Abstract
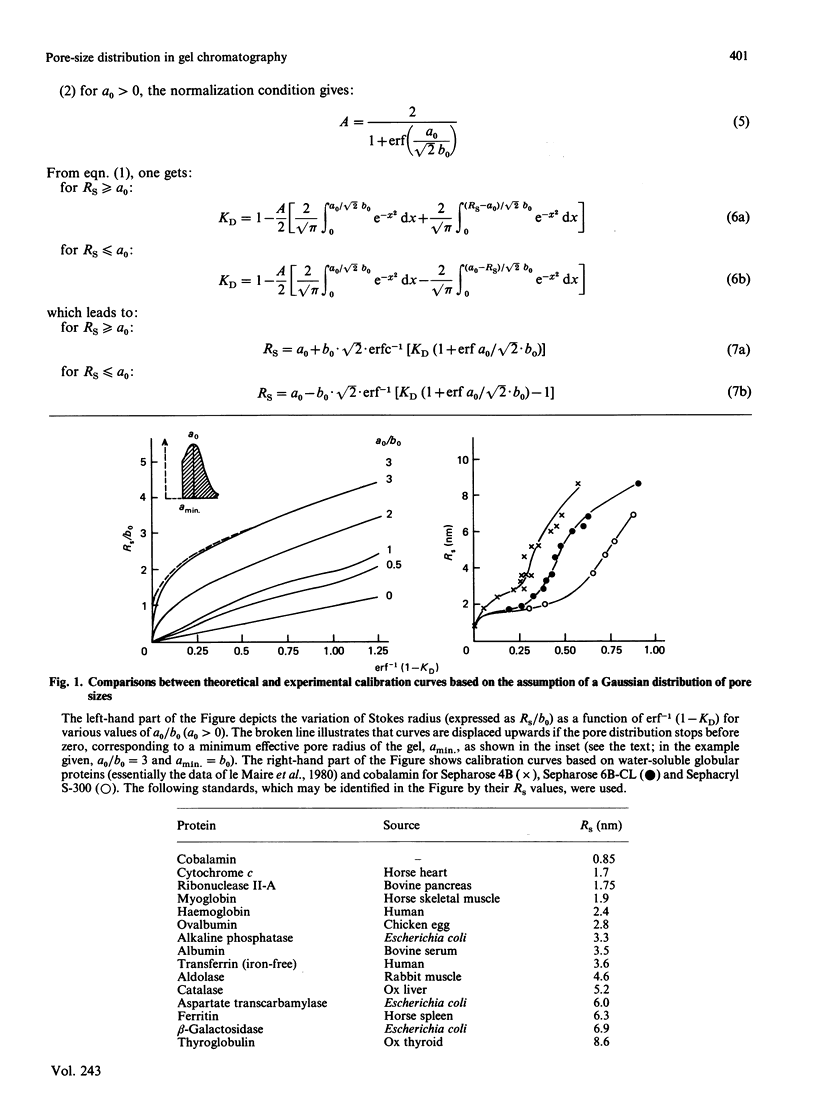
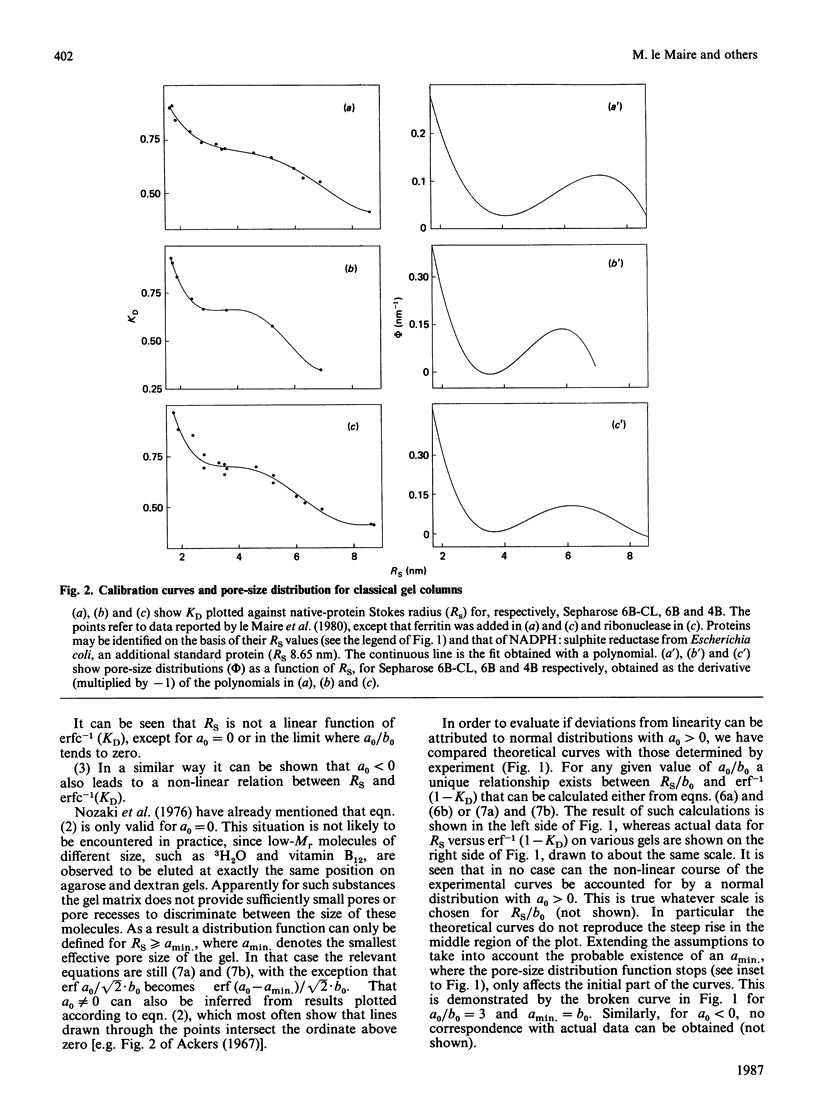
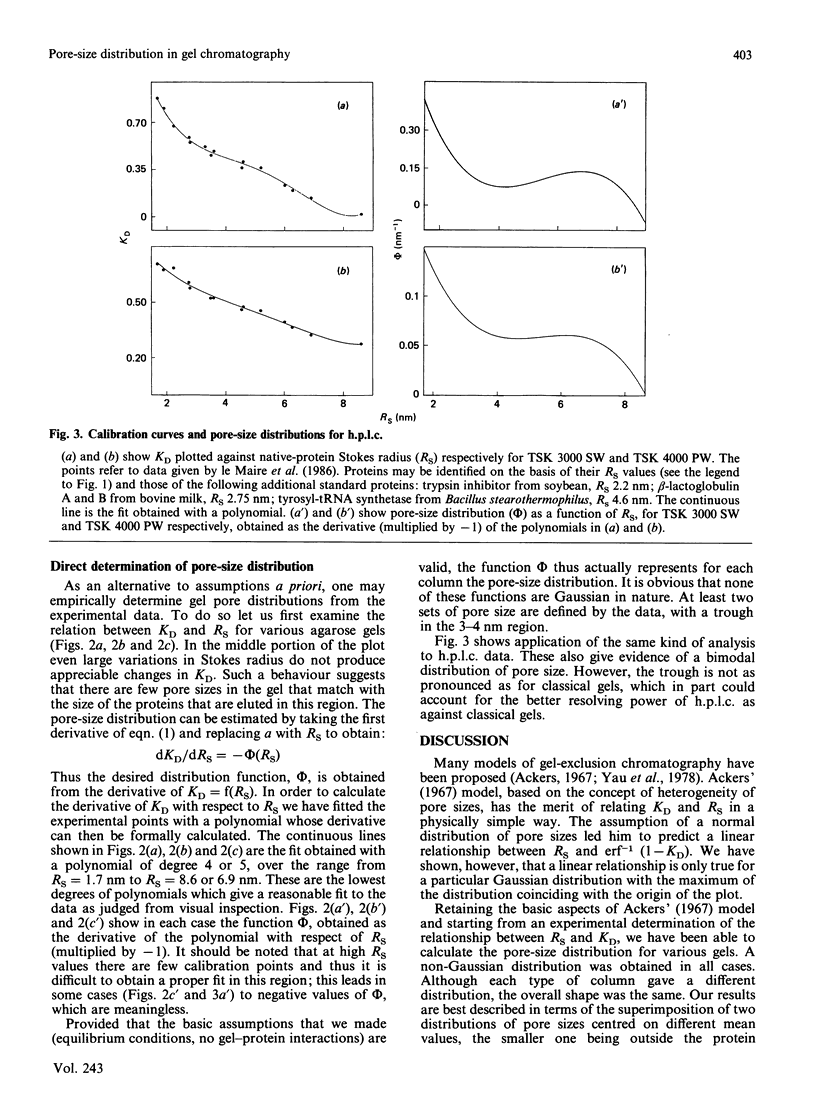
The separation of proteins by gel-exclusion chromatography has been explained in terms of partitioning of the macromolecules within the gel by a distribution of pores of various radii. The assumption that the distribution of pore sizes is Gaussian has led to the prediction of a linear relationship between the molecular Stokes radius (RS) of the protein and the function erf-1 (1-KD), where KD is the partition coefficient [Ackers (1967) J. Biol. Chem. 242, 3237-3238]. Since careful calibrations of classical (agarose and dextran) gels and h.p.l.c. gels have shown that such a linear relationship is not verified experimentally over a wide range of native protein sizes, we have reinvestigated the model of Ackers (above reference). We show that Ackers' (above reference) derivation is not valid except for a particular Gaussian distribution of pore sizes centred at the origin. Relaxation of this restriction to allow for other types of Gaussian distributions cannot account for the non-linear calibration curves that we have obtained. Instead we show that the pore-size distribution can be calculated from the experimentally determined function KD = f(RS) and that this distribution is bimodal (non-Gaussian). One distribution is centred below 2 nm, whereas the mean value of the second one is around 6-8 nm. The minimum in this bimodal distribution corresponds, for some gels, to a region of poor resolution, which needs to be appreciated for the proper use of gel chromatography in the determination of molecular size.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K. Analytical gel chromatography of proteins. Adv Protein Chem. 1970;24:343–446. doi: 10.1016/s0065-3233(08)60245-4. [DOI] [PubMed] [Google Scholar]
- Arnott S., Fulmer A., Scott W. E., Dea I. C., Moorhouse R., Rees D. A. The agarose double helix and its function in agarose gel structure. J Mol Biol. 1974 Dec 5;90(2):269–284. doi: 10.1016/0022-2836(74)90372-6. [DOI] [PubMed] [Google Scholar]
- Fish W. W., Reynolds J. A., Tanford C. Gel chromatography of proteins in denaturing solvents. Comparison between sodium dodecyl sulfate and guanidine hydrochloride as denaturants. J Biol Chem. 1970 Oct 10;245(19):5166–5168. [PubMed] [Google Scholar]
- HODGKIN D. C., KAMPER J., MACKAY M., PICKWORTH J., TRUEBLOOD K. N., WHITE J. G. Structure of vitamin B12. Nature. 1956 Jul 14;178(4524):64–66. doi: 10.1038/178064a0. [DOI] [PubMed] [Google Scholar]
- Horiike K., Tojo H., Yamano T., Nozaki M. Interpretation of the stokes radius of macromolecules determined by gel filtration chromatography. J Biochem. 1983 Jan;93(1):99–106. doi: 10.1093/oxfordjournals.jbchem.a134183. [DOI] [PubMed] [Google Scholar]
- Houssin C., le Maire M., Aggerbeck L. P., Shechter E. The lactose permease of Escherichia coli: evidence in favor of a dimer. Arch Biochem Biophys. 1985 Aug 1;240(2):593–606. doi: 10.1016/0003-9861(85)90066-9. [DOI] [PubMed] [Google Scholar]
- Le Maire M., Aggerbeck L. P., Monteilhet C., Andersen J. P., Møller J. V. The use of high-performance liquid chromatography for the determination of size and molecular weight of proteins: a caution and a list of membrane proteins suitable as standards. Anal Biochem. 1986 May 1;154(2):525–535. doi: 10.1016/0003-2697(86)90025-4. [DOI] [PubMed] [Google Scholar]
- Le Maire M., Moller J. V., Tanford C. Retention of enzyme activity by detergent-solubilized sarcoplasmic Ca2+ -ATPase. Biochemistry. 1976 Jun 1;15(11):2336–2342. doi: 10.1021/bi00656a014. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Fish W. W. Protein polypeptide chain molecular weights by gel chromatography in guanidinium chloride. Methods Enzymol. 1972;26:28–42. doi: 10.1016/s0076-6879(72)26004-9. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Schechter N. M., Reynolds J. A., Tanford C. Use of gel chromatography for the determination of the Stokes radii of proteins in the presence and absence of detergents. A reexamination. Biochemistry. 1976 Aug 24;15(17):3884–3890. doi: 10.1021/bi00662a036. [DOI] [PubMed] [Google Scholar]
- Robinson N. C., Talbert L. Triton X-100 induced dissociation of beef heart cytochrome c oxidase into monomers. Biochemistry. 1986 May 6;25(9):2328–2335. doi: 10.1021/bi00357a005. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Tanford C., Nozaki Y., Reynolds J. A., Makino S. Molecular characterization of proteins in detergent solutions. Biochemistry. 1974 May 21;13(11):2369–2376. doi: 10.1021/bi00708a021. [DOI] [PubMed] [Google Scholar]
- Valdes R., Jr, Ackers G. K. Study of protein subunit association equilibria by elution gel chromatography. Methods Enzymol. 1979;61:125–142. doi: 10.1016/0076-6879(79)61011-x. [DOI] [PubMed] [Google Scholar]
- le Maire M., Rivas E., Møller J. V. Use of gel chromatography for determination of size and molecular weight of proteins: further caution. Anal Biochem. 1980 Jul 15;106(1):12–21. doi: 10.1016/0003-2697(80)90112-8. [DOI] [PubMed] [Google Scholar]


