Abstract
Introduction
In cervical cancer treatment, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and albumin-globulin ratio (AGR) are being studied as potential prognostic markers for predicting the effectiveness of concurrent chemoradiotherapy (CCRT). This study aims to investigate the relationship between these biomarkers and survival outcomes in cervical cancer patients undergoing CCRT.
Materials and methods
This retrospective study was conducted at Amrita Institute of Medical Sciences between January 2016 and December 2019. It included patients at any stage who received definitive CCRT and were followed for at least two years post-treatment. Patients who had initial surgery and those lost to follow-up were excluded.
Results
The study included 123 patients with a median age of 68. Most patients had stage IIB (39%) and squamous cell carcinoma (76.4%). With a median follow-up of 56 months, the five-year overall survival (OS) was 66.8%, progression-free survival (PFS) was 94%, and recurrence-free survival (RFS) was 81.2%. AGR (p = 0.001), NLR (p = 0.0001), and PLR (p = 0.001) were found to be significantly associated with OS, NLR (p = 0.002) and AGR (p = 0.001) significantly affected RFS, while only PLR (p = 0.02) significantly affected PFS on univariate analysis. NLR significantly impacted OS (p = 0.003) and RFS (p = 0.03) on multivariate analysis.
Conclusion
The results of our study showed that increased NLR and elevated levels of albumin indicate a higher likelihood of mortality. Furthermore, a higher NLR was linked to an increased probability of recurrence in patients with cervical cancer who received primary treatment with CCRT. Therefore, the identification of predictive biomarkers could significantly improve the assessment of progression risk, aiding in the selection of the most suitable treatment and personalized therapy.
Keywords: concurrent chemoradiation therapy, haematological indices, locally advanced cervical cancer, long-term prognosis, survival outcomes
Introduction
The incidence of cervical cancer has decreased in the past 10 years. However, it still stands as the second most prevalent cancer among women in India, with a 9% incidence rate, according to GLOBOCAN (Global Cancer Observatory) 2022 [1]. The typical course of action for locally advanced cervical cancer (stage ≥ IB3 according to the International Federation of Gynecology and Obstetrics 2018 staging) involves undergoing concomitant chemoradiotherapy (CCRT) first, followed by receiving image-guided adaptive brachytherapy (IGABT) [2]. While the FIGO (International Federation of Gynecology and Obstetrics) staging is generally reliable for predicting outcomes before surgery, the clinical stage can be inaccurate, particularly in advanced cases [3,4]. Various factors, including patient characteristics, tumor attributes, and treatment-related variables, such as HPV (human papillomavirus) status, FIGO stage, tumor size, lymph node involvement, and histology, have been recognized as predictors of outcomes following radiation therapy or concurrent chemoradiotherapy [3,5]. However, these factors are typically assessed post-biopsy or surgery in histopathology reports. It is essential to have a preoperative test that is noninvasive and easily accessible to accurately forecast the survival probability and prognosis for cervical cancer [5].
Cancer can change hematological parameters due to inflammatory and immunosuppressive factors [1,6-8]. While the exact processes are not entirely understood, there is evidence indicating that it affects the prognosis of various cancer types, such as cervical cancer [9]. Elevated release of proinflammatory cytokines leads to widespread inflammatory reactions and changes in blood-related elements such as serum albumin, serum globulin, hemoglobin (Hb), neutrophil counts, lymphocyte counts, and platelet counts [10-15]. Recent studies suggest that certain pretreatment blood cell levels can indicate outcomes for cervical cancer patients. For example, higher neutrophil and monocyte levels are linked to poorer outcomes, while higher hemoglobin and lymphocyte levels are associated with improved outcomes. Ratios like the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and albumin-globulin ratio (AGR) may help predict a patient's response to CCRT [14,15]. However, the results regarding the relation of these parameters with prognosis are inconsistent. Investigating readily available baseline tests for forecasting overall survival (OS) and progression-free survival (PFS) in resource-constrained countries such as India is essential. Therefore, we conducted this study to investigate the clinical predictive significance of pretreatment hematological factors and their association with clinical outcomes in cervical cancer.
Materials and methods
Target population
In this study, 123 patients diagnosed with cervical cancer between January 2016 and December 2019 were included. The data was gathered retrospectively from the hospital's electronic database with the approval of the Institutional Ethics Committee (IEC-AIIMS-2024-GYNECONCO-179). The inclusion criteria were as follows: age above 18 years, histopathologically confirmed cervical cancer of FIGO 2018 staging IB or above [4], complete clinicopathological information, received definitive concurrent chemoradiotherapy, and having at least two years of follow-up either in person or via phone. Exclusion criteria included receiving a blood transfusion within two months before treatment; having a history of acute infection in the last three months; having chronic inflammatory conditions such as HIV infection, systemic lupus erythematosus (SLE), inflammatory bowel disease (IBD), rheumatoid arthritis (RA), or sepsis; and missing clinicopathological or laboratory information or being lost to follow-up.
Management protocol and follow-up
In our hospital, patients with locally advanced or advanced-stage cancer underwent routine blood tests. Imaging such as CE-MRI of the abdomen and pelvis, CECT of the whole abdomen and pelvis, or PET-CT scans were performed to determine the disease stage as per availability and convenience. Cervical biopsies were taken during pelvic examinations or guided by imaging, as appropriate. After confirming the stage and histopathology, patients received CCRT based on the institutional protocol. Routine blood tests were conducted one week before the treatment and four weeks after completion. Follow-ups occurred every three months for the first two years, every six months for the following three years, and annually after that. Patients underwent history-taking and pelvic examinations during each follow-up, and imaging was performed accordingly as indicated.
CCRT protocol
All patients received cisplatin-based chemotherapy. They began with at least one cycle of weekly intravenous cisplatin (40 mg/m2), aiming to complete five or six cycles during external beam radiotherapy (EBRT). If patients had impaired kidney function, they were given carboplatin instead of cisplatin. Treatment involved three-dimensional conformal radiation therapy (3DCRT) or image-guided radiation therapy (IGRT), with 1.8-2 Gy fractions administered up to five times per week. EBRT for the paraaortic region was planned based on imaging. High-dose-rate (HDR) brachytherapy (BT) was administered using an intracavitary technique. CT scans were used to plan both EBRT and BT. The total radiation therapy time was calculated from the first day of EBRT to the last day of BT. Starting from April 2019, all patients received treatment using IGRT.
Data collection
The following information was collected for each patient: basic demographic profile, age, comorbidities, ECOG (Eastern Cooperative Oncology Group) performance status, clinical presentation, BMI (body mass index), clinical stage, blood investigation parameters, histopathology, treatment given, chemotherapy drug used, and treatment response. Follow-up was conducted via phone until August 10, 2023. During follow-ups, the current status of the patients, progression, recurrences, progression/recurrence sites, and treatment received were noted. Hematological parameters such as Hb (hemoglobin) levels, WBC (white blood cell) count, neutrophil count, lymphocyte count, platelet count, serum albumin, and serum globulin levels were noted for all patients before the front-line treatment and four weeks after treatment completion. Additionally, the AGR, NLR, and PLR were calculated.
Statistical analysis
The data was analyzed using SPSS Statistics 20.0 software (IBM Corp., Armonk, NY). Categorical variables were described using frequencies and percentages, and ratios were compared using the Chi-square test. Survival curves were plotted using the Kaplan-Meier method, and Spearman’s rank correlation coefficient was utilized to test the correlation between different factors. OS, PFS, and RFS (recurrence-free survival) were calculated from the treatment onset date. Hematological parameters were associated with OS, PFS, and RFS. A p-value of <0.05 was considered statistically significant.
Results
Basic demographic, clinical, and pathological characteristics
We enrolled 123 patients in our study who met our inclusion and exclusion criteria. Table 1 summarizes the initial patient characteristics and clinicopathological aspects of the disease.
Table 1. Demographic and clinical-pathological characteristics of patients included in the study (N = 123).
The data has been represented as N and %.
ECOG: Eastern Cooperative Oncology Group; FIGO: The International Federation of Gynecology and Obstetrics; PET: Positron emission tomography; CT: Computed tomography; MRI: Magnetic resonance imaging.
| Variables | Category | N | Percentage |
| Age (years) | ≤50 | 18 | 14.6 |
| >50 | 105 | 85.4 | |
| ECOG performance status | 0 | 19 | 15.4 |
| 1 | 92 | 74.8 | |
| 2 | 12 | 9.8 | |
| Comorbidities | No | 53 | 43.1 |
| Yes | 70 | 56.9 | |
| Clinical presentation | Postmenopausal bleeding | 71 | 57.7 |
| Abnormal uterine bleeding | 22 | 17.8 | |
| Discharge | 13 | 10.5 | |
| Abdominal symptoms | 10 | 8.1 | |
| Asymptomatic | 4 | 3.2 | |
| Postcoital bleeding | 2 | 1.6 | |
| Urinary symptoms | 1 | 0.8 | |
| Histology type | Squamous cell carcinoma | 94 | 76.4 |
| Adenocarcinoma | 15 | 12.2 | |
| Poorly differentiated | 14 | 11.4 | |
| FIGO 2018 stage | IB2 | 9 | 7.3 |
| IIA | 8 | 6.5 | |
| IIB | 48 | 39 | |
| IIIB | 19 | 15.4 | |
| IIIC1 | 13 | 10.5 | |
| IIIC2 | 9 | 7.3 | |
| IVA | 17 | 13.8 | |
| Preoperative imaging | MRI | 101 | 82.1 |
| PET-CT | 22 | 17.9 | |
| Number of chemotherapy cycles | 1 | 3 | 0.8 |
| 2 | 4 | 3.2 | |
| 3 | 8 | 6.5 | |
| 4 | 2 | 1.6 | |
| 5 | 17 | 13.8 | |
| 6 | 77 | 62.6 | |
| Treatment course | Completed | 104 | 84.5 |
| Stopped | 19 | 15.4 |
Their median age was 61 (38-78) years, and the most common symptom reported was postmenopausal bleeding (57.7%). Other symptoms included menstrual irregularities (n = 22, 17.8%), abnormal vaginal discharge (n = 13, 10.5%), abdominal complaints (n = 10, 8.1%), postcoital bleeding (n = 2, 1.6%), and urinary symptoms (n = 1, 0.8%). However, four patients (3.2%) were symptom-free and were only diagnosed incidentally through imaging. Nineteen (15.4%) patients demonstrated an ECOG score of 0, while 92 (74.8%) patients had a score of 1, and 12 (9.8%) patients had a score of 2. The majority of patients presented with FIGO (the International Federation of Gynecology and Obstetrics) stage IIB (n = 48, 39%), followed by stage IIIC (n = 21, 17.8%), stage IIIB (n = 19, 15.4%), stage IVA (n = 17, 13.8%), stage IB2 (n = 9, 7.3%), and stage IIA (n = 8, 6.5%). Squamous cell carcinoma, adenocarcinoma, and poorly differentiated carcinoma were observed in 94 (76.4%), 15 (12.2%), and 14 (11.4%) patients, respectively.
Treatment received
All patients who underwent CCRT were administered at least one cycle of cisplatin- or carboplatin-based chemotherapy. Cisplatin was given to most patients (n = 104, 84.6%), whereas carboplatin was given to 19 (15.3%) patients. The patients were given a median radiation dose of 46 Gy, ranging from 42 to 52.4 Gy. Most patients received either 45 Gy (n = 64, 52%) or 50.4 Gy (n = 59, 47.9%). The median duration of EBRT was 38 days, with a range of 25-64 days. The median total cumulative dose for the complete radiation treatment was 84 Gy, administered over 48 days, with a range of 28-102 days. The median period between biopsy-based diagnosis and the commencement of CCRT was 26 days, ranging from 12 to 42 days. The treatment course was completed by 84.5% (n = 104) of patients, with 15.4% (n = 19) of patients experiencing treatment withholdings due to intolerance. Additionally, 13 patients received blood transfusions. All patients received BT, with a median dose of 38 Gy.
Hematological parameters and their calculated cut-off values
The cut-off values for each parameter were determined using the ROC (receiver operating characteristic) curve, as depicted in Table 2.
Table 2. Hematological parameters with cut-off values analyzed using receiver operating curve analysis in patients with cervical cancer and treated with CCRT (N = 123).
p-value is considered significant if <0.05.
NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; AGR: Albumin-globulin ratio; CCRT: Concurrent chemoradiotherapy.
| Category | NLR | PLR | Albumin (g/dl) | Globulin (g/dl) | AGR |
| Area under curve | 0.875 | 0.647 | 0.649 | 0.654 | 0.696 |
| 95% Confidence interval | 0.804-0.946 | 0.542-0.752 | 0.548-0.749 | 0.548-0.760 | 0.596-0.795 |
| Standard error | 0.036 | 0.054 | 0.05 | 0.054 | 0.051 |
| Sensitivity | 91.4% | 70.6% | 80% | 65.7% | 51.4% |
| Specificity | 84.4% | 60.2% | 47.7% | 59.1% | 78.4% |
| Cut-off value | 4.28 | 150 | 4.28 | 3.32 | 1.08 |
| P-value | 0.0001 | 0.012 | 0.01 | 0.008 | 0.001 |
The cut-off value for Hb was 11.4 g/dl, WBC was 8.4 K/uL, albumin was 4.28 g/dl, globulin was 3.32 g/dl, NLR was 4.28, PLR was 150, and AGR was 1.08. Using these cut-off values, survival analyses were compared between the groups, as listed in Table 3.
Table 3. Univariate and multivariate analysis for correlation between clinical-pathological and hematological parameters with survival outcomes (N = 123).
The data has been represented as N and %.
*p-value considered significant (p < 0.05).
**p-value considered highly significant (p < 0.001).
(Ref)^: Reference value for calculating hazard ratio.
OS: Overall survival; PFS: Progression-free survival; RFS: Recurrence-free survival; HR: Hazard ratio; CI: Confidence interval; UVA: Univariate analysis; MVA: Multivariate analysis; WBC: White blood cell; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; AGR: Albumin-globulin ratio.
| Category (N = 123) | Subcategories | N | Percentage | OS | PFS | RFS | ||||||
| HR (95% CI) | UVA | MVA | HR (95% CI) | UVA | MVA | HR (95% CI) | UVA | MVA | ||||
| Age (years) | ≤50 (Ref)^ | 18 | 14.6 | 6.94 (0.94-10.45) | 0.05* | 0.03* | 1.06 (0.12-8.81) | 0.09 | - | 1.20 (0.24-3.91) | 0.07 | - |
| >50 | 105 | 85.4 | ||||||||||
| Stage | ≤IIB (Ref)^ | 65 | 52.8 | 2.30 (1.15-4.50) | 0.01* | 0.65 | 5.42 (0.17-10.82) | 0.05* | 0.32 | 3.35 (1.17-9.52) | 0.16 | - |
| >IIB | 58 | 47.1 | ||||||||||
| Hemoglobin (g/dL) | ≤11.5 | 52 | 42.2 | 1.83 (0.94-3.56) | 0.07 | 0.09 | 1.43 (0.11-4.32) | 0.42 | - | 1.41 (0.54-3.66) | 0.08 | - |
| >11.5 (Ref)^ | 71 | 57.7 | ||||||||||
| WBC (× 109/L) | ≤8.4 (Ref)^ | 75 | 60.9 | 1.16 (0.59-2.26) | 0.60 | - | 2.05 (0.45-9.17) | 0.34 | - | 1.80 (0.69-4.67) | 0.06 | - |
| >8.4 | 48 | 39 | ||||||||||
| NLR | ≤4.28 (Ref)^ | 77 | 62.6 | 21.4 (8.8-38.2) | <0.001** | 0.003* | 2.56 (0.57-11.4) | 0.19 | - | 4.24 (1.6-11.2) | 0.004* | 0.014* |
| >4.28 | 46 | 37.4 | ||||||||||
| PLR | ≤150 (Ref)^ | 67 | 54.5 | 3.10 (1.51-6.34) | 0.002* | 0.32 | 7.56 (0.91-14.2) | 0.02* | 0.06 | 2.20 (0.83-5.70) | 0.10 | - |
| >150 | 56 | 45.5 | ||||||||||
| Albumin (g/dL) | ≤4.28 | 74 | 60.2 | 2.96 (1.29-6.80) | 0.01* | 0.04* | 0.91 (0.2-4.08) | 0.90 | - | 1.88 (0.66-5.35) | 0.20 | - |
| >4.28 (Ref)^ | 49 | 39.8 | ||||||||||
| Globulin (g/dL) | ≤3.32 (Ref)^ | 64 | 52.0 | 2.79 (1.37-5.66) | 0.004* | 0.37 | 10.4 (0.45-28.4) | 0.005* | 0.06 | 4.81 (1.56-14.83) | 0.006* | 0.018* |
| >3.32 | 59 | 48.0 | ||||||||||
| AGR | ≤1.13 (Ref)^ | 38 | 30.9 | 3.16 (1.61-6.20) | <0.001** | 0.69 | 5.8 (0.78-12.31) | 0.10 | - | 4.34 (1.64-11.47) | 0.003* | 0.36 |
| >1.13 | 85 | 69.1 | ||||||||||
OS and its association with hematological parameters
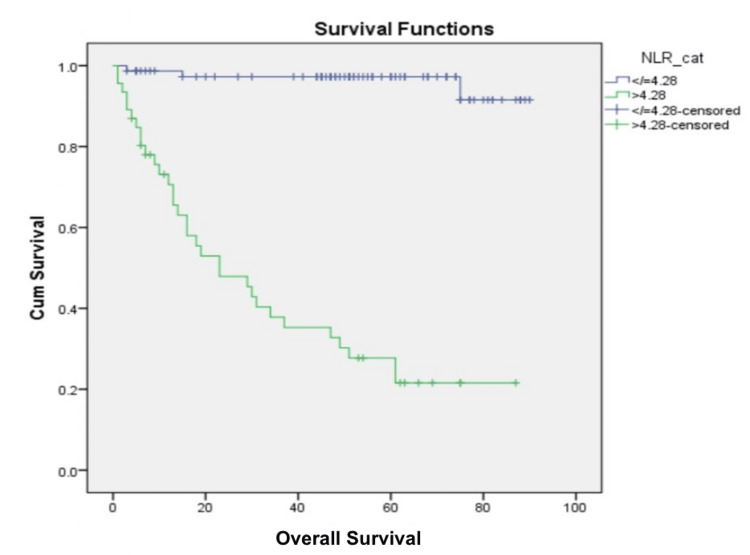
The median follow-up duration was 56 months (38-129 months). Of 123 patients, 35 (28.4%) expired within the defined follow-up period. The five-year OS rate was 66.8%. Univariate analysis explored the connection between various factors and OS. Patients aged ≤50 years (p = 0.05), with FIGO stage ≤ IIB (p = 0.01), serum albumin levels > 4.28 g/dL (p = 0.01), serum globulin levels ≤ 3.32 (p = 0.004), AGR > 1.13 (p = 0.001), NLR ≤ 4.28 (p = <0.001) (Figure 1), and PLR ≤ 150 (p = 0.002) exhibited significantly higher OS rates. No significant associations were found with WBC count (p = 0.60) or Hb levels (p = 0.07). In a Cox proportional hazards model, age (p = 0.03), NLR (p = 0.003), and serum albumin levels (p = 0.04) showed independent effects on OS.
Figure 1. Kaplan-Meier curve showing overall survival outcomes using NLR cut-off value of 4.28.
NLR: Neutrophil-to-lymphocyte ratio.
PFS and its association with hematological parameters
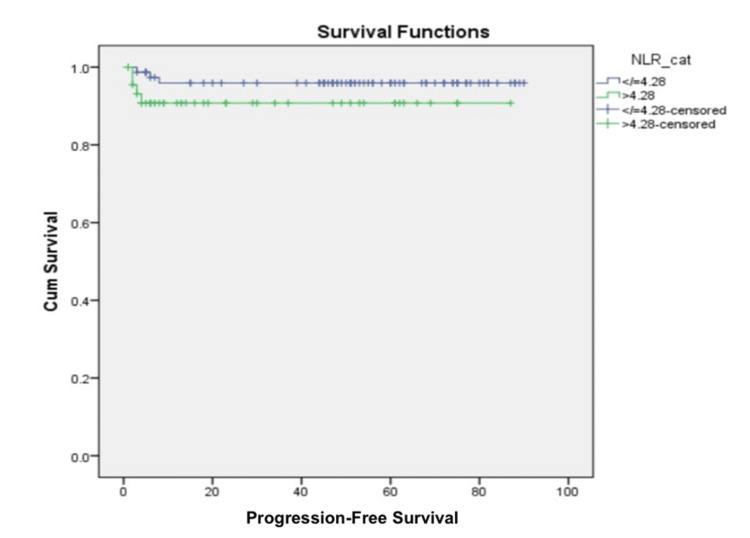
Eight patients (6.5%) experienced disease progression during the treatment, with six patients having stage IVA disease and two patients with stage IIIC disease. The five-year PFS rate stood at 94%. Upon analysis, only stage (p = 0.05) and PLR (p = 0.02) showed a significant association with PFS. In contrast, age (p = 0.09), Hb (p = 0.42), WBC count (p = 0.34), serum albumin (p = 0.90), serum globulin (p = 0.50), AGR (p = 0.10), and NLR (p = 0.19) displayed insignificant associations (Figure 2). Interestingly, on multivariate analysis, PLR (p = 0.06) was not identified as an independent risk factor affecting PFS.
Figure 2. Kaplan-Meier curve showing progression-free survival outcomes using NLR cut-off value of 4.28.
NLR: Neutrophil-to-lymphocyte ratio.
RFS and its association with hematological parameters
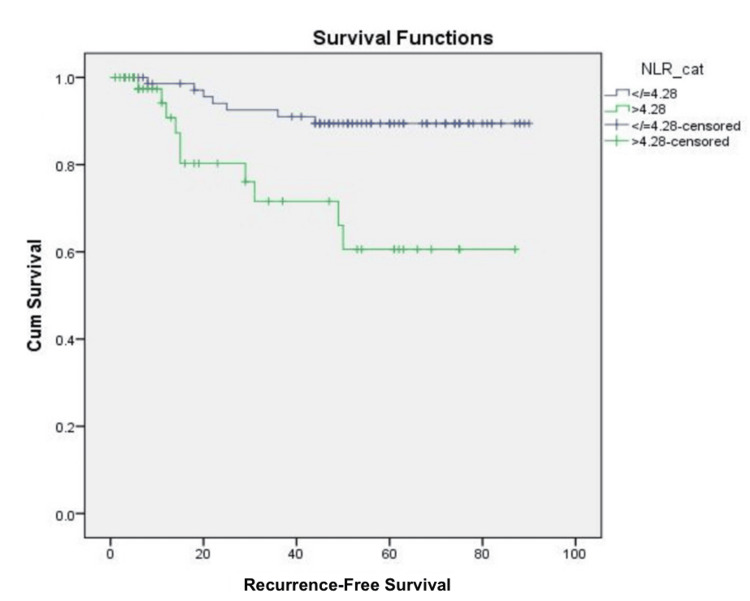
After receiving treatment, 17 patients (13.8%) experienced recurrences during follow-up. The five-year RFS rate was 81.2%. Most recurrences occurred at distant sites (n = 13, 76.4%), while others were observed in loco-regional areas (n = 8, 47%) and the abdominal regions (n = 2, 11.7%). The recurrences were managed using various chemotherapy drugs, including the paclitaxel-carboplatin combination (n = 4, 23.5%); paclitaxel-carboplatin and bevacizumab combination (n = 2, 11.7%); cisplatin, ifosfamide, and paclitaxel (TIP) combination (n = 3, 17.6%); gemcitabine and carboplatin combination (n = 1, 5.8%); and whole-brain radiation therapy (WBRT) for brain metastasis (n = 3, 17.6%). It was observed that serum globulin (p = 0.006), AGR (p = 0.003), and NLR (p = 0.004) significantly impacted the univariate analysis (Figure 3). However, only serum globulin (p = 0.018) and NLR (p = 0.014) affected RFS independently in the multivariate analysis.
Figure 3. Kaplan-Meier curve showing recurrence-free survival outcome using NLR cut-off value of 4.28.
NLR: Neutrophil-to-lymphocyte ratio.
Subgroup analysis for radiation techniques and its influence over hematological parameters
In the past, 3DCRT was the primary radiotherapy technique at our center. However, in 2019, we began using IGRT. The patients treated with radiotherapy through 3DCRT or IGRT accounted for 98 (79.6%) and 25 (20.3%) of the total, respectively. Notably, detailed pelvic volume information was available for only 25 IGRT patients. No variation was observed in hematological parameters with IGRT.
Discussion
Systemic inflammation plays a crucial role in shaping the tumor environment by involving cytokines, inflammatory cells, and chemokines [16,17]. Notably, neutrophils and platelets aid in tumor expansion and migration. Lymphocytes assist in combating cancer cells [18]. Therefore, elevated NLR and PLR levels may indicate tumor hostility and reflect the defense mechanism of an individual. Several studies have been conducted to evaluate NLR and/or PLR as predictive factors in cervical cancer [19-34]. Table 4 summarizes previous studies assessing the effect of PLR or NLR on survival outcomes in cervical cancer patients.
Table 4. Review of literature comparing previous studies assessing the effect of PLR or NLR on survival outcomes in cervical cancer patients.
*p-value < 0.05 was considered significant.
SCC: Squamous cell carcinoma; AC: Adenocarcinoma; CCRT: Concurrent chemoradiotherapy; BT: Brachytherapy; RH: Radical hysterectomy; PLND: Pelvic lymphadenectomy; NACT: Neoadjuvant chemotherapy; RT: Radiotherapy; F/B: Followed by; PLR: Neutrophil-to-lymphocyte ratio; NLR: Neutrophil-to-lymphocyte ratio; OS: Overall survival; DFS: Disease-free survival; PFS: Progression-free survival; N/A: Not available; NS: Non-significant; LRR: Lower relative risk.
| Study | Type of the study | Study duration | Sample size (N) | Stages | Histology | Treatment | PLR cut-off | NLR cut-off | Median follow-up period (months) | Univariate analysis | Multivariate analysis | ||
| PLR | NLR | PLR | NLR | ||||||||||
| Wang et al. [19] | Retrospective, single-center | 1999-2010 | 111 | IB2-IIB | All | NACT F/B RH+PLND | 142.2 | 2.4 | N/A | NS | NS | NS | NS |
| Zhang et al. [20] | Retrospective, single-center | 2005-2008 | 460 | I-II | SCC, AC | RH+PLND | 150.9 | 2.2 | 69 | NS | OS, PFS | NS | OS |
| Jain et al. [13] | Retrospective | 2005-2013 | 56 | I-IV | SCC | RT/CCRT | N/A | 2.5 | NR | N/A | OS, PFS | N/A | OS, PFS |
| Chen et al. [21] | Retrospective, single-center | 2006-2009 | 407 | IB1–IIA | All | Any | 143.47 (OS) 152.02 (RFS) | 2.09 (OS) 2.59 (RFS) | NR | OS, RFS, LMN | OS, RFS | OS, RFS, LMN | OS, RFS |
| Onal et al. [22] | Retrospective, single-center | 2006-2014 | 235 | IB2-IVA | SCC, AC | CCRT | 133.02 | 3.03 | 31.7 | OS | OS, PFS | NS | OS, PFS |
| Zhu et al. [11] | Retrospective, single-center | 2003-2008 | 96 | IA–IV | AC | RT/CCRT | 158, | 2.32 | N/A | OS (III-IV) | OS, PFS | NR | NR |
| Choi et al. [12] | Retrospective, single-center | 2012-2014 | 339 | I-IV | SCC | RH+PLND | 138.8 | 2.5 | 44 | OS, PFS | NS | PFS | NS |
| Nuchpramool et al. [23] | Retrospective, single-center | 2001-2016 | 484 | IA2-IB1 | All | RH+PLND | NS | NS | 56.9 | NS | NS | NS | NS |
| Holub and Biete [24] | Retrospective, single-center | 2009-2016 | 151 | I-IV | All | Any | 210 | 3.8 | 43.8 | OS | OS | NS | NS |
| Huang et al. [25] | Meta-analysis | 2010-2013 | 2804 | I-IV | All | Any | N/A | NR | N/A | N/A | OS, PFS | N/A | OS, PFS |
| Wu et al. [26] | Meta-analysis | 2012-2016 | 2452 | I-IV | All | Any | N/A | N/A | N/A | N/A | OS, PFS | N/A | OS, PFS |
| Ittiamornlert and Ruengkhachorn [27] | Retrospective, single-center | 2006-2017 | 355 | IVB | All | NACT | N/A | 3.6 | NR | N/A | OS, PFS | N/A | N/A |
| Trinh et al. [28] | Retrospective, single-center | 2008-2019 | 99 | I-IV | All | Any | 186.93 | 1.65 | 48.9 | NS | OS, PFS | NS | OS |
| Jonska-Gmyrek et al. [10] | Retrospective, single-center | 2008-2018 | 148 | I-IV | All | CCRT+BT | 148.89 | 2.34 | 75 | Combined NLR and PLR significantly associated with OS and DFS | Combined NLR and PLR significantly associated with OS and DFS | ||
| Du et al. [29] | Retrospective, single-center | 2012-2017 | 203 | I-IIA | SCC, AC, ASC | RH | N/A | 3.75 | NR | N/A | OS, PFS | N/A | NR |
| Sabyasachi et al. [30] | Retrospective, single-center | 2017-2019 | 208 | IB3-IIIC1 | SCC | CCRT+BT | 140.6 | 2.45 | N/A | LRR | LRR | LRR | LRR |
| Zhao et al. [31] | Retrospective, single-center | 2008-2018 | 202 | I-IV | All | RT/CCRT | N/A | 3.029 | 71 | N/A | OS, PFS | N/A | OS, PFS |
| Gao et al. [32] | Retrospective, single-center | 2001-2016 | 110 | I–IV | All | RH+PLND | 186.88 | N/A | N/A | OS | N/A | OS | N/A |
| Jin et al. [33] | Retrospective, single-center | 2012-2016 | 190 | IB2-IVA | N/A | CCRT+BT | N/A | 2.52 | 46 | N/A | OS, PFS | N/A | OS, PFS |
| Kumar et al. [34] | Retrospective, single-center | 2003-2017 | 1051 | IB2-IVA | All | CCRT+BT | N/A | N/A | 69 | OS, DFS | N/A | OS, DFS | N/A |
Despite varying inclusion criteria such as different cancer stages, histologies, treatments, and cut-off values, most of these studies separately analyzed NLR and PLR and consistently identified a correlation with survival outcomes. Our research also observed that a high NLR value (>4.28) was associated with lower OS and RFS in univariate and multivariate analyses. Similarly, a higher PLR (>150) was linked to a significant decrease in OS as well as the PFS.
The albumin concentration is a crucial clinical marker, providing insights into an individual's nutritional status. Low levels of albumin can negatively impact metabolism and the function of immune cells by reducing their effectiveness. In addition to its role in the immune system, albumin also regulates the inflammatory response by acting as an antioxidant agent in the development of tumors [35]. A decrease in serum albumin levels has been linked to an increased inflammatory response to cancer cells and the heightened release of various cytokines that lead to progress in tumor infiltration [36]. Few studies have shown an inverse relationship between serum albumin and oncological consequences [37]. However, Yoshino et al. did not find any connection between albumin levels and survival rates [38]. Our research, on the other hand, uncovered a significant impact of decreased albumin levels on OS in both univariate (p = 0.01) and multivariate analyses (p = 0.04).
Globulin is another marker for immune and inflammatory status, and higher levels have been linked to advanced cancer, leading to a negative impact on the immune system of cancer patients [38]. Yoshino et al. found that higher serum globulin levels were significantly linked to lower OS in patients with cervical cancer [38]. However, other studies did not find a significant correlation between elevated globulin levels and treatment outcomes in cervical cancer [37]. On the other hand, our study observed that higher globulin levels were associated with poorer OS, PFS, and RFS in univariate analyses and poorer RFS in multivariate analyses. This discrepancy could be due to a false increase in serum globulin levels, possibly caused by urinary tract infections or other inflammatory processes resulting from infections. Some researchers have calculated that the lower AGR denotes lower survival for cervical cancer patients [39]. However, other studies failed to demonstrate that AGR was an independent predictor for survival [38]. Our study, however, only showed a low AGR association with poor OS in univariate analysis.
Other significant poor prognostic factors associated with OS were age > 50 years and stage > IIB in our study. Age above 50 years was associated with a poorer OS in the multivariate analysis. However, pretreatment decreased hemoglobin levels, and higher WBC counts did not influence OS or PFS. A study conducted by Lee et al. found that, in their analysis, cancer stage, age, and pretreatment hemoglobin levels were linked to OS when looked at individually, but only the cancer stage was linked to poorer overall survival in their combined analysis [10].
Our study showed that IGRT administered to the patients did not affect hematological parameters. However, to our knowledge, no other research has demonstrated the effect of radiation dose on NLR, PLR, albumin, globulin, and AGR levels.
Strengths and limitations
Our study identified a strong association between elevated NLR levels and increased albumin levels with reduced OS and RFS, as confirmed by multivariate analysis. We also calculated AGR, a parameter rarely studied in previous literature. The study was conducted in a South Indian state with one of the lowest age-standardized cervical cancer incidence rates, at 9.35% per 100,000 women, compared to other states [40]. There is limited research in India on the prognosis of cervical cancer using hematological parameters, making our study an essential contribution to the development of noninvasive markers for highlighting the prognosis of locally advanced and advanced-stage cervical cancer patients.
However, our study has some limitations. First, the study was retrospective with a limited sample size. We also ruled out infection based solely on the patient's history without conducting prior tests, which could have potentially skewed the data on serum globulin levels. Additionally, we included all histologies and advanced stages, which could have influenced the survival outcomes. Lastly, our study was conducted in a single institution. Further studies involving more stratified, larger populations and multicenters are needed to reduce selection bias and confirm our findings.
Conclusions
Our research revealed that higher NLR and elevated albumin levels predict a greater risk of mortality, and a higher NLR was associated with higher chances of recurrence in cervical cancer patients who underwent primary treatment with CCRT. Thus, identifying predictive biomarkers could significantly enhance the assessment of progression risk, assisting in the selection of the most appropriate treatment and personalized therapy. Further research is crucial to validate our findings and gain a deeper understanding of how they can be applied in clinical practice.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. Institutional Ethics Committee of All India Institute of Medical Sciences issued approval IEC-AIIMS-2024-GYNECONCO-179.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Monal Garg, Priya Bhati, Debnarayan Dutta
Acquisition, analysis, or interpretation of data: Monal Garg, Gautham Balaji, Ajay Sasidharan, Sruthi Kalavagunta, Sheejamol VS, Debnarayan Dutta
Drafting of the manuscript: Monal Garg, Gautham Balaji, Sheejamol VS, Debnarayan Dutta
Critical review of the manuscript for important intellectual content: Monal Garg, Priya Bhati, Ajay Sasidharan, Sruthi Kalavagunta
Supervision: Monal Garg, Priya Bhati, Gautham Balaji
References
- 1.Immunity, inflammation, and cancer. Grivennikov SI, Greten FR, Karin M. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treatment strategies and prognostic factors of 2018 FIGO stage IIIC cervical cancer: a review. Qin F, Pang H, Yu T, Luo Y, Dong Y. Technol Cancer Res Treat. 2022;21:15330338221086403. doi: 10.1177/15330338221086403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Predictors of radiation field failure after definitive chemoradiation in patients with locally advanced cervical cancer. Bae HS, Kim YJ, Lim MC, et al. Int J Gynecol Cancer. 2016;26:737–742. doi: 10.1097/IGC.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 4.Implications of the revised cervical cancer FIGO staging system. Bhatla N, Singhal S, Dhamija E, Mathur S, Natarajan J, Maheshwari A. Indian J Med Res. 2021;154:273–283. doi: 10.4103/ijmr.IJMR_4225_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Integration pattern of human papillomavirus is a strong prognostic factor for disease-free survival after radiation therapy in cervical cancer patients. Joo J, Shin HJ, Park B, et al. Int J Radiat Oncol Biol Phys. 2017;98:654–661. doi: 10.1016/j.ijrobp.2017.02.226. [DOI] [PubMed] [Google Scholar]
- 6.Inflammation and cancer: back to Virchow? Balkwill F, Mantovani A. Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 7.Tumor microenvironments, the immune system and cancer survival. Strausberg RL. Genome Biol. 2005;6:211. doi: 10.1186/gb-2005-6-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pre-treatment haemoglobin and peripheral blood lymphocyte count as independent predictors of outcome in carcinoma of cervix. Hoskin PJ, Rojas AM, Peiris SN, Mullassery V, Chong IY. Clin Oncol (R Coll Radiol) 2014;26:179–184. doi: 10.1016/j.clon.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Pretreatment neutrophil-to-lymphocyte ratio combined with platelet-to-lymphocyte ratio as a predictor of survival outcomes after definitive concurrent chemoradiotherapy for cervical cancer. Lee JW, Seol KH. J Clin Med. 2021;10:2199. doi: 10.3390/jcm10102199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pretreatment neutrophil to lymphocyte and platelet to lymphocyte ratios as predictive factors for the survival of cervical adenocarcinoma patients. Jonska-Gmyrek J, Gmyrek L, Zolciak-Siwinska A, Kowalska M, Fuksiewicz M, Kotowicz B. Cancer Manag Res. 2018;10:6029–6038. doi: 10.2147/CMAR.S178745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pretreatment neutrophil-lymphocyte and platelet-lymphocyte ratio predict clinical outcome and prognosis for cervical cancer. Zhu M, Feng M, He F, et al. Clin Chim Acta. 2018;483:296–302. doi: 10.1016/j.cca.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Prognostic significance of VEGF expression in patients with bulky cervical carcinoma undergoing neoadjuvant chemotherapy. Choi CH, Song SY, Choi JJ, et al. BMC Cancer. 2008;8:295. doi: 10.1186/1471-2407-8-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Role of monocyte and lymphocyte counts in prognosis of cervical cancer. Jain A, Bobdey S, Sathwara J, et al. Int J Reprod Contracept Obstet Gynecol. 2016;5:2243–2249. [Google Scholar]
- 14.The pretreatment neutrophil-to-lymphocyte ratio predicts therapeutic response to radiation therapy and concurrent chemoradiation therapy in uterine cervical cancer. Mizunuma M, Yokoyama Y, Futagami M, Aoki M, Takai Y, Mizunuma H. Int J Clin Oncol. 2015;20:989–996. doi: 10.1007/s10147-015-0807-6. [DOI] [PubMed] [Google Scholar]
- 15.Relationship between low hemoglobin levels and outcomes after treatment with radiation or chemoradiation in patients with cervical cancer: has the impact of anemia been overstated? Bishop AJ, Allen PK, Klopp AH, Meyer LA, Eifel PJ. Int J Radiat Oncol Biol Phys. 2015;91:196–205. doi: 10.1016/j.ijrobp.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Cancer-related inflammation. Mantovani A, Allavena P, Sica A, Balkwill F. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 17.Inflammation and cancer: triggers, mechanisms, and consequences. Greten FR, Grivennikov SI. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Could the platelet-to-lymphocyte ratio be a novel marker for predicting invasiveness of cervical pathologies? Kose M, Celik F, Kose SK, Arioz DT, Yilmazer M. Asian Pac J Cancer Prev. 2015;16:923–926. doi: 10.7314/apjcp.2015.16.3.923. [DOI] [PubMed] [Google Scholar]
- 19.Pretreatment neutrophil-to-lymphocyte and platelet-tolymphocyte ratios do not predict survival in patients with cervical cancer treated with neoadjuvant chemotherapy and radical hysterectomy. Wang D, Wu M, Feng FZ, et al. Chin Med J. 2013;126:1464–1468. [PubMed] [Google Scholar]
- 20.Preoperative neutrophil-lymphocyte ratio before platelet-lymphocyte ratio predicts clinical outcome in patients with cervical cancer treated with initial radical surgery. Zhang Y, Wang L, Liu Y, Wang S, Shang P, Gao Y, Chen X. Int J Gynecol Cancer. 2014;24:1319–1325. doi: 10.1097/IGC.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 21.Peripheral platelet/lymphocyte ratio predicts lymph node metastasis and acts as a superior prognostic factor for cervical cancer when combined with neutrophil: lymphocyte. Chen L, Zhang F, Sheng XG, Zhang SQ, Chen YT, Liu BW. Medicine (Baltimore) 2016;95:0. doi: 10.1097/MD.0000000000004381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prognostic utility of pretreatment hematologic parameters in patients receiving definitive chemoradiation therapy for cervical cancer. Onal C, Guler OC, Yildirim BA. Int J Radiat Oncol Biol Phys. 2016;96:0. doi: 10.1097/IGC.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 23.Preoperative neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are not clinically useful in predicting prognosis in early stage cervical cancer. Nuchpramool P, Hanprasertpong J. Surg Res Pract. 2018;2018:9162921. doi: 10.1155/2018/9162921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Impact of systemic inflammation biomarkers on the survival outcomes of cervical cancer patients. Holub K, Biete A. Clin Transl Oncol. 2019;21:836–844. doi: 10.1007/s12094-018-1991-4. [DOI] [PubMed] [Google Scholar]
- 25.Prognostic significance of neutrophil-to-lymphocyte ratio in cervical cancer: a systematic review and meta-analysis of observational studies. Huang QT, Man QQ, Hu J, et al. Oncotarget. 2017;8:16755–16764. doi: 10.18632/oncotarget.15157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio in cervical cancer: a meta-analysis and systematic review. Wu J, Chen M, Liang C, Su W. Oncotarget. 2017;8:13400–13412. doi: 10.18632/oncotarget.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neutrophil-lymphocyte ratio as a predictor of oncologic outcomes in stage IVB, persistent, or recurrent cervical cancer patients treated by chemotherapy. Ittiamornlert P, Ruengkhachorn I. BMC Cancer. 2019;19:51. doi: 10.1186/s12885-019-5269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prognostic value of changes in neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR) for patients with cervical cancer undergoing definitive chemoradiotherapy (dCRT) Trinh H, Dzul SP, Hyder J, et al. Clin Chim Acta. 2020;510:711–716. doi: 10.1016/j.cca.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Effects of peripheral blood neutrophil/lymphocyte ratio levels and their changes on the prognosis of patients with early cervical cancer. Du JQ, Zhang F, Wang CQ, et al. Front Oncol. 2023;13:1139809. doi: 10.3389/fonc.2023.1139809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pretreatment systemic inflammatory markers, neutrophil lymphocyte ratio, and platelet lymphocyte ratio as a prognostic factor in cervical cancer: a retrospective study. Sabyasachi S, Behjet M, Sumana MD, et al. South Asian J Cancer. 2023:1–7. [Google Scholar]
- 31.Predictive significance of lymphocyte level and neutrophil-to-lymphocyte ratio values during radiotherapy in cervical cancer treatment. Zhao M, Gao Z, Gu X, Yang X, Wang S, Fu J. Cancer Med. 2023;12:15820–15830. doi: 10.1002/cam4.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Assessment of peripheral platelet to lymphocyte ratio and prognostic nutritional index in the efficacy and prognosis of radiotherapy for cervical cancer. Gao Z, Zhao M, Yang X, Fu J. Curr Oncol. 2023;30:2834–2844. doi: 10.3390/curroncol30030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nomograms for predicting prognostic value of combined neutrophil-to-lymphocyte ratio and SCC-Ag in locally advanced cervical cancer. Jin L, Cao F, Zhang Y, Dang Y, Wang F. Transl Cancer Res. 2024;13:1323–1335. doi: 10.21037/tcr-23-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Correlation of hematological parameters with clinical outcomes in cervical cancer patients treated with radical radio(chemo)therapy: a retrospective study. Kumar A, Gurram L, Naga Ch P, et al. Int J Radiat Oncol Biol Phys. 2024;118:182–191. doi: 10.1016/j.ijrobp.2023.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Fibrinogen and albumin score changes during preoperative treatment can predict prognosis in patients with locally advanced rectal cancer. Tang MJ, Ding SB, Hu WY. Gastroenterol Res Pract. 2019;2019:1–8. doi: 10.1155/2019/3514586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albumin concentration controls cancer. Seaton K. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2594053/ J Natl Med Assoc. 2001;93:490–493. [PMC free article] [PubMed] [Google Scholar]
- 37.Low preoperative albumin-globulin score predicts favorable survival in esophageal squamous cell carcinoma. Zhang F, Sun P, Wang ZQ, et al. Oncotarget. 2016;7:30550–30560. doi: 10.18632/oncotarget.8868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.A low albumin to globulin ratio with a high serum globulin level is a prognostic marker for poor survival in cervical cancer patients treated with radiation based therapy. Yoshino Y, Taguchi A, Shimizuguchi T, et al. Int J Gynecol Cancer. 2019;29:17–22. doi: 10.1136/ijgc-2018-000025. [DOI] [PubMed] [Google Scholar]
- 39.A low preoperative albumin-to-globulin ratio is a negative prognostic factor in patients with surgically treated cervical cancer. Kawata A, Taguchi A, Baba S, et al. Int J Clin Oncol. 2021;26:980–985. doi: 10.1007/s10147-021-01861-8. [DOI] [PubMed] [Google Scholar]
- 40.Secular trends in incidence and mortality of cervical cancer in India and its states, 1990-2019: data from the Global Burden of Disease 2019 Study. Singh M, Jha RP, Shri N, Bhattacharyya K, Patel P, Dhamnetiya D. BMC Cancer. 2022;22:149. doi: 10.1186/s12885-022-09232-w. [DOI] [PMC free article] [PubMed] [Google Scholar]