Abstract
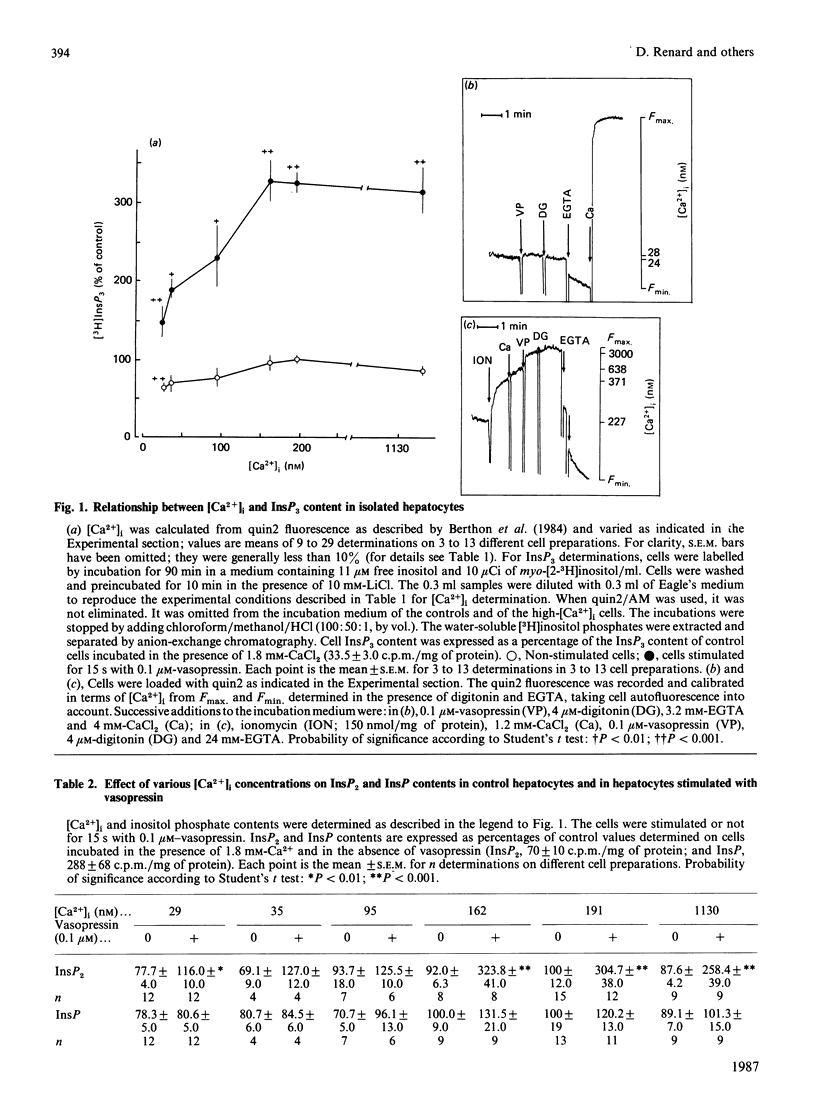
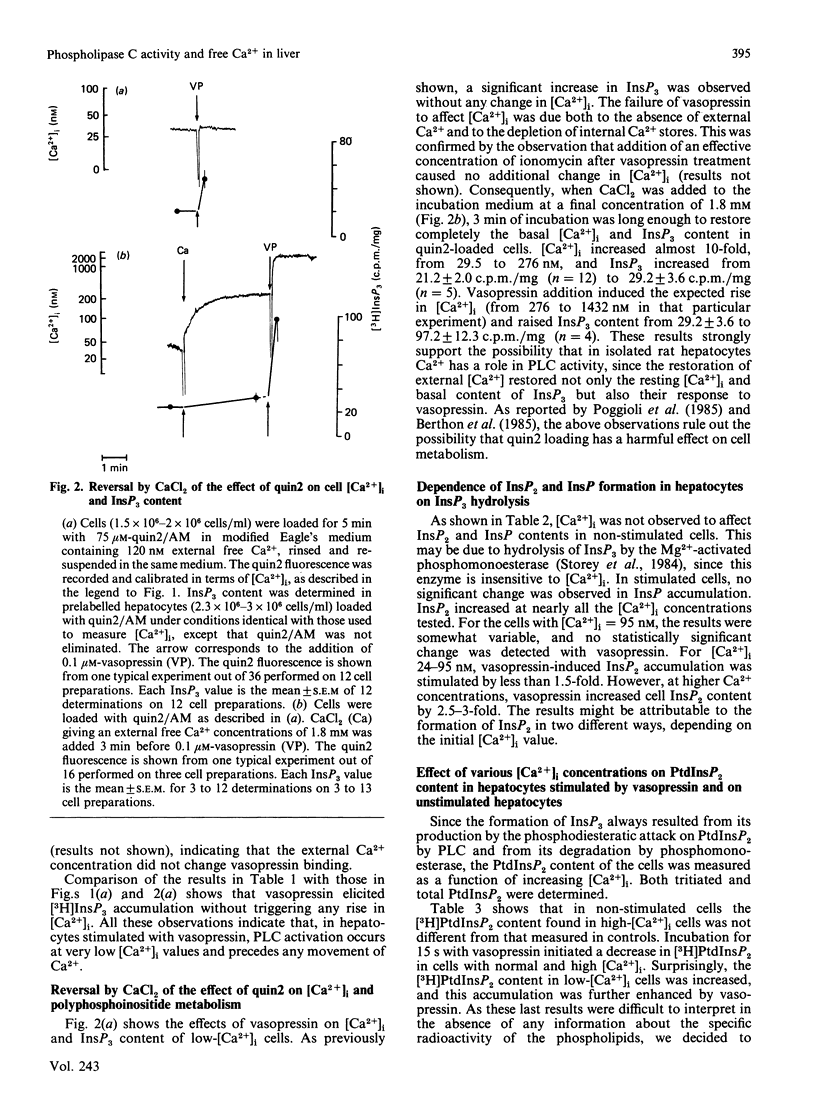
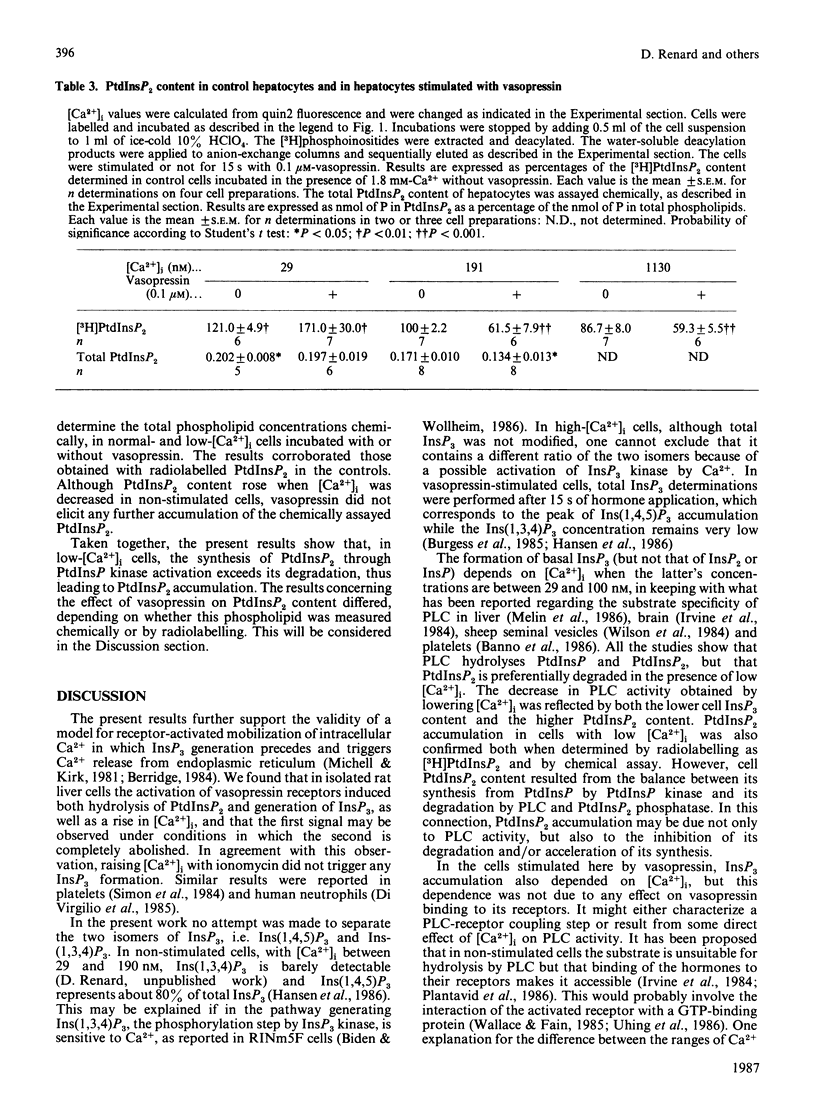
The dependence of phospholipase C activity on the cytosolic Ca2+ concentration ([Ca2+]i) was studied in intact liver cells treated with the Ca2+-mobilizing hormone vasopressin, or not so treated. Phospholipase C (PLC) activity was estimated from the formation of [3H]inositol trisphosphate (InsP3) and the degradation of [3H]phosphatidylinositol 4,5-bisphosphate (PtdInsP2). The [Ca2+]i of the cells was clamped from 29 to 1130 nM by quin2 loading. This wide concentration range was obtained by loading the hepatocytes with a high concentration of the Ca2+ indicator in low-Ca2+ medium or by using the Ca2+ ionophore ionomycin in medium containing Ca2+. In resting cells, in which [Ca2+]i was 193 nM, treatment with 0.1 microM-vasopressin which stimulates liver PLC maximally, tripled InsP3 content and raised [Ca2+]i to 2 microM within 15 s. Lowering [Ca2+]i partially decreased cell InsP3 content as well as the ability of vasopressin to stimulate InsP3 formation maximally. At 29 nM, the lowest Ca2+ concentration obtained in isolated liver cells, basal InsP3 content was 64% of that measured in control cells. Addition of vasopressin no longer affected [Ca2+]i, but significantly increased InsP3 by 200%, although less than in the controls (300%). The maintenance of the greater part of the PLC response at constant [Ca2+]i indicated that, in the liver, InsP3 formation does not result from an increase in [Ca2+]i. The effects of lowering [Ca2+]i were reversible. When low cell [Ca2+]i was restored to a normal value, resting InsP3 content and the ability of vasopressin to stimulate InsP3 formation maximally by 300% were also restored. Raising [Ca2+]i from 193 to 1130 nM had little effect on the InsP3 content or the vasopressin-mediated increase in InsP3. In agreement with the stimulation of PLC activity by vasopressin, cell [3H]PtdInsP2 and total PtdInsP2 were degraded by application of this hormone for 15 s. In contrast, when [Ca2+]i was lowered to 29 nM, basal [3H]PtdInsP2 and total PtdInsP2 were increased by about 30%, [3H]PtdInsP2 was further increased by vasopressin, but total PtdInsP2 was not changed. These results show that, in intact hepatocytes, PLC is little affected by [Ca2+]i concentrations above 193 nM, but is partially dependent on Ca2+ below that value. They suggest that, in addition to activating PLC activity, vasopressin might stimulate PtdInsP2 synthesis, presumably via phosphatidylinositol-phosphate kinase, and that this pathway might predominate in cells with low [Ca2+]i.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banno Y., Nakashima S., Nozawa Y. Partial purification of phosphoinositide phospholipase C from human platelet cytosol; characterization of its three forms. Biochem Biophys Res Commun. 1986 Apr 29;136(2):713–721. doi: 10.1016/0006-291x(86)90498-5. [DOI] [PubMed] [Google Scholar]
- Bartfai T. Preparation of metal-chelate complexes and the design of steady-state kinetic experiments involving metal nucleotide complexes. Adv Cyclic Nucleotide Res. 1979;10:219–242. [PubMed] [Google Scholar]
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthon B., Binet A., Mauger J. P., Claret M. Cytosolic free Ca2+ in isolated rat hepatocytes as measured by quin2. Effects of noradrenaline and vasopressin. FEBS Lett. 1984 Feb 13;167(1):19–24. doi: 10.1016/0014-5793(84)80824-8. [DOI] [PubMed] [Google Scholar]
- Berthon B., Capiod T., Claret M. Effects of noradrenaline, vasopressin and angiotensin on the Na-K pump in rat isolated liver cells. Br J Pharmacol. 1985 Sep;86(1):151–161. doi: 10.1111/j.1476-5381.1985.tb09445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Ca2+ regulates the inositol tris/tetrakisphosphate pathway in intact and broken preparations of insulin-secreting RINm5F cells. J Biol Chem. 1986 Sep 15;261(26):11931–11934. [PubMed] [Google Scholar]
- Binet A., Berthon B., Claret M. Hormone-induced increase in free cytosolic calcium and glycogen phosphorylase activation in rat hepatocytes incubated in normal and low-calcium media. Biochem J. 1985 Jun 15;228(3):565–574. doi: 10.1042/bj2280565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Irvine R. F., Berridge M. J., McKinney J. S., Putney J. W., Jr Actions of inositol phosphates on Ca2+ pools in guinea-pig hepatocytes. Biochem J. 1984 Dec 15;224(3):741–746. doi: 10.1042/bj2240741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest R., Prpić V., Exton J. H., Blackmore P. F. Stimulation of inositol trisphosphate formation in hepatocytes by vasopressin, adrenaline and angiotensin II and its relationship to changes in cytosolic free Ca2+. Biochem J. 1985 Apr 1;227(1):79–90. doi: 10.1042/bj2270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Vicentini L. M., Treves S., Riz G., Pozzan T. Inositol phosphate formation in fMet-Leu-Phe-stimulated human neutrophils does not require an increase in the cytosolic free Ca2+ concentration. Biochem J. 1985 Jul 15;229(2):361–367. doi: 10.1042/bj2290361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The control by Ca2+ of the polyphosphoinositide phosphodiesterase and the Ca2+-pump ATPase in human erythrocytes. Biochem J. 1982 Jan 15;202(1):53–58. doi: 10.1042/bj2020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud F., M'Zali H., Chailley B., Mazet F. Changes in morphology and in polyphosphoinositide turnover of human erythrocytes after cholesterol depletion. Biochim Biophys Acta. 1984 Nov 21;778(1):191–200. doi: 10.1016/0005-2736(84)90462-0. [DOI] [PubMed] [Google Scholar]
- Hansen C. A., Mah S., Williamson J. R. Formation and metabolism of inositol 1,3,4,5-tetrakisphosphate in liver. J Biol Chem. 1986 Jun 25;261(18):8100–8103. [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Dawson R. M. Phosphatidylinositol-4,5-bisphosphate phosphodiesterase and phosphomonoesterase activities of rat brain. Some properties and possible control mechanisms. Biochem J. 1984 Feb 15;218(1):177–185. doi: 10.1042/bj2180177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Lundberg G. A., Jergil B., Sundler R. Subcellular localization and enzymatic properties of rat liver phosphatidylinositol-4-phosphate kinase. Biochim Biophys Acta. 1985 Sep 30;846(3):379–387. doi: 10.1016/0167-4889(85)90009-6. [DOI] [PubMed] [Google Scholar]
- Martin T. F., Lucas D. O., Bajjalieh S. M., Kowalchyk J. A. Thyrotropin-releasing hormone activates a Ca2+-dependent polyphosphoinositide phosphodiesterase in permeable GH3 cells. GTP gamma S potentiation by a cholera and pertussis toxin-insensitive mechanism. J Biol Chem. 1986 Feb 25;261(6):2918–2927. [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Claret M. Synergistic stimulation of the Ca2+ influx in rat hepatocytes by glucagon and the Ca2+-linked hormones vasopressin and angiotensin II. J Biol Chem. 1985 Sep 25;260(21):11635–11642. [PubMed] [Google Scholar]
- Melin P. M., Sundler R., Jergil B. Phospholipase C in rat liver plasma membranes. Phosphoinositide specificity and regulation by guanine nucleotides and calcium. FEBS Lett. 1986 Mar 17;198(1):85–88. doi: 10.1016/0014-5793(86)81189-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Nakanishi H., Nomura H., Kikkawa U., Kishimoto A., Nishizuka Y. Rat brain and liver soluble phospholipase C: resolution of two forms with different requirements for calcium. Biochem Biophys Res Commun. 1985 Oct 30;132(2):582–590. doi: 10.1016/0006-291x(85)91173-8. [DOI] [PubMed] [Google Scholar]
- Plantavid M., Rossignol L., Chap H., Douste-Blazy L. Studies of endogenous polyphosphoinositide hydrolysis in human platelet membranes. Evidence that polyphosphoinositides remain inaccessible to phosphodiesterase in the native membrane. Biochim Biophys Acta. 1986 Feb 12;875(2):147–156. doi: 10.1016/0005-2760(86)90163-3. [DOI] [PubMed] [Google Scholar]
- Poggioli J., Mauger J. P., Claret M. Effect of cyclic AMP-dependent hormones and Ca2+-mobilizing hormones on the Ca2+ influx and polyphosphoinositide metabolism in isolated rat hepatocytes. Biochem J. 1986 May 1;235(3):663–669. doi: 10.1042/bj2350663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggioli J., Mauger J. P., Guesdon F., Claret M. A regulatory calcium-binding site for calcium channel in isolated rat hepatocytes. J Biol Chem. 1985 Mar 25;260(6):3289–3294. [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Rhodes D., Prpić V., Exton J. H., Blackmore P. F. Stimulation of phosphatidylinositol 4,5-bisphosphate hydrolysis in hepatocytes by vasopressin. J Biol Chem. 1983 Mar 10;258(5):2770–2773. [PubMed] [Google Scholar]
- Rink T. J., Pozzan T. Using quin2 in cell suspensions. Cell Calcium. 1985 Apr;6(1-2):133–144. doi: 10.1016/0143-4160(85)90040-5. [DOI] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Siess W., Binder H. Thrombin induces the rapid formation of inositol bisphosphate and inositol trisphosphate in human platelets. FEBS Lett. 1985 Jan 21;180(1):107–112. doi: 10.1016/0014-5793(85)80241-6. [DOI] [PubMed] [Google Scholar]
- Simon M. F., Chap H., Douste-Blazy L. Activation of phospholipase C in thrombin-stimulated platelets does not depend on cytoplasmic free calcium concentration. FEBS Lett. 1984 May 7;170(1):43–48. doi: 10.1016/0014-5793(84)81365-4. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Cox C. C., Snyderman R. Receptor-coupled activation of phosphoinositide-specific phospholipase C by an N protein. Science. 1986 Apr 4;232(4746):97–100. doi: 10.1126/science.3006254. [DOI] [PubMed] [Google Scholar]
- Steiner S., Lester R. L. Studies on the diversity of inositol-containing yeast phospholipids: incorporation of 2-deoxyglucose into lipid. J Bacteriol. 1972 Jan;109(1):81–88. doi: 10.1128/jb.109.1.81-88.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Uhing R. J., Prpic V., Jiang H., Exton J. H. Hormone-stimulated polyphosphoinositide breakdown in rat liver plasma membranes. Roles of guanine nucleotides and calcium. J Biol Chem. 1986 Feb 15;261(5):2140–2146. [PubMed] [Google Scholar]
- Wallace M. A., Fain J. N. Guanosine 5'-O-thiotriphosphate stimulates phospholipase C activity in plasma membranes of rat hepatocytes. J Biol Chem. 1985 Aug 15;260(17):9527–9530. [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Joseph S. K., Thomas A. P. Inositol trisphosphate and diacylglycerol as intracellular second messengers in liver. Am J Physiol. 1985 Mar;248(3 Pt 1):C203–C216. doi: 10.1152/ajpcell.1985.248.3.C203. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Bross T. E., Hofmann S. L., Majerus P. W. Hydrolysis of polyphosphoinositides by purified sheep seminal vesicle phospholipase C enzymes. J Biol Chem. 1984 Oct 10;259(19):11718–11724. [PubMed] [Google Scholar]


