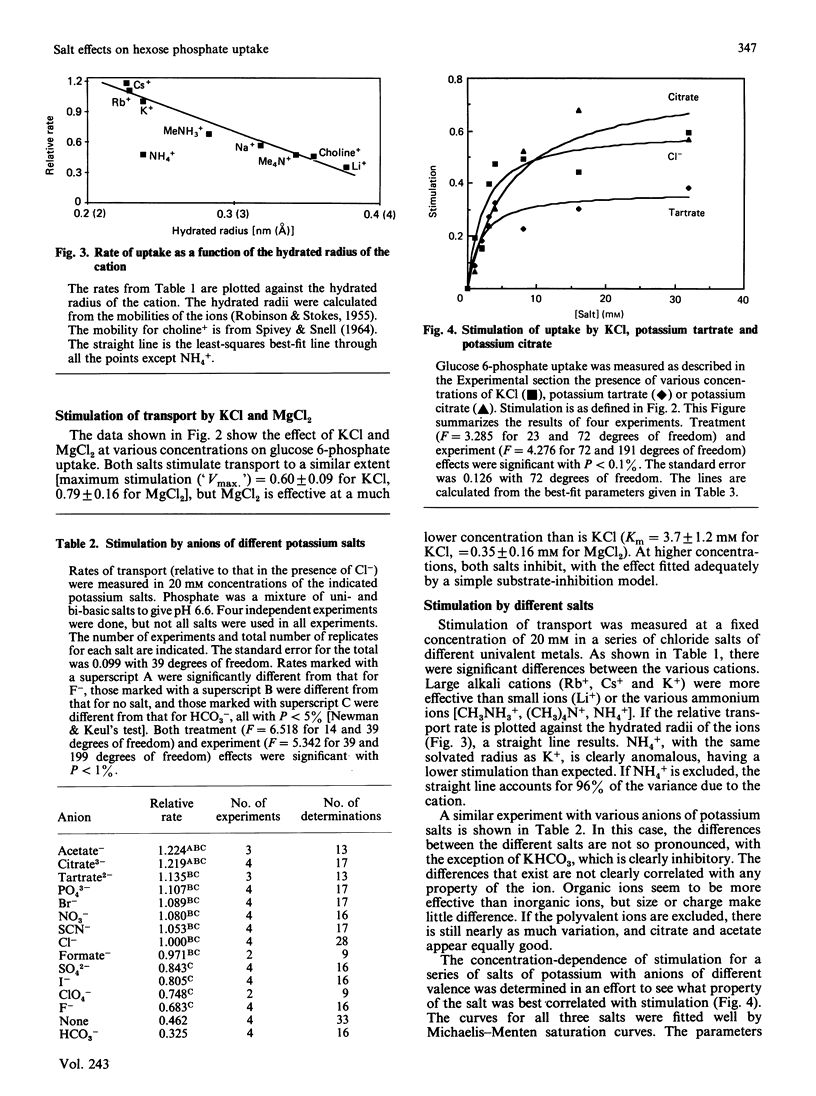
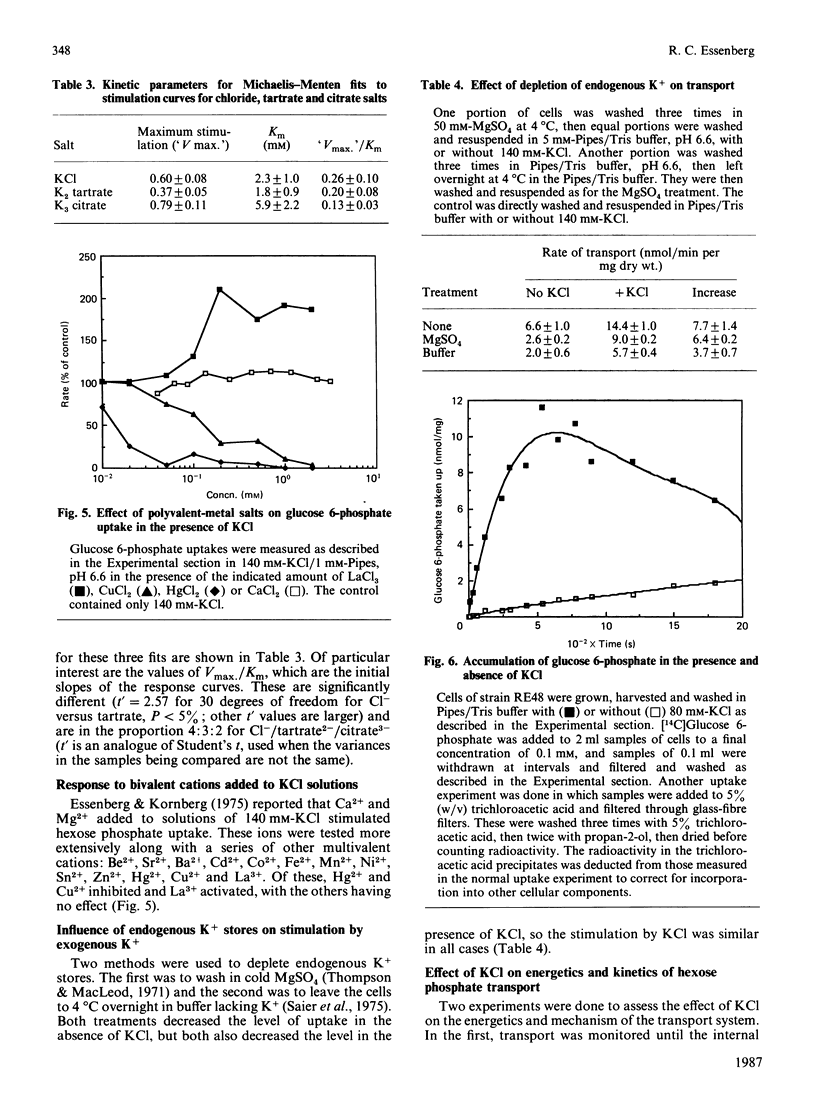
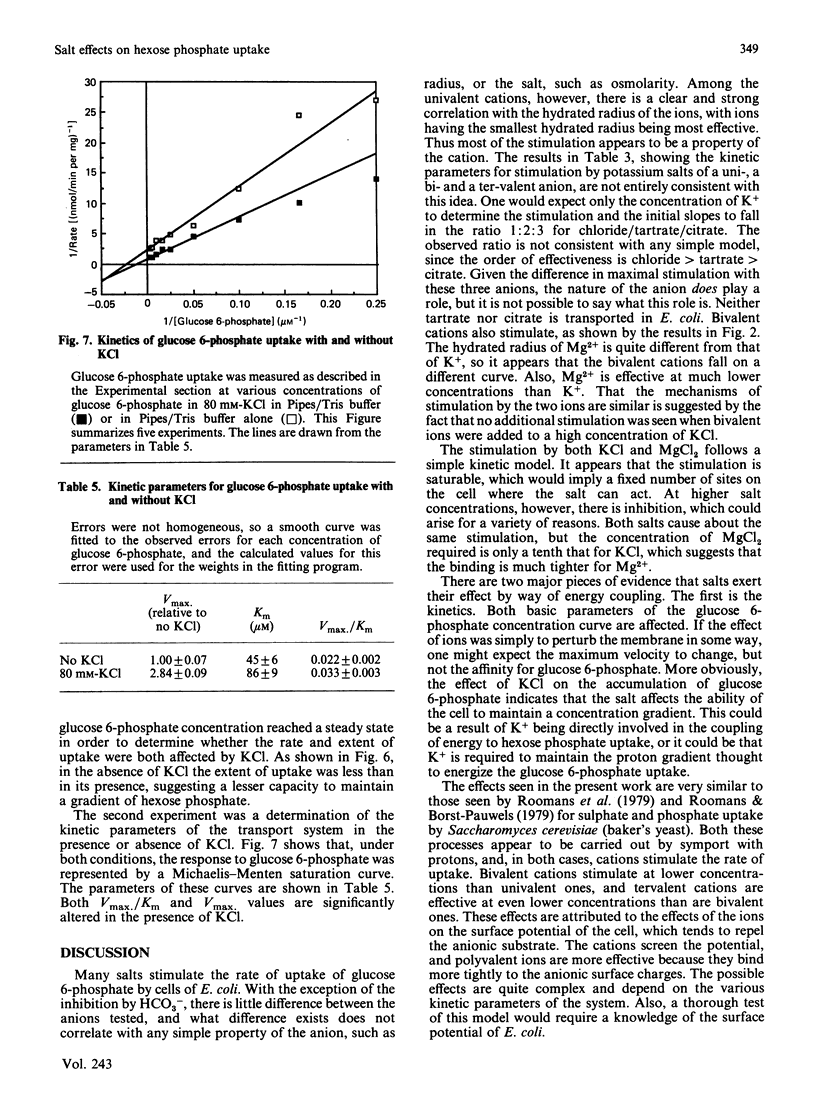
Hexose phosphate uptake in Escherichia coli is stimulated by salts. KCl and MgCl2 stimulate to about the same extent, but Mg2+ is effective at a tenth the concentration of K+. At higher concentrations, both salts inhibit. The stimulation by a series of salts correlates strongly with the hydrated radius of the cation, with small ions more effective than large. There are effects by the anion, but they do not correlate with any simple property. Cells accumulate glucose 6-phosphate to a higher concentration in the presence of KCl than in its absence. The maximum velocity of glucose 6-phosphate uptake is stimulated by KCl, as is the ratio V/Km.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Essenberg R. C., Kornberg H. L. Energy coupling in the uptake of hexose phosphates by Escherichia coli. J Biol Chem. 1975 Feb 10;250(3):939–945. [PubMed] [Google Scholar]
- Lanyi J. K. The role of Na+ in transport processes of bacterial membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):377–397. doi: 10.1016/0304-4157(79)90011-x. [DOI] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The relationship between the electrochemical proton gradient and active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):854–859. doi: 10.1021/bi00624a007. [DOI] [PubMed] [Google Scholar]
- Roomans G. M., Borst-Pauwels G. W. Interaction of cations with phosphate uptake by Saccharomyces cerevisiae. Effects of surface potential. Biochem J. 1979 Mar 15;178(3):521–527. doi: 10.1042/bj1780521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roomans G. M., Kuypers G. A., Theuvenet A. P., Borst-Pauwels G. W. Kinetics of sulfate uptake by yeast. Biochim Biophys Acta. 1979 Feb 20;551(1):197–206. doi: 10.1016/0005-2736(79)90365-1. [DOI] [PubMed] [Google Scholar]
- Russell L. M., Rosenberg H. Linked transport of phosphate, potassium ions and protons in Escherichia coli. Biochem J. 1979 Oct 15;184(1):13–21. doi: 10.1042/bj1840013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. M., Rosenberg H. The nature of the link between potassium transport and phosphate transport in Escherichia coli. Biochem J. 1980 Jun 15;188(3):715–723. doi: 10.1042/bj1880715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Wentzel D. L., Feucht B. U., Judice J. J. A transport system for phosphoenolpyruvate, 2-phosphoglycerate, and 3-phosphoglycerate in Salmonella typhimurium. J Biol Chem. 1975 Jul 10;250(13):5089–5096. [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Functions of Na+ and K+ in the active transport of -aminoisobutyric acid in a marine pseudomonad. J Biol Chem. 1971 Jun 25;246(12):4066–4074. [PubMed] [Google Scholar]


