Abstract
Although efforts have been undertaken to produce polyhydroxyalkanoates (PHA) with various monomers, the low yield of PHAs because of complex metabolic pathways and inhibitory substrates remains a major hurdle in their analyses and applications. Therefore, we investigated the feasibility of mass production of PHAs containing 5-hydroxyvalerate (5HV) using δ-valerolactone (DVL) without any pretreatment along with the addition of plant oil to achieve enough biomass. We identified that PhaCBP-M-CPF4, a PHA synthase, was capable of incorporating 5HV monomers and that C. necator PHB−4 harboring phaCBP-M-CPF4 synthesized poly(3HB-co-3HHx-co-5HV) in the presence of bean oil and DVL. In fed-batch fermentation, the supply of bean oil resulted in the synthesis of 49 g/L of poly(3HB-co-3.7 mol% 3HHx-co-5.3 mol%5HV) from 66 g/L of biomass. Thermophysical studies showed that 3HHx was effective in increasing the elongation, whereas 5HV was effective in decreasing the melting point. The contact angles of poly(3HB-co-3HHx-co-5HV) and poly(3HB-co-3HHx) were 109 and 98°, respectively. In addition, the analysis of microbial degradation confirmed that poly(3HB-co-3HHx-co-5HV) degraded more slowly (82% over 7 days) compared to poly(3HB-co-3HHx) (100% over 5 days). Overall, the oil-based fermentation strategy helped produce more PHA, and the mass production of novel PHAs could provide more opportunities to study polymer properties.
Keywords: polyhydroxyalkanoates, 5-hydroxyvalerate, δ-valerolactone, plant oil
1. Introduction
Polyhydroxyalkanoates (PHAs) are polymers that accumulate within microorganisms in nutrient-limited environments, and they have been studied as promising alternatives to conventional petroleum-based plastics because of their biodegradability and bio-based characteristics [1]. Moreover, the excellent biodegradability of PHAs, which decompose not only in soil but also in marine environments, makes them attractive candidates for use as bio-plastics [2]. PHAs are composed of various hydroxyalkanoate monomers that are present in microorganisms [3]. The most common PHA is poly(3-hydroxybutyrate) (P(3HB)), which consists of a 3HB monomer. P(3HB) had the advantage of being naturally produced in various microorganisms without the need for engineering [4]. However, its rigid and brittle nature, along with its high melting point, makes industrial utilization of PHB difficult [5]. These issues have been addressed by integrating other monomers into the polymer chains to synthesize copolymers, terpolymers, and tetrapolymers [6].
Poly(3-hydroxybutyrate-co-5-hydroxyvalerate) (PHB5HV or P(3HB-co-5HV)) is an actively researched PHA copolymer. 5-hydroxyvalerate (5HV) contributes to the flexibility of PHA polymers and decreases their melting points [7]. Additionally, the incorporation of 5HV into a polymer enhances its degradation rate by lipases; P(3HB-co-3HP-co-5HV) has been reported to exhibit low cytotoxicity and support cell proliferation [8]. Poly(3HB-co-5HV) can be synthesized by Aneurinibacillus thermoaerophilus and Methylocystis parvus [9,10]. One of the most studied PHA-producing strains, wild-type Cupriavidus necator, was also reported to be able to synthesize PHA containing 5HV; however, the mole fraction of 5HV in PHA produced by C. necator was very low [11]. Nonetheless, PHB5HV with a high mole fraction of 5HV could be synthesized by simply introducing PHA synthase with broad specificity into C. necator. Recently, in our lab, we successfully produced poly(3HB-co-5HV) with the mole fraction of 5HV reaching 70% using an engineered strain of C. necator. The PHB5HV produced by this strain had an increased elongation at a break of up to 1400% [12]. However, despite the favorable properties of PHAs containing 5HV monomers, their production is still relatively low, and further research on large-scale production is needed.
C. necator, also known as Ralstonia eutropha, can utilize various carbon sources, such as fructose, CO2, and plant oils, to produce PHA [13]. To date, various studies have been conducted to synthesize different types of PHA from fructose by constructing pathways for monomer synthesis through genetic engineering in Cupriavidus necator or by supplying precursors alongside fructose [14,15,16,17]. However, producing various PHAs from fructose through genetic modification significantly reduces the yield of PHA, and in subsequent scale-up processes, it may not be possible to produce PHA with the desired mole fraction of monomers [18,19] (Table 1). Moreover, considering that the highest reported PHB yield from fructose fermentation is 18.46 g/L from 350 g/L of fructose, the method of supplying precursors along with fructose is also not suitable for the mass production of various PHAs.
Table 1.
Production of co- or terpolymers with various monomers using Cupriavidus necator strains.
| C-Source | PHA Type | DCW (g/L) | PHA (g/L) | Content (%) | Ref. | |
|---|---|---|---|---|---|---|
| Copolymers | fructose | P (3HB-co-2.1 mol% 3HP) | 2.5 | - | 31 | [18] |
| fructose | P (3HB-co-37.7 mol% 3HHx) | 1.42 ± 0.05 | - | 41.1 ± 3.0 | [19] | |
| fructose | P (3HB-co-64.9 mol% 3HV) | 1.5 ± 0.1 | - | 42.5 ± 3.8 | [20] | |
| fructose + ε-CL | P (3HB-co-4HB) | 7.5 | - | - | [14] | |
| waste rapeseed oil + propanol | P (3HB-co-3HV) | 14.7 ± 0.3 | 11.7 ± 0.7 | 80 | [21] | |
| P (3HB-co-3HV) * | 138 | 105 | 76 | |||
| fructose + coconut oil | P (3HB-co-3HHx) | 19 | 15 | - | [22] | |
| palm kernel oil + butyrate | P (3HB-co-3HHx) * | 153–175 | 113–138 | 73.3–78.6 | [23] | |
| Terpolymers | fructose + 4HVA + 5HVA | P (3HB-co-3HV-co-4HV-co-5HV) | 8.7 ± 0.1 | 6.3 ± 0.1 | 72 | [12] |
| fructose + GVL | P (3HB-co-3HV-co-4HV) | 8.2 ± 0.2 | - | 80 ± 2 | [14] | |
| fructose | P (3HB-co-3HV-co-3H4MV-co-3H2MP) | 1.67 ± 0.03 | 0.93 ± 0.03 | 55.9 ± 1.8 | [16] | |
| tung oil | P (3HB-co-3HV-co-3HHx) | 1.65 | 0.68 | 41.2 | [24] | |
| fructose + bean oil + DVL | P (3HB-co-3HHx-co-5HV) * | 66 | 49 | 73 | this work | |
| fructose + bean oil + DVL | P (3HB-co-3HHx-co-5HV) * | 90 | 69 | 77 | this work |
Abbreviation: ε-CL, ε-caprolactone; GVL, γ-valerolactone; 4HVA, 4-hydroxyvaleric acid; 5HVA, 5-hydroxyvaleric acid; DVL, δ-valerolactone; 3HB, 3-hydroxybutyrate; 3HV, 3-hydroxyvalerate; 3H4MV, 3-hydroxy-4-methylvalerate; 3H2MP, 3-hydroxy-2-methylpropionate; 3HP, 3-hydroxypropionate; 3HHx, 3-hydroxyhexanoate; 4HB, 4-hydroxybutyrate; 4HV, 4-hydroxyvalerate; 5HV, 5-hydroxyvalerate. PHA with the mark * was produced through fed-batch fermentation.
Plant oils, such as soybean oil, palm oil, rapeseed oil, and jatropha oil, have been widely used as inexpensive carbon sources for PHA production in C. necator, aiming to reduce the cost of PHA [25]. Furthermore, plant oils yield higher amounts of PHA than sugars, and with simple genetic modifications to C. necator, plant oils can be used to produce poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx or poly(3HB-co-3HHx)) with properties superior to those of PHB [26,27]. Engineered C. necator H16 produced 125.9 g/L of PHBHHx from 164.7 g/L of dry cell weight using palm kernel oil. Another study reported the production of 86% PHBHHx from 124 g/L biomass of C. necator Re2058/pCB113 using fructose and rapeseed oil [28,29]. Therefore, using plant oil instead of fructose for the synthesis of various PHAs in C. necator seems more advantageous in terms of yield and cost-effectiveness.
While plant oils have been widely utilized to produce poly(3HB-co-3HHx), research on their use for producing PHAs with other monomers remains limited. Therefore, this study aims to assess the feasibility of using vegetable oil and precursors to enable large-scale production of various PHAs, particularly focusing on 5HV monomers by co-supplying DVL with vegetable oil. Furthermore, by analyzing the thermal and physical properties of the resulting polymer, this research provides a deeper understanding of the role of 5HV within PHA.
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
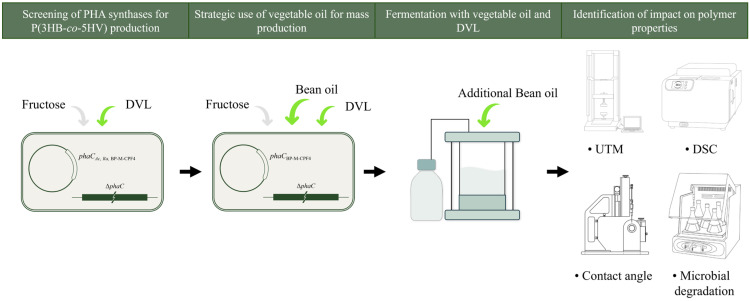
The C. necator PHB−4 wild-type strain and PHB−4 strains harboring various PHA synthase genes (phaCBP-M-CPF4, phaCAc, and phaCRa) were cultured in 5 mL of tryptic soy broth (TSB) at 30 °C for 24 h to obtain the seed culture (Figure 1).
Figure 1.
The overall methodological framework of the experiment. The experiment proceeded in the following order: establishing a strain capable of producing poly(3HB-co-5HV), confirming the increase in PHA production containing 5HV through vegetable oil feeding, verifying the feasibility of mass-producing PHA containing 5HV through vegetable oil-based fermentation, and finally, analyzing the physical properties of the produced PHA.
The culture was supplemented with 50 µg/mL kanamycin for plasmid activity. For PHA production, 5× Ralstonia eutropha minimal medium (5× ReMM; 20 g/L NaH2PO4, 23 g/L Na2HPO4, 2.25 g/L K2SO4), 100× MgSO4 (39 g/L MgSO4), 100× CaCl2 (6.2 g/L CaCl2), and 1000× trace element (15 g/L FeSO4·7H2O, 2.4 g/L MnSO4·H2O, 2.4 g/L ZnSO4·7H2O, 0.48 g/L CuSO4·5H2O dissolved in 0.1 M hydrochloric acid) solutions were used. The 5× ReMM was sterilized at 121 °C for 15 min using an autoclave, and the 100× MgSO4, 100× CaCl2, and 1000× trace element solutions were filtered using a 28-mm syringe filter with a 0.22-μm polyethersulfone (PES) membrane (Sartorious, Goettingen, Germany). A fructose solution (200 g/L) was used as the carbon source, and 50 g/L of urea solution was used as the nitrogen source. The production test of PHA with 3HHx and 5HV units was carried out in a 5 mL culture in a 14 mL round test tube, and the main culture conditions were as follows: 4 g/L NaH2PO4, 4.6 g/L Na2HPO4, 0.45 g/L K2SO4; 0.39 g/L MgSO4; 0.062 g/L CaCl2; 0.0015 g/L FeSO4·7H2O, 0.0024 g/L MnSO4·H2O, 0.0024 g/L ZnSO4·7H2O, 0.00048 g/L CuSO4·5H2O; 10 g/L fructose; 1 g/L urea. Additionally, various concentrations of 5-hydroxyvaleric acid (5HVA), δ-valerolactone (DVL), and bean oil were added, and 50 µg/mL kanamycin was added for plasmid activity. Unless otherwise specified, medium components were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Analytical Methods
For PHA analysis, the culture broth was centrifuged, and the resulting pellet was washed twice with 1 mL distilled water (DW) and 1 mL hexane. Hexane was used to remove the residual bean oil and was not used when bean oil was not added to the culture. The cell pellet was washed once again with 1 mL of distilled water. The washed cells were then transferred into a glass vial for lyophilization, and the dry cell weight was measured. Next, 1 mL of chloroform and 1 mL of 15% (v/v) H2SO4/85% methanol solution were added to a glass vial, and methanolysis was performed at 100 °C for 2 h, followed by cooling to room temperature. Then, 1 mL of DW was added to the methyl ester solution, and the mixture was vortexed twice for 5 s each. The bottom of the chloroform layer was transferred to a microtube containing anhydrous Na2SO4 to remove residual water. The filtered 1 mL sample was analyzed using GC-FID (Young In Chromass 6500, Seoul, Republic of Korea) equipped with a fused silica capillary column (DB-FFAP, 30-mm length, 0.320-mm internal diameter, and 0.25 film, Agilent, Santa Clara, CA, USA). The injection volume was 1 µL, and the split ratio was 1/10. Helium was used as the carrier gas at a flow rate of 3.0 mL/min. The oven program for PHA analysis was as follows: 80 °C for 5 min, then increased from 80 °C to 220 °C at a rate of 20 °C/min, and held at 220 °C for 5 min. During the analysis, the injector temperature was maintained at 210 °C, and the FID temperature was maintained at 230 °C.
2.3. Gel Permeation Chromatography (GPC)
GPC was used to determine the number-average molar mass (Mn), weight-average molar mass (MW), and dispersity. GPC was performed using an HPLC system (Young In Chromass, Seoul, Republic of Korea) comprising a loop injector (Rheodyne 7725i), dual-headed isocratic pump system (YL9112), column oven (YL9131) with three columns (K-G 4A, guard column; K-804 8.0 × I.D. × 300 mm; K-805, 8.0 × 300 mm; Sho-dex), and a refractive index detector (YL9170). Chloroform was used as the mobile phase at a flow rate of 1 mL/min at 40 °C. The injection volume of the prepared samples was 20 µL. Polystyrene standards ranging from 5000 to 2,000,000 Da were used to calculate the MW and construct the calibration curve.
2.4. Analysis of the Physical and Thermal Characteristics
A Universal Testing Machine (UTM) Model was used to measure the tensile strength, Young’s modulus, and elongation at break of the samples. The samples were cut into 10 × 60-mm pieces, and the gauge length was defined accordingly. The tests were conducted at a crosshead speed of 10 mm/min. The elongation at break was calculated using Equation (1):
| EL = (dafter − dbefore)/dbefore × 100 | (1) |
where d represents the distance between the grips holding the sample before and after the sample break.
Differential scanning calorimetry (DSC) was performed using a NEXTA DSC 200 instrument (Hitachi high-tech, Hitachi, Japan) to analyze the thermal properties of the PHAs. Approximately 5 mg of the PHA film containing 3HHx and 5HV units was measured in an aluminum pan for DSC. The experiment was conducted under a N2 atmosphere. The heating and cooling rates were all 10 °C/min. The temperature program is as follows: 30 °C, 7 min → −60 °C, 10 min → 190 °C, 10 min (first heating) → −60 °C, 2 min → 30 °C, 10 min → −60 °C, 10 min → 190 °C, 10 min (second heating) → −60 °C, 0 min. The crystallization temperature (Tc), glass transition temperature (Tg), and melting temperature (Tm) of the polymer were determined via second heating.
2.5. Culture Conditions for a 5-L Fermenter
Precultures for the fermenter were prepared at a volume of 50 mL (TSB) in two 250-mL baffled flasks at 30 °C for 24 h. Each culture was centrifuged at 3511× g at 4 °C. The cell pellet was washed twice with 10 mL of DW. Each cell pellet was then suspended in 10 mL of DW to inoculate the fermenter with a total of 20 mL of cell culture. Fed-batch fermentation was conducted on a 2-L scale in a 5-L fermenter. The main culture conditions at the beginning of the culture were as follows: 4 g/L NaH2PO4, 4.6 g/L Na2HPO4, 0.45 g/L K2SO4; 0.39 g/L MgSO4; 0.062 g/L CaCl2; 0.0015 g/L FeSO4·7H2O, 0.0024 g/L MnSO4·H2O, 0.0024 g/L ZnSO4·7H2O, 0.00048 g/L CuSO4·5H2O; 10 g/L fructose; 5 g/L Bean oil; 1 g/L NH4NO3. Additionally, 100 g/L of bean oil was gradually supplied for 10–20 h, which is the exponential phase, and 5 g/L of DVL was supplied at 48 h in one stroke. For pH control, phosphoric acid was used as the acid, and ammonia water as the base. The pH was adjusted to 6.8, and the dissolved oxygen (DO) level was set at 20%. The initial stirring rate was 200 rpm and was increased to 600 rpm to maintain the DO level during culture.
3. Results
3.1. PhaC Screening for Efficient 5HV Polymerization in PHA
Wild-type C. necator has been reported to synthesize PHB5HV [11]. However, because of the high specificity of C. necator phaC for 3HB, the mole fraction of 5HV was very low. Additionally, during PHA mass production, if a large amount of carbon source was supplied to produce 3HB monomers, it was expected that the mole fraction of 5HV would decrease further because of the accumulation of large amounts of 3HB in PHA. Therefore, for the efficient production of PHAs containing 5HV, screening for phaC with broad specificity not only toward 3HB but also toward 5HV was necessary. In addition, as 5HV is obtained by an additional saponification process requiring an increase in pH using DVL for ring opening and then neutralization of the pH for biological utilization, the ability of strains to use DVL as a precursor was also important [12,30].
To confirm the individual activity of phaC, a plasmid carrying three types of phaC (phaCBP-M-CPF4, phaCAc, and phaCAt) was inserted into C. necator PHB−4. C. necator PHB−4 is a strain that cannot produce PHB because of the abnormal expression of PHA synthase from C. necator H16, achieved through random mutagenesis techniques [31,32]. Next, we tested the ability of the constructed strains to synthesize poly (3HB-co-5HV) using delta-valerolactone as a precursor; along with wild-type C. necator H16; 1% fructose was used to support cell growth.
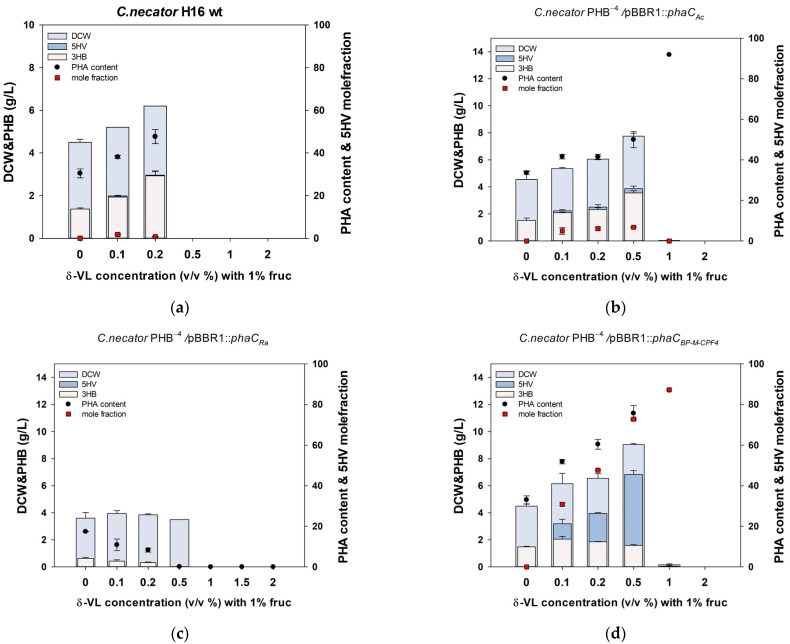
Wild-type C. necator H16 was able to synthesize poly (3HB-co-5HV) from DVL. The highest achieved mole fraction of 5HV in PHA was 1.7%, which was noted at a DVL concentration of 0.1%; however, it decreased to 0.7% when the DVL concentration was 0.2% (Figure 2a). As the DVL concentration increased, both dry cell weight (DCW) and PHB also increased. This indicates that DVL contributes to 3HB accumulation in C. necator. However, when the concentration of DVL exceeded 0.5%, C. necator H16 wild type did not grow.
Figure 2.
Validation of the 5HV polymerization ability of wild-type Cupriavidus necator H16 and C. necator PHB−4 harboring various PHA synthases. (a) Wild-type C. necator H16 (b) C. necator PHB−4 harboring phaCAc (c) C. necator PHB−4 harboring phaCRa (d) C. necator PHB−4 harboring phaCBP-M-CPF4. Statistical analysis was performed by applying ANOVA with the level of significance at 5%.
phaCAc and phaCRa from Aeromonas caviae and Rhodococcus aetherivorans, respectively, have been widely used to synthesize poly(3HB-co-3HHx) in C. necator because of their broad specificity [33,34,35,36]. phaCAc and phaCRa were codon-optimized and inserted into the pBBR1MCS2 vector along with a high-expression ribosome binding site (RBS). As expected, C. necator PHB−4 with inserted phaCAc produced PHA with a 5HV mole fraction of approximately 6% from DVL, with the highest 5HV mole fraction of 6.7% being achieved at a DVL concentration of 0.5% (Figure 2b). However, C. necator PHB−4 carrying phaCRa was unable to synthesize poly(3HB-co-5HV). Additionally, as the concentration of 5HV increased, the PHA content decreased (Figure 2c).
phaCBP-M-CPF4, discovered by a research team in Malaysia, is a PHA synthase derived from uncultured bacteria found through the metagenomic analysis of mangrove soil. phaCBP-M-CPF4 has been reported to possess broad substrate specificity and is capable of polymerizing 3HB, but also 3HV, 4HB, 4HV, 5HV, 3HHx, and others [37,38,39]. Furthermore, when codon-optimized phaCBP-M-CPF4 is expressed in C. necator PHB−4 with a high-expression RBS, it can synthesize poly(3HB-co-5HV) with a mole fraction of 70% 5HV from 5-hydroxyvaleric acid (5-HVA) [12]. The PHA synthase with the highest 5HV polymerization ability was phaCBP-M-CPF4. When we confirmed the production of poly (3HB-co-5HV) from DVL in C. necator PHB−4 with inserted phaCBP-M-CPF4 (PHB−4/BP), we observed that with increasing DVL concentration, both the production of PHA and the mole fraction of 5HV in PHA increased (Figure 2d). PHB−4/BP produced poly(3HB-co-5HV) with a 5HV mole fraction of 72.7% at a concentration of 0.5% DVL, yielding 6.85 ± 0.20 g/L. This is comparable to a previous study in which the same strain produced PHA with a 5HV mole fraction of 72.2%, yielding 5.87 ± 0.10 g/L when 0.5% 5HVA was used as the precursor [12]. The similarity in the 5HV mole fraction and PHA production when DVL and its ring-opening form, 5HVA, were used suggests that C. necator PHB−4 expresses a lactonase capable of opening lactone rings, such as DVL, and its activity was already sufficient.
3.2. Validation of Plant Oil for Increased Production of PHA Containing 5HV
Plant oil has been widely used to enhance the price competitiveness of PHA because of its cost-effectiveness and high conversion rate to PHA compared to sugar [40,41]. In addition, plant oils could help in the production of considerable amounts of cell mass and PHA [21,29,42,43,44]. Therefore, we aimed to investigate whether additional supplementation with plant oil could increase the production of poly(3HB-co-5HV) by the PHB−4/BP strain to maximize the production of PHA containing 5HV. Additionally, in a previous study, considering the replacement of the native-phaC of C. necator H16 with the broad-specificity phaC from Rhodococcus aetheriborans, resulting in the production of PHA with a 1–1.5% mole fraction of 3HHx, we anticipated that feeding the PHB−4/BP strain with an oil source would lead to the integration of additional 3HHx into PHA, thereby enhancing the flexibility of polymer [45].
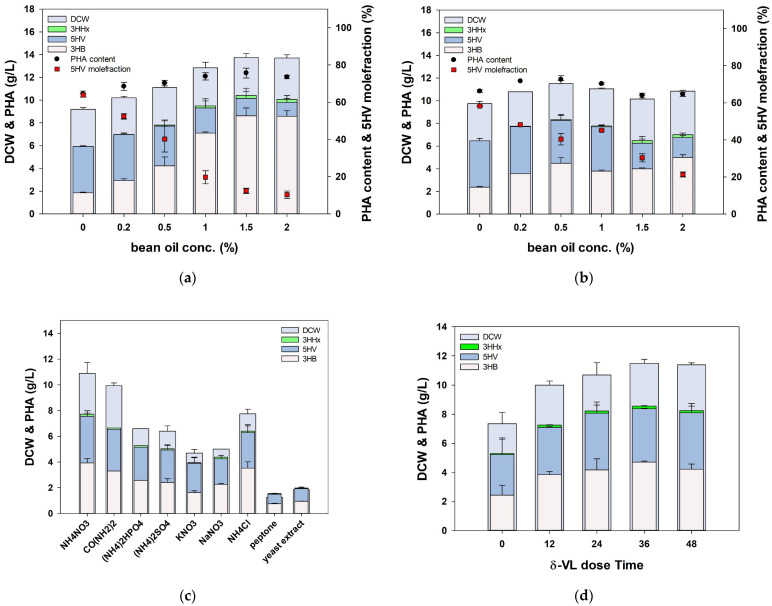
In the previous experiment, we confirmed that the optimal concentration of DVL to produce poly(3HB-co-5HV) with a high 5HV mole fraction was 0.5% (Figure 2). Therefore, we used 1% fructose as the carbon source and 0.5% 5HV or DVL as the precursors, with bean oil supplemented at concentrations ranging from 0% to 2%. We observed that with the use of 5HV as the precursor, the addition of bean oil from 0% to 1.5% led to an increase in both PHA production and DCW (Figure 3a). Additionally, as expected, a small amount of 3HHx was polymerized, resulting in the formation of a poly(3HB-co-3HHx-co-5HV) ter-polymer. However, as the concentration of bean oil increased beyond 1.5%, the mole fraction of 5HV in PHA decreased from 64% to 10%.
Figure 3.
Confirmation of increased production of poly(3HB-co-3HHx-co-5HV) after supplying bean oil. (a) 5-hydroxyvaleric acid (5HVA) or (b) δ-valerolactone (DVL) was used as the 5HV precursor. (c) N-source optimization for poly(3HB-co-3HHx-co-5HV) production. (d) DVL feeding time optimization. Statistical analysis was performed by applying ANOVA with a level of significance at 5%.
On the contrary, when DVL was used as the precursor, increasing the concentration of bean oil from 0% to 0.5% resulted in an approximately 30% increase in PHA production (Figure 3b). However, when bean oil was added at concentrations of 1% or higher, both DCW and PHA decreased. This decrease might be attributed to the fact that 5HVA, as a free fatty acid, could directly participate in beta-oxidation along with bean oil, whereas DVL, as a lactone, could not directly participate in beta-oxidation. Therefore, the reduced compatibility between bean oil and DVL may be attributed to the requirement for lactonase, an enzyme essential for the ring-opening process of DVL. However, 5HVA is generally difficult to obtain, and an additional saponification process is required. Therefore, we focused on the 30% increase in PHA production when bean oil was added to the DVL precursor and designed further experiments.
The type of nitrogen source is crucial for PHA production from various substrates [46]. Furthermore, since the carbon source has changed, the corresponding control of the nitrogen source is also necessary. To enhance PHA production from bean oil and DVL, after including 1% fructose for initial cell growth, nine different nitrogen sources were administered at a concentration of 0.1%, and the production of poly(3HB-co-3HHx-co-5HV) was compared (Figure 3c). When urea and NH4Cl were employed, the PHA production was similar, yielding 6.66 g/L and 6.41 ± 0.35 g/L, respectively. However, the PHA content when urea was used was 67.9%, whereas it was higher at 82.6 ± 1.85% when NH4Cl was used. On the contrary, when NH4NO3 was used, PHB−4/BP accumulated the highest PHA at 7.73 ± 0.19 g/L, and the DCW was also the highest at 10.9 ± 0.6 g/L. In our study, the type of nitrogen source used resulted in significant differences in PHA production from bean oil and DVL. Notably, the highest cell mass and PHA yield were achieved when using NH4NO3, a less commonly used nitrogen source, instead of the more commonly used sources such as urea and (NH4)2SO4 in Cupriavidus necator fermentation. This highlights the importance of optimizing the nitrogen source as a key aspect of fermentation optimization.
The precursors used in the synthesis of PHA copolymers are known to inhibit cell growth [47]. Previous experiments revealed that DVL, the precursor of 5HV, also inhibited cell growth at concentrations of 0.5–1%. The toxicity of such precursors could be mitigated by supplying precursors after the cells had grown to a certain extent following the initiation of the culture. To confirm that the delayed supply of the DVL precursor could overcome its toxicity, DVL was supplied at 0, 12, 24, 36, and 48 h after the start of the culture, and PHA production was subsequently observed. It was observed that the delayed supply of DVL resulted in higher PHA production, with the production increasing with the extent of delay. When DVL was supplied at 0 h, the DCW and PHA production were 7.35 ± 0.55 g/L and 5.31 ± 0.75 g/L, respectively (Figure 3d). When DVL supply was delayed, both DCW and PHA production increased, reaching a maximum of 11.5 ± 0.2 g/L DCW and 8.56 ± 0.02 PHA, respectively.
3.3. Fed-Batch Production of Poly(3HB-co-3HHx-co-5HV)
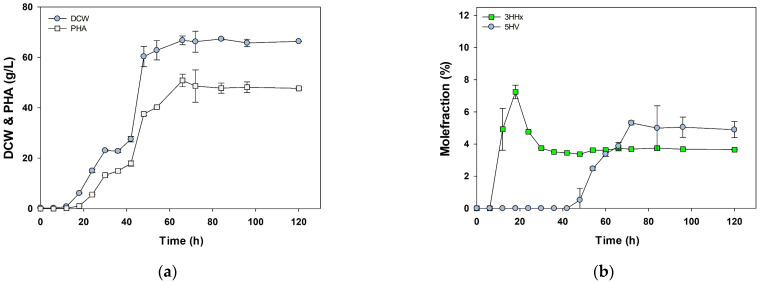
Based on the previously identified conditions, the feasibility of mass-producing poly(3HB-co-3HHx-co-5HV) using PHB−4/BP was tested on a 5-L scale. The working volume in the 5-L fermenter was 2 L. Initially, 1% fructose, 0.5% bean oil, and 0.1% NH4NO3 were fed during cultivation, with 0.5% DVL added after 48 h of fermentation, and terpolymer production and cell growth of the PHB−4/BP strain were observed. At 42 h, i.e., 6 h before DVL feeding, a PHA production of 0.57 ± 0.01 g/L was observed, with a PHA content of 9.18 ± 0.34%. The low PHA production and PHA content in the fermenter were attributed to the use of ammonia water as a pH regulator. Owing to the continuous decrease in pH with the growth of PHB−4/BP, ammonia water was continuously supplied to maintain the pH, leading to excessive N-source feeding and a subsequent decrease in PHA content. However, after DVL feeding at 48 h, the 5HV molar fraction continued to increase until 84 h, resulting in 3.17 ± 0.06 g/L of poly(3HB-co-2 mol% 3HHx-co-76 mol% 5HV) at 84 h.
To increase PHA production and content, an additional 100 g/L of bean oil was supplied from 10 h to 20 h with all other conditions remaining unchanged. Consequently, both DCW and PHA increased until 72 h, accumulating 48.63 ± 4.53 g/L of poly(3HB-co-3.7 mol% 3HHx-co-5.3 mol% 5HV) with 66.25 ± 2.95 g/L of DCW (Figure 4). Subsequently, when the supply of bean oil was increased to 200 g/L from 10 h to 20 h, 68.6 ± 7.8 g/L of poly(3HB-co- 5.6 mol% 3HHx-co- 2.7 mol% 5HV) was accumulated at 144 h with 90.3 ± 0.9 g/L of DCW (Supplementary Figure S1). However, the growth rate decreased, possibly because of the reduced oxygen supply resulting from the rapid supply of oil over a short period. Through this experiment, we confirmed the potential for the mass production of PHA-containing 5HV monomers using the PHB−4/BP strain with bean oil supplementation.
Figure 4.
Fed-batch Poly(3HB-co-3HHx-co-5HV) production by C. necator PHB−4 harboring phaCBP-M-CPF4 in a 5-L jar fermenter. The initial culture conditions included 1% fructose, 0.5% bean oil, and 0.1% NH4NO3. From 10 h to 20 h of culture, 100 g/L of bean oil was supplied, and 5 g/L of DVL was added after 48 h. The changes in (a) DCW (Dry Cell Weight) and PHA, as well as (b) the molar fractions of 3HHx and 5HV, were monitored over the cultivation period. Statistical analysis was performed by applying ANOVA with the level of significance at 5%.
3.4. Physical and Mechanical Properties of Poly(3HB-co-3HHx-co-5HV)
To characterize the produced Poly (3HB-co-3.6 mol% 3HHx-co-4.9 mol% 5HV), the cell pellet was concentrated using a continuous centrifuge. PHA was then extracted using chloroform and cast into films. The thermal and physical properties of the poly(3HB-co-3HHx-co-5HV) film were analyzed using a UTM, DSC, and GPC and compared with those of the poly(3HB-co-7.1 mol% 3HHx) film.
In a previous study, a poly(3HB-co-5HV) film with a 70% 5HV mole fraction was reported to exhibit an elongation at a break of 1400% [12]. However, the increase in elongation at break owing to the 5HV content in PHA was found to be minimal at low 5HV mole fractions. Upon confirming the elongation at break using UTM for both films, the elongation at break of poly(3HB-co-7.1 mol% 3HHx) was 176.6 ± 18.2%, whereas that of Poly(3HB-co- 3.6 mol% 3HHx-co- 4.9 mol% 5HV) was 16.6 ± 1.4% (Table 2). This indicates that at low mole fractions, 3HHx has a more significant effect on elongation at break than 5HV.
Table 2.
Physical and mechanical properties of poly(3HB-co-3.6 mol% 3HHx-co-4.9 mol% 5HV) produced by C. necator PHB−4 harboring phaCBP-M-CPF4 and poly(3HB-co-7.1 mol% 3HHx).
| PHA Composition | Properties | |||||||
|---|---|---|---|---|---|---|---|---|
| 3HB | 3HHx | 5HV | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) | Mn (103) | Mw (103) | Dispersity |
| 91.5 | 3.6 | 4.9 | 9.03 ± 0.18 | 16.64 ± 1.36 | 112.14 ± 4.65 | 60 ± 10 | 306 ± 19 | 5.2 ± 0.5 |
| 92.9 | 7.1 | - | 6.81 ± 0.42 | 176.6 ± 18.24 | 81.71 ± 5.25 | 53 ± 3 | 354 ± 11 | 6.7 ± 0.2 |
The high melting point of PHB hinders its industrial application because it requires more energy for plastic molding. Additionally, the degradation of PHA at high temperatures is unavoidable, which can lead to changes in the polymer’s properties. Therefore, to facilitate the molding of PHA to increase its industrial applicability and prevent degradation during the molding process, it is necessary to decrease its melting point. Analysis of the thermal behavior of the two films using DSC revealed that the Tm of Poly(3HB-co-3HHx-co-5HV) was 151.5 °C, and no Tg and Tc were observed (Table 3). In contrast, for poly(3HB-co-3HHx), the Tm was 175.5 °C, with Tg and Tc values of 2.5 °C and 48.3 °C, respectively. Considering the significantly lower Tm of Poly(3HB-co-3HHx-co-5HV) than that of poly(3HB-co-3HHx), it can be inferred that the 5HV monomer in PHA contributes more to the decrease in Tm than 3HHx.
Table 3.
Thermal properties of poly(3HB-co-3.6 mol% 3HHx-co-4.9 mol% 5HV) produced by C. necator PHB−4 harboring phaCBP-M-CPF4 and poly(3HB-co-7.1 mol% 3HHx).
| Tg (°C) | Tc (°C) | Tm (°C) | ΔH (mJ/mg) | |
|---|---|---|---|---|
| Poly(3HB-co-3HHx-co-5HV) | n.a. | n.a. | 151.5 ± 0.1 | 16.6 ± 0.3 |
| Poly(3HB-co-3HHx) | 2.6 ± 0.2 | 48.3 ± 0.3 | 175.5 ± 0.0 | 19.2 ± 0.2 |
Abbreviations: Tg, glass transition temperature; Tc, crystallization temperature, Tm, melting temperature.
The roles of 3HHx and 5HV as PHA monomers have also been confirmed in two previous studies that produced poly(3HB-co-33 mol% 5HV) and poly(3HB-co-31.1 mol% 3HHx) using engineered C. necator [12,22]. The elongation at break for poly(3HB-co-31.1 mol% 3HHx) produced from lauric acid was 243.4 ± 36.4%, whereas poly(3HB-co-33 mol% 5HV) exhibited an elongation at break of 157.7%. Furthermore, PHBHHx achieved an elongation at a break of 132.2 ± 17.3%, similar to that of poly(3HB-co-33 mol% 5HV), with just the incorporation of 9.4% of 3HHx. Regarding thermal properties, poly(3HB-co-33 mol% 5HV) exhibited melting points of 31.5 °C and 153.3 °C, whereas poly(3HB-co-31.1 mol% 3HHx) showed a relatively higher melting point of 166.4 °C. A relatively lower melting point was observed in the presence of 5HV, even at low mole fractions. Poly(3HB-co-12.0 mol% 3HHx) produced from Cupriavidus eutrophus B10646 had a melting point of 170 °C, whereas poly(3HB-co-5HV) produced from Escherichia coli showed a melting point of 159.2 °C with only 4.7 mol% polymerization of 5HV [48,49].
For commercialization and improved processability of PHA, enhancement of flexibility and reduction of melting point should be achieved simultaneously. Typically, increasing the content of monomers other than 3HB in PHA copolymers improves the flexibility of the polymer and reduces its melting point. However, the mole fraction of the monomers required to achieve ideal mechanical properties and melting points may vary. For instance, in PHBHHx, a mole fraction of approximately 10% 3HHx may be optimal because higher 3HHx mole fractions result in amorphous polymers with reduced tensile strength and Young’s modulus. However, a higher molar fraction of 3HHx may be required to achieve a lower melting point. In such cases, the production of PHA terpolymers by incorporating additional monomers with different properties is a good solution.
This study confirmed that 3HHx is effective in increasing elongation even at low molar fractions, whereas 5HV is effective in reducing the melting point at low molar fractions. Therefore, it is anticipated that optimizing the molar fraction of 3HHx to achieve the desired mechanical properties, followed by the addition of 5HV to achieve a lower melting point, can be an effective approach.
3.5. Comparison of PHBHHx5HV and Conventional PHBHHx Degradation and Contact Angle
Previous reports have identified the role of 5HV units in facilitating polymer degradation of PHA by pig pancreatic lipase [50]. However, the role of 5HV in PHA degradation by microorganisms has not been reported. Microbulbifer sp. Sol66, a PHB-degrading strain isolated from the coastal area of Korea, demonstrated a rapid degradation rate, reaching up to 98% in just 4 days of cultivation [51]. To verify the role of 5HV in PHA degradation by microorganisms, the degradation rate of Poly(3HB-co-3.6 mol% 3HHx-co-4.9 mol% 5HV) by Sol66 was compared to that of the Poly (3HB-co-7.1 mol% 3HHx) film.
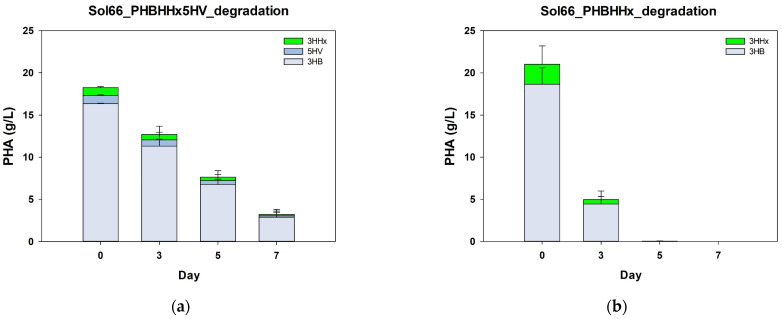
The Poly(3HB-co-3.6 mol% 3HHx-co-4.9 mol% 5HV) film exhibited a lower degradation rate than the Poly(3HB-co-7.1 mol% 3HHx) film. The Poly(3HB-co-7.1 mol% 3HHx) film degraded by 76.4% in just 3 days, whereas the Poly(3HB-co-3.6 mol% 3HHx-co-4.9 mol% 5HV) film degraded by only 30.4% (Figure 5). Additionally, while the PHBHHx film was completely degraded by the fifth day, the Poly(3HB-co-3.6 mol% 3HHx-co-4.9 mol% 5HV) film degraded by only 82.3% by the seventh day. Throughout the 7-day degradation period, the molar fraction of 5HV remained between 4 and 5%, and the molar fraction of 3HHx remained between 3 and 4%. This indicates that the degradation of PHA terpolymers by Sol66 did not specifically involve certain monomers.
Figure 5.
Time-dependent degradation rate of (a) poly(3HB-co-3.7 mol% 3HHx-co-5.3 mol% 5HV) and (b) poly(3HB-co-7.1 mol% 3HHx) films by Microbulbifer sp. Sol66. Statistical analysis was performed by applying ANOVA with the level of significance at 5%.
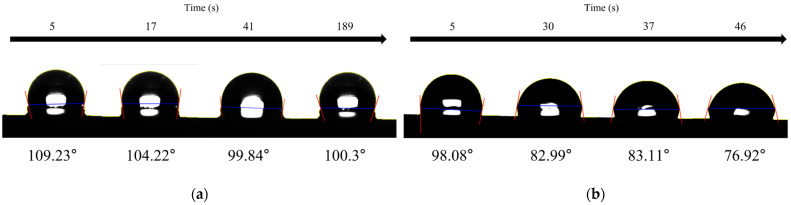
The interaction of the two PHA films with liquids was analyzed using a contact angle meter. For poly(3HB-co-3HHx-co-5HV), the initial contact angle was 109.23°, which is higher than that of 98.08° for poly(3HB-co-3HHx) (Figure 6). This indicated that the poly(3HB-co-3HHx-co-5HV) film had a more hydrophobic surface. Furthermore, when observing the change in contact angle over time, Poly(3HB-co-3HHx-co-5HV) maintained a contact angle of approximately 100° for about 3 min. In contrast, the contact angle of the Poly(3HB-co-3HHx) film decreased to 76.92° after 46 s of measurement, and after 3 min, the water droplet was completely absorbed by the polymer. The PHA films showed differences in the contact angle depending on the presence of 5HV monomers. However, because the films may contain components derived from plant oils or microbial sources that can be extracted with chloroform and may influence the contact angle, it may be difficult to attribute these changes solely to the presence of 5HV.
Figure 6.
Analysis of changes in contact angle over time for extracted (a) poly(3HB-co-3.7 mol% 3HHx-co-5.3 mol% 5HV) and (b) poly(3HB-co-7.1 mol% 3HHx) films. Statistical analysis was performed by applying ANOVA with the level of significance at 5%.
4. Conclusions
Previous efforts to produce PHA copolymers or terpolymers through genetic modification of organisms aimed at synthesizing precursors from fructose or adding precursors alongside fructose have been limited by low production yields. Therefore, in this study, we aimed to explore the feasibility of mass-producing PHA containing 5HV by using vegetable oil together with DVL, a precursor of 5HV.
First, to efficiently incorporate 5HV into PHA, we tested the ability of three PHA synthases, known for their broad substrate specificity, to synthesize P(3HB-co-5HV). As a result, we developed a Cupriavidus necator PHB−4 strain harboring phaCBP-M-CPF4, which exhibited a very high ability to synthesize P(3HB-co-5HV). Additionally, by feeding bean oil, we found that while the addition of vegetable oil leads to further synthesis of 3HHx in the PHA, the overall PHA production increased by about 30% when DVL was used.
In a 5-L jar fermenter, the potential for large-scale production of poly(3HB-co-3HHx-co-5HV) through the addition of plant oil was confirmed. Under fed-batch fermentation, PHB−4/BP produced 48.63 ± 4.53 g/L of poly(3HB-co-3.7 mol% 3HHx-co-5.3 mol% 5HV) from 66.25 ± 2.95 g/L of DCW when 100 g/L of bean oil was additionally supplied. Furthermore, although the lag phase was long because the conditions were not thoroughly optimized, when 200 g/L of bean oil was supplied, 90.3 ± 0.9 g/L of DCW yielded 68.6 ± 7.8 g/L of poly(3HB-co-5.6 mol% 3HHx-co-2.7 mol% 5HV) at 144 h. The terpolymers were collected by continuous centrifugation and extracted with chloroform. By comparing the physical properties of the produced terpolymer and conventional PHBHHx, it was confirmed that, at lower mole fractions, the 3HHx monomer in PHA effectively increased the elongation at break, whereas the 5HV monomer effectively reduced the melting point.
The roles of 5HV and 3HHx in the produced PHA were elucidated through a comparison with conventional Poly(3HB-co-3HHx). Thermal and physical property analyses revealed that at low molar fractions, 3HHx effectively enhanced the elongation of PHA, while 5HV reduced its melting point. Additionally, PHA containing 5HV exhibited slower degradation by marine microorganisms compared to Poly(3HB-co-3HHx), with a higher contact angle, indicating improved water resistance.
Vegetable oil is an inexpensive feedstock known to produce significantly higher PHA yield and biomass in Cupriavidus necator fermentation compared to sugars. However, despite these advantages, the use of vegetable oil for PHA production has been primarily limited to P(3HB-co-3HHx), with few examples of its use for large-scale production of PHAs containing other monomers. In this study, we strategically utilized vegetable oil to enhance biomass production for the mass production of PHA containing 5HV. Furthermore, we propose using a combination of vegetable oil and precursors as an effective strategy for the large-scale production of PHAs with diverse monomers. The analysis of polymer properties suggests that incorporating 5HV can lower the melting point, regulate degradation rates in marine environments, and enhance water resistance. Given the demand for large quantities of samples in polymer applications, this oil-based fermentation strategy facilitates greater PHA production, offering expanded opportunities to investigate the properties of novel PHAs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym16192773/s1, Figure S1: Fed-batch Poly(3HB-co-3HHx-co-5HV) production by Cupriavidus necator PHB-4 harboring phaCBP-M-CPF4 in a 5-L jar fermenter. The initial culture conditions included 1% fructose, 0.5% bean oil, and 0.1% NH4NO3. From 10 h to 20 h of culture, 200 g/L of bean oil was supplied, and 5 g/L of DVL was added after 48 h. The changes in (A) DCW (Dry Cell Weight) and PHA, as well as (B) the molar fractions of 3HHx and 5HV were monitored over the cultivation period.
Author Contributions
S.-J.O.: Writing—Original Draft, Conceptualization, Investigation, Y.S.: Visualization, J.O.: Methodology, S.K.: Formal analysis, Y.L.: Formal analysis, S.C.: Investigation, G.L.: Investigation, J.-C.J.: Validation, J.-M.J.: Validation, J.-J.Y.: Writing—Reviewing and Editing, S.K.B.: Writing—Reviewing and Editing, J.A.: conceptualization. H.-T.K.: supervision, funding, Y.-H.Y.: Supervision, Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was supported by the R&D Program of KEIT (20009508, 20018072, 20025698), the Cooperative Research Program for Agriculture Science and Technology Development (PJ01708201) through the Rural Development Administration and the support of ‘The R&D Program for Forest Science Technology (Project No. “2023473E10-2325-EE02”)’ provided by Korea Forest Service (Korea Forestry Promotion Institute).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rueda E., Gonzalez-Flo E., Mondal S., Forchhammer K., Arias D.M., Ludwig K., Drosg B., Fritz I., Gonzalez-Esquer C.R., Pacheco S., et al. Challenges, Progress, and Future Perspectives for Cyanobacterial Polyhydroxyalkanoate Production. Rev. Environ. Sci. Biotechnol. 2024;23:321–350. doi: 10.1007/s11157-024-09689-0. [DOI] [Google Scholar]
- 2.Meereboer K.W., Misra M., Mohanty A.K. Review of Recent Advances in the Biodegradability of Polyhydroxyalkanoate (PHA) Bioplastics and Their Composites. Green Chem. 2020;22:5519–5558. doi: 10.1039/D0GC01647K. [DOI] [Google Scholar]
- 3.Prados E., Maicas S. Bacterial Production of Hydroxyalkanoates (PHA) Univers. J. Microbiol. Res. 2016;4:23–30. doi: 10.13189/ujmr.2016.040104. [DOI] [Google Scholar]
- 4.Alves M.I., Macagnan K.L., Rodrigues A.A., De Assis D.A., Torres M.M., De Oliveira P.D., Furlan L., Vendruscolo C.T., Moreira A.D.S. Poly(3-Hydroxybutyrate)-P(3HB): Review of Production Process Technology. Indus. Biotechnol. 2017;13:192–208. doi: 10.1089/ind.2017.0013. [DOI] [Google Scholar]
- 5.Briassoulis D., Tserotas P., Athanasoulia I.G. Alternative Optimization Routes for Improving the Performance of Poly(3-Hydroxybutyrate) (PHB) Based Plastics. J. Clean. Prod. 2021;318:128555. doi: 10.1016/j.jclepro.2021.128555. [DOI] [Google Scholar]
- 6.Volova T., Kiselev E., Nemtsev I., Lukyanenko A., Sukovatyi A., Kuzmin A., Ryltseva G., Shishatskaya E. Properties of Degradable Polyhydroxyalkanoates with Different Monomer Compositions. Int. J. Biol. Macromol. 2021;182:98–114. doi: 10.1016/j.ijbiomac.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Yan X., Liu X., Yu L.P., Wu F., Jiang X.R., Chen G.Q. Biosynthesis of Diverse α,ω-Diol-Derived Polyhydroxyalkanoates by Engineered Halomonas Bluephagenesis. Metab. Eng. 2022;72:275–288. doi: 10.1016/j.ymben.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Lakshmanan M., Foong C.P., Abe H., Sudesh K. Biosynthesis and Characterization of Co and Ter-Polyesters of Polyhydroxyalkanoates Containing High Monomeric Fractions of 4-Hydroxybutyrate and 5-Hydroxyvalerate via a Novel PHA Synthase. Polym. Degrad. Stab. 2019;163:122–135. doi: 10.1016/j.polymdegradstab.2019.03.005. [DOI] [Google Scholar]
- 9.Rehakova V., Pernicova I., Kourilova X., Sedlacek P., Musilova J., Sedlar K., Koller M., Kalina M., Obruca S. Biosynthesis of Versatile PHA Copolymers by Thermophilic Members of the Genus Aneurinibacillus. Int. J. Biol. Macromol. 2023;225:1588–1598. doi: 10.1016/j.ijbiomac.2022.11.215. [DOI] [PubMed] [Google Scholar]
- 10.Myung J., Flanagan J.C.A., Waymouth R.M., Criddle C.S. Expanding the Range of Polyhydroxyalkanoates Synthesized by Methanotrophic Bacteria through the Utilization of Omega-Hydroxyalkanoate Co-Substrates. AMB Express. 2017;7:118. doi: 10.1186/s13568-017-0417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doi Y., Tamaki A., Kunioka M., Soga K. Biosynthesis of Terpolyesters of 3-Hydroxybutyrate, 3-Hydroxyvalerate, and 5-Hydroxyvalerate in Alcaligenes eutrophus from 5-Chloropentanoic and Pentanoic Acids. Die Makromol. Chem. Rapid Commun. 1987;8:631–635. doi: 10.1002/marc.1987.030081209. [DOI] [Google Scholar]
- 12.Oh S.J., Kim S., Lee Y., Shin Y., Choi S., Oh J., Bhatia S.K., Joo J.C., Yang Y.H. Controlled Production of a Polyhydroxyalkanoate (PHA) Tetramer Containing Different Mole Fraction of 3-Hydroxybutyrate (3HB), 3-Hydroxyvalerate (3 HV), 4 HV and 5 HV Units by Engineered Cupriavidus necator. Int. J. Biol. Macromol. 2024;266:131332. doi: 10.1016/j.ijbiomac.2024.131332. [DOI] [PubMed] [Google Scholar]
- 13.Sohn Y.J., Son J., Jo S.Y., Park S.Y., Yoo J.I., Baritugo K.A., Na J.G., Choi J.-i., Kim H.T., Joo J.C., et al. Chemoautotroph Cupriavidus necator as a Potential Game-Changer for Global Warming and Plastic Waste Problem: A Review. Bioresour. Technol. 2021;340:125693. doi: 10.1016/j.biortech.2021.125693. [DOI] [PubMed] [Google Scholar]
- 14.Zhila N.O., Sapozhnikova K.Y., Kiselev E.G., Shishatskaya E.I., Volova T.G. Biosynthesis of Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate) from Different 4-Hydroxybutyrate Precursors by New Wild-Type Strain Cupriavidus necator IBP/SFU-1. Processes. 2023;11:1423. doi: 10.3390/pr11051423. [DOI] [Google Scholar]
- 15.Duvigneau S., Dürr R., Behrens J., Kienle A. Advanced Kinetic Modeling of Bio-Co-Polymer Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Production Using Fructose and Propionate as Carbon Sources. Processes. 2021;9:1260. doi: 10.3390/pr9081260. [DOI] [Google Scholar]
- 16.Wang C.-T., Sivashankari R.M., Miyahara Y., Tsuge T. Polyhydroxyalkanoate Copolymer Production by Recombinant Ralstonia Eutropha Strain 1F2 from Fructose or Carbon Dioxide as Sole Carbon Source. Bioengineering. 2024;11:455. doi: 10.3390/bioengineering11050455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S., Roh S., Yoo J., Ahn J.H., Gong G., Lee S.M., Um Y., Han S.O., Ko J.K. Tailored Polyhydroxyalkanoate Production from Renewable Non-Fatty Acid Carbon Sources Using Engineered Cupriavidus necator H16. Int. J. Biol. Macromol. 2024;263:130360. doi: 10.1016/j.ijbiomac.2024.130360. [DOI] [PubMed] [Google Scholar]
- 18.Fukui T., Suzuki M., Tsuge T., Nakamura S. Microbial Synthesis of Poly((R)-3-Hydroxybutyrate-Co- 3-Hydroxypropionate) from Unrelated Carbon Sources by Engineered Cupriavidus necator. Biomacromolecules. 2009;10:700–706. doi: 10.1021/bm801391j. [DOI] [PubMed] [Google Scholar]
- 19.Insomphun C., Xie H., Mifune J., Kawashima Y., Orita I., Nakamura S., Fukui T. Improved Artificial Pathway for Biosynthesis of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) with High C6-Monomer Composition from Fructose in Ralstonia eutropha. Metab. Eng. 2015;27:38–45. doi: 10.1016/j.ymben.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Jo Y.Y., Park S., Gong G., Roh S., Yoo J., Ahn J.H., Lee S.M., Um Y., Kim K.H., Ko J.K. Enhanced Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) with Modulated 3-Hydroxyvalerate Fraction by Overexpressing Acetolactate Synthase in Cupriavidus necator H16. Int. J. Biol. Macromol. 2023;242:125166. doi: 10.1016/j.ijbiomac.2023.125166. [DOI] [PubMed] [Google Scholar]
- 21.Obruca S., Marova I., Snajdar O., Mravcova L., Svoboda Z. Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) by Cupriavidus necator from Waste Rapeseed Oil Using Propanol as a Precursor of 3-Hydroxyvalerate. Biotechnol. Lett. 2010;32:1925–1932. doi: 10.1007/s10529-010-0376-8. [DOI] [PubMed] [Google Scholar]
- 22.Oh S.J., Choi T.R., Kim H.J., Shin N., Hwang J.H., Kim H.J., Bhatia S.K., Kim W., Yeon Y.J., Yang Y.H. Maximization of 3-Hydroxyhexanoate Fraction in Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Using Lauric Acid with Engineered Cupriavidus necator H16. Int. J. Biol. Macromol. 2024;256:128376. doi: 10.1016/j.ijbiomac.2023.128376. [DOI] [PubMed] [Google Scholar]
- 23.Sato S., Maruyama H., Fujiki T., Matsumoto K. Regulation of 3-Hydroxyhexanoate Composition in PHBH Synthesized by Recombinant Cupriavidus necator H16 from Plant Oil by Using Butyrate as a Co-Substrate. J. Biosci. Bioeng. 2015;120:246–251. doi: 10.1016/j.jbiosc.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Lee H.S., Lee S.M., Park S.L., Choi T.R., Song H.S., Kim H.J., Bhatia S.K., Gurav R., Kim Y.G., Kim J.H., et al. Tung Oil-Based Production of High 3-Hydroxyhexanoate-Containing Terpolymer Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate-Co-3-Hydroxyhexanoate) Using Engineered Ralstonia eutropha. Polymers. 2021;13:1084. doi: 10.3390/polym13071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chien Bong C.P., Alam M.N.H.Z., Samsudin S.A., Jamaluddin J., Adrus N., Mohd Yusof A.H., Muis Z.A., Hashim H., Salleh M.M., Abdullah A.R., et al. A Review on the Potential of Polyhydroxyalkanoates Production from Oil-Based Substrates. J. Environ. Manag. 2021;298:113461. doi: 10.1016/j.jenvman.2021.113461. [DOI] [PubMed] [Google Scholar]
- 26.Tang H.J., Neoh S.Z., Sudesh K. A Review on Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) [P(3HB-Co-3HHx)] and Genetic Modifications That Affect Its Production. Front. Bioeng. Biotechnol. 2022;10:1057067. doi: 10.3389/fbioe.2022.1057067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciesielski S., Mozejko J., Pisutpaisal N. Plant Oils as Promising Substrates for Polyhydroxyalkanoates Production. J. Clean. Prod. 2015;106:408–421. doi: 10.1016/j.jclepro.2014.09.040. [DOI] [Google Scholar]
- 28.Sato S., Fujiki T., Matsumoto K. Construction of a Stable Plasmid Vector for Industrial Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) by a Recombinant Cupriavidus necator H16 Strain. J. Biosci. Bioeng. 2013;116:677–681. doi: 10.1016/j.jbiosc.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Santolin L., Waldburger S., Neubauer P., Riedel S.L. Substrate-Flexible Two-Stage Fed-Batch Cultivations for the Production of the PHA Copolymer P(HB-Co-HHx) With Cupriavidus necator Re2058/PCB113. Front. Bioeng. Biotechnol. 2021;9:623890. doi: 10.3389/fbioe.2021.623890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J., Li C., Zou Y., Yan Y. Bacterial Synthesis of C3-C5 Diols via Extending Amino Acid Catabolism. Korea Adv. Inst. Sci. Technol. 2020;117:19159–19167. doi: 10.1073/pnas.2003032117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SelL H.G. The Isolation of Mutants Not Accumulating Poly-β-Hydroxybutyric Acid. Arch. Mikrobiol. 1970;71:283–294. doi: 10.1007/BF00410161. [DOI] [PubMed] [Google Scholar]
- 32.Raberg M., Voigt B., Hecker M., Steinbüchel A. A Closer Look on the Polyhydroxybutyrate- (PHB-) Negative Phenotype of Ralstonia eutropha PHB-4. PLoS ONE. 2014;9:e95907. doi: 10.1371/journal.pone.0095907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chek M.F., Hiroe A., Hakoshima T., Sudesh K., Taguchi S. PHA Synthase (PhaC): Interpreting the Functions of Bioplastic-Producing Enzyme from a Structural Perspective. Appl. Microbiol. Biotechnol. 2019;103:1131–1141. doi: 10.1007/s00253-018-9538-8. [DOI] [PubMed] [Google Scholar]
- 34.Bhatia S.K., Kim J.H., Kim M.S., Kim J., Hong J.W., Hong Y.G., Kim H.J., Jeon J.M., Kim S.H., Ahn J., et al. Production of (3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Copolymer from Coffee Waste Oil Using Engineered Ralstonia eutropha. Bioprocess Biosyst. Eng. 2018;41:229–235. doi: 10.1007/s00449-017-1861-4. [DOI] [PubMed] [Google Scholar]
- 35.Wang H., Ye J.W., Chen X., Yuan Y., Shi J., Liu X., Yang F., Ma Y., Chen J., Wu F., et al. Production of PHA Copolymers Consisting of 3-Hydroxybutyrate and 3-Hydroxyhexanoate (PHBHHx) by Recombinant Halomonas bluephagenesis. Chem. Eng. J. 2023;466:143261. doi: 10.1016/j.cej.2023.143261. [DOI] [Google Scholar]
- 36.Harada K., Kobayashi S., Oshima K., Yoshida S., Tsuge T., Sato S. Engineering of Aeromonas Caviae Polyhydroxyalkanoate Synthase Through Site-Directed Mutagenesis for Enhanced Polymerization of the 3-Hydroxyhexanoate Unit. Front. Bioeng. Biotechnol. 2021;9:627082. doi: 10.3389/fbioe.2021.627082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foong C.P., Lakshmanan M., Abe H., Taylor T.D., Foong S.Y., Sudesh K. A Novel and Wide Substrate Specific Polyhydroxyalkanoate (PHA) Synthase from Unculturable Bacteria Found in Mangrove Soil. J. Polym. Res. 2018;25:23. doi: 10.1007/s10965-017-1403-4. [DOI] [Google Scholar]
- 38.Tan H.T., Chek M.F., Lakshmanan M., Foong C.P., Hakoshima T., Sudesh K. Evaluation of BP-M-CPF4 Polyhydroxyalkanoate (PHA) Synthase on the Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) from Plant Oil Using Cupriavidus necator Transformants. Int. J. Biol. Macromol. 2020;159:250–257. doi: 10.1016/j.ijbiomac.2020.05.064. [DOI] [PubMed] [Google Scholar]
- 39.Tan H.T., Chek M.F., Miyahara Y., Kim S.Y., Tsuge T., Hakoshima T., Sudesh K. Characterization of an (R)-Specific Enoyl-CoA Hydratase from Streptomyces Sp. Strain CFMR 7: A Metabolic Tool for Enhancing the Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) J. Biosci. Bioeng. 2022;134:288–294. doi: 10.1016/j.jbiosc.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Lim S.W., Kansedo J., Tan I.S., Tan Y.H., Nandong J., Lam M.K., Ongkudon C.M. Microbial Valorization of Oil-Based Substrates for Polyhydroxyalkanoates (PHA) Production—Current Strategies, Status, and Perspectives. Process Biochem. 2023;130:715–733. doi: 10.1016/j.procbio.2023.05.013. [DOI] [Google Scholar]
- 41.Du C., Sabirova J., Soetaert W., Ki S., Lin C. Polyhydroxyalkanoates Production From Low-Cost Sustainable Raw Materials. Curr. Chem. Biol. 2012;6:14–25. [Google Scholar]
- 42.Surendran A., Lakshmanan M., Chee J.Y., Sulaiman A.M., Van Thuoc D., Sudesh K. Can Polyhydroxyalkanoates Be Produced Efficiently from Waste Plant and Animal Oils? Front. Bioeng. Biotechnol. 2020;8:169. doi: 10.3389/fbioe.2020.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fadzil F.I.B.M., Tsuge T. Microbial Applications. Volume 2. Springer International Publishing; Cham, Switzerland: 2017. Bioproduction of Polyhydroxyalkanoate from Plant Oils; pp. 231–260. [Google Scholar]
- 44.Ng K.S., Ooi W.Y., Goh L.K., Shenbagarathai R., Sudesh K. Evaluation of Jatropha Oil to Produce Poly(3-Hydroxybutyrate) by Cupriavidus necator H16. Polym. Degrad. Stab. 2010;95:1365–1369. doi: 10.1016/j.polymdegradstab.2010.01.021. [DOI] [Google Scholar]
- 45.Budde C.F., Riedel S.L., Willis L.B., Rha C.K., Sinskey A.J. Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) from Plant Oil by Engineered Ralstonia Eutropha Strains. Appl. Environ. Microbiol. 2011;77:2847–2854. doi: 10.1128/AEM.02429-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shenbagarathai R., Saranya V. Effect of Nitrogen and Calcium Sources on Growth and Production of PHA of Pseudomonas Sp. LDC-5 and Its Mutant. Curr. Res. J. Biol. Sci. 2010;2:164–167. [Google Scholar]
- 47.Gumel A.M., Annuar M.S.M., Chisti Y. Recent Advances in the Production, Recovery and Applications of Polyhydroxyalkanoates. J. Polym. Environ. 2013;21:580–605. doi: 10.1007/s10924-012-0527-1. [DOI] [Google Scholar]
- 48.Satoh K., Kawakami T., Isobe N., Pasquier L., Tomita H., Zinn M., Matsumoto K. Versatile Aliphatic Polyester Biosynthesis System for Producing Random and Block Copolymers Composed of 2-, 3-, 4-, 5-, and 6-Hydroxyalkanoates Using the Sequence-Regulating Polyhydroxyalkanoate Synthase PhaCAR. Microb. Cell. Fact. 2022;21:84. doi: 10.1186/s12934-022-01811-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volova T.G., Syrvacheva D.A., Zhila N.O., Sukovatiy A.G. Synthesis of P(3HB-Co-3HHx) Copolymers Containing High Molar Fraction of 3-Hydroxyhexanoate Monomer by Cupriavidus Eutrophus B10646. J. Chem. Technol. Biotechnol. 2016;91:416–425. doi: 10.1002/jctb.4592. [DOI] [Google Scholar]
- 50.Chuah J.A., Yamada M., Taguchi S., Sudesh K., Doi Y., Numata K. Biosynthesis and Characterization of Polyhydroxyalkanoate Containing 5-Hydroxyvalerate Units: Effects of 5HV Units on Biodegradability, Cytotoxicity, Mechanical and Thermal Properties. Polym. Degrad. Stab. 2013;98:331–338. doi: 10.1016/j.polymdegradstab.2012.09.008. [DOI] [Google Scholar]
- 51.Park S.L., Cho J.Y., Kim S.H., Bhatia S.K., Gurav R., Park S.H., Park K., Yang Y.H. Isolation of Microbulbifer Sp. Sol66 with High Polyhydroxyalkanoate-Degrading Activity from the Marine Environment. Polymers. 2021;13:4257. doi: 10.3390/polym13234257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available on request.