Abstract
Naturally occurring human immunodeficiency virus (HIV-1) variants require the presence of CD4 and specific chemokine receptors to enter a cell. In the laboratory, HIV-1 variants that are capable of bypassing CD4 and utilizing only the CCR5 chemokine receptor for virus entry have been generated. Here we report that these CD4-independent viruses are significantly more sensitive to neutralization by soluble CD4 and a variety of antibodies. The same amino acid changes in the HIV-1 gp120 envelope glycoprotein determined CD4 independence and neutralization sensitivity. The CD4-independent envelope glycoproteins exhibited higher affinity for antibodies against CD4-induced gp120 epitopes but not other neutralizing ligands. The CD4-independent envelope glycoproteins did not exhibit increased lability relative to the wild-type envelope glycoproteins. The utilization of two receptors apparently allows HIV-1 to maintain a more neutralization-resistant state prior to engaging CD4 on the target cell, explaining the rarity of CD4 independence in wild-type HIV-1.
Human immunodeficiency virus types 1 and 2 (HIV-1 and HIV-2) are the etiologic agents of AIDS in humans (6, 15, 34), and related simian immunodeficiency viruses (SIVs) can cause AIDS-like illness in monkeys (22, 40, 49). AIDS is associated with the depletion of CD4-positive T lymphocytes, which are the major target cells of viral infection in vivo (30).
The entry of primate immunodeficiency viruses into target cells is mediated by the viral envelope glycoproteins, gp120 and gp41, which are organized into trimeric complexes on the virion surface (2, 11, 68, 86). Viral entry usually requires the binding of the exterior envelope glycoprotein, gp120, to the primary receptor CD4 (18, 42, 51). The gp120 glycoprotein is heavily glycosylated and contains protruding variable loops (48), features that are thought to decrease the susceptibility of the virus to host immune responses (88, 91). The interaction between gp120 and CD4 promotes a series of conformational changes in gp120 that result in the formation or exposure of a binding site for particular members of the chemokine receptor family that serve as coreceptors (85, 91). The chemokine receptor CCR5 is the major coreceptor for primary HIV-1 isolates (1, 13, 21, 23, 24) and can be utilized by HIV-2 and SIV isolates as well (12, 52). Some HIV-1 isolates use the CXCR4 chemokine receptor as a coreceptor (31). Binding of gp120 to the coreceptor is thought to induce additional conformational changes that lead to activation of the transmembrane glycoprotein gp41 and subsequent fusion of the viral and cellular membranes (10, 79, 86).
The study of receptor binding for the primate immunodeficiency viruses has been facilitated by the creation of soluble forms of the CD4 glycoprotein (sCD4) (20, 32, 38, 75, 84). In addition to anchoring and orienting the viral envelope glycoproteins with respect to the target cell membrane, binding to CD4 initiates changes in the conformation of the envelope glycoproteins (3, 4, 19, 26, 70, 71, 74, 81, 83, 87, 93). Some of these conformational changes allow high-affinity interaction with CCR5 (85, 91). The CD4-induced movement of the V1/V2 loops results in the exposure of conserved, discontinuous structures on the HIV-1 gp120 glycoprotein recognized by the 17b and 48d monoclonal antibodies (83, 93). The 17b and 48d epitopes are proximal to a gp120 region implicated in chemokine receptor binding (46, 64, 94). A plausible model based on current structural and mutagenic data (46, 93, 94), is that CD4 binding repositions the V1/V2 stem, allowing formation of an antiparallel β sheet that contributes to the 17b and 48d epitopes and to chemokine receptor binding. Other gp120 elements such as the third variable (V3) loop also contribute to interaction with the chemokine receptor (7, 13, 16, 78).
Infection by primate immunodeficiency viruses is generally more efficient when CD4 is expressed on the surface of the target cells. However, some viral isolates are able to achieve reasonably efficient infection of cells lacking CD4. For example, some HIV-2 isolates have been shown to enter CD4-negative cells by using CXCR4 (14, 28). Some SIV strains can infect CD4-negative brain capillary endothelial cells or other cell types by using CCR5 as a primary receptor (27, 70). The gp120 glycoproteins of some SIV isolates can efficiently bind rhesus monkey CCR5 in the absence of sCD4 (53). Naturally occurring, CD4-independent HIV-1 isolates appear to be rare, but CXCR4-using HIV-1 isolates have been derived by passage on CD4-negative cultured cells (25, 37, 47). We have previously derived a CD4-independent variant of the HIV-1 ADA strain that utilizes the CCR5 coreceptor and demonstrated that changes in the gp120 V2 loop and/or V1/V2 stem region were responsible for both CD4-independent entry into cells and gp120 binding to CCR5 in the absence of CD4 (43). Recently, we have shown that the removal of a single N-linked glycosylation site in the gp120 V1/V2 stem is sufficient for CD4 independence (43a).
The small number of changes required in the wild-type HIV-1 envelope glycoproteins to achieve CD4 independence contrasts with the rarity with which CD4-independent HIV-1 apparently arise in vivo. One explanation is that CD4 independence is associated with negative consequences that are operative in vivo but not in the tissue culture settings in which CD4-independent HIV-1 isolates have been selected. One such property is viral susceptibility to host immune responses. Here we test the hypothesis that CD4-independent HIV-1 variants exhibit altered sensitivity to neutralization by antibodies compared with the parental wild-type virus, which is CD4 dependent.
MATERIALS AND METHODS
Cell lines.
HeLa, 293T, and Cf2Th cells were obtained from the American Type Culture Collection and maintained as previously described (13). Cf2Th cells expressing human CCR5 (Cf2Th-CCR5) were generated and maintained in medium containing G418 (0.6 mg/ml) as previously described (57). Cf2Th-CD4/CCR5 cells, which express both human CD4 and human CCR5, were generated and maintained in medium containing G418 (0.6 mg/ml) and hygromycin (0.15 mg/ml) as previously described (29).
Site-directed mutagenesis.
All mutagenesis was done using the Stratagene QuikChange protocol as previously described (43). To generate the C-terminally deleted HIV-1 envelope glycoproteins used for antibody binding, gp120 shedding, and syncytium formation assays, the primer ActXba (5′-CTATAGTGAATAGAGTTAGGCAGTGATCTAGACCATTATCGTTTCAGACCCACC -3′) was used to introduce a stop codon at position 711 in the intracellular region of the gp41 glycoprotein. HIV-1 envelope glycoprotein residues are numbered according to the sequence of the prototypic HXBc2 isolate, as currently recommended (45).
Antibody binding to cell surface envelope glycoproteins.
Antibody binding to 293T cells expressing cytoplasmic tail-deficient envelope glycoproteins was measured by fluorescence-activated cell sorting (FACS). A serum mixture from HIV-1-infected individuals was used at a 1:200 dilution in Dulbecco modified Eagle medium (DMEM) with 0.2% azide (DMEM-azide) as a control.
For FACS analysis, 0.5 × 106 to 1.0 × 106 cells were resuspended in DMEM-azide containing different concentrations of an anti-gp120 monoclonal antibody. After 60 min of incubation at 37°C, cells were washed twice with DMEM-azide, resuspended in DMEM-azide containing a 1:200 dilution of phycoerythrin-conjugated goat anti-human antibody, and incubated at 37°C for another 60 min. Cells were then washed twice with FACS buffer (2% fetal bovine serum plus 0.2% azide in phosphate-buffered saline [PBS]) and resuspended in PBS–2% formaldehyde before being analyzed by FACS. As a control, mock-transfected 293T cells were stained with primary and secondary antibodies. As an additional control, env-transfected 293T cells were stained with secondary antibody only.
Immunoprecipitations.
Approximately 40-μl aliquots of protein A-Sepharose beads resuspended in 1× PBS (50% of bead volume) were added to cell supernatants containing radiolabeled gp120 glycoproteins together with the indicated amounts of human anti-gp120 antibodies or a sCD4-immunoglobulin (Ig) fusion protein (CD4-lg). The mixture was incubated by rocking for 24 h at room temperature. Beads were washed four times with a solution containing 150 mM NaCl, 10 mM Tris-HCl (pH 7.5), and 0.5% detergent NP-40. Beads were resuspended in 40 μl of 2× sodium dodecyl sulfate (SDS) sample buffer, boiled, and centrifuged briefly. The samples were analyzed under denaturing conditions on an SDS–10% polyacrylamide gel followed by autoradiography.
CCR5 binding assay.
The binding of radiolabeled gp120 to Cf2Th-CCR5 cells was assayed as previously described (43) at 4°C and 37°C.
Virus entry and neutralization assay.
Recombinant reporter viruses (38) were generated by transfecting 293T cells with 2 μg of an env-expressing pSVIIIenv plasmid, 5 μg of a packaging plasmid, and 15 μg of a vector plasmid expressing firefly luciferase, using the calcium phosphate method. Seventy-two hours after transfection, the virus-containing supernatant was harvested and cleared by low-speed centrifugation. The virus in the supernatant was quantitated by measuring reverse transcriptase (RT) as described elsewhere (36).
Target cells were seeded at a density of 6,000 cells/well in 96-well, luminometer-compatible tissue culture plates (Dynex). Twenty-four hours later, 20,000 RT units of virus were preincubated with various concentrations of anti-envelope glycoprotein antibody or ligand in a volume of 50 μl of medium for 1 h at 37°C. The medium was removed from the wells containing the target cells, and 20,000 RT units of virus were added to the cells. In the case of the receptor-directed antibodies OKT4a and 2D7, the antibodies were added to the target cells in a 50-μl volume for 1 h at 37°C prior to addition of 20,000 RT units of virus. After 8 h of virus-cell incubation, the wells were washed with DMEM and fresh medium (200 μl) was added. Seventy-two hours after infection, the medium was removed from each well and the cells were lysed by agitation in 20 μl of lysis buffer. Luciferase activity was measured using an EG&G Berthold Microplate Luminometer LB 96V in accordance with the Promega “Luciferase Assay System Technical Bulletin.”
Syncytium inhibition assay.
Cf2Th-CCR5/CD4 cells were cultured in 96-well tissue culture plates at a density of 2 × 104 cells/well. Twenty-four hours later, 3 × 104 envelope glycoprotein-expressing 293T cells were added to the Cf2Th-CCR5/CD4 cells. The 293T cells had been transfected 48 h earlier with 18 μg of a plasmid expressing HIV-1 envelope glycoproteins with cytoplasmic tail deletions and 2 μg of a Tat-expressing plasmid. After 24 h of cocultivation at 37°C, syncytia were counted. For testing antibodies or sCD4 for the ability to inhibit syncytium formation, the 293T cells expressing the envelope glycoproteins were preincubated with various concentrations of antibody or sCD4 for 1 h at 37°C prior to addition to the 96-well plate.
gp120 shedding assay.
Approximately 5 × 106 293T cells were transfected with 18 μg of a plasmid expressing the HIV-1 envelope glycoprotein with cytoplasmic tail deletions and 2 μg of a Tat-expressing plasmid. After 24 h, cells were replated into six-well plates at a density of 106 cells/well and radiolabeled with [35S]methionine-cysteine overnight. The labeling medium was then replaced with medium alone or with medium containing sCD4 (30 μg/ml) or 17b antibody (10 μg/ml). The cells were incubated at 37°C for 2 h with gentle rocking. The medium was harvested, and shed gp120 was precipitated by the C11 anti-gp120 antibody and protein A-Sepharose beads. Samples were analyzed by SDS-polyacrylamide gel electrophoresis and a phosphorimager.
RESULTS
Relative sensitivities of CD4-independent viruses to neutralization.
The HIV-1 envelope glycoproteins used in this study are shown in Fig. 1. In the initial experiments investigating neutralization sensitivity, we used the wild-type envelope glycoproteins derived from the ADA R5 primary HIV-1 isolate and the 197 N/K and 190 S/R-197 N/S CD4-independent variants (43). The single amino acid change in the 197 N/K gp120 envelope glycoprotein, relative to the wild-type ADA envelope glycoprotein, results in a loss of the N-linked glycosylation site at position 197 in the V1/V2 stem. In the 190 S/R-197 N/S glycoprotein, the serine-to-arginine change at position 190 results in a loss of an N-linked glycosylation site at asparagine 188 in the V2 variable loop, and the asparagine-to-serine change at residue 197 results in an N-terminal shift of the glycosylation site at position 197. These envelope glycoproteins were used to pseudotype recombinant HIV-1 variants expressing luciferase. The viruses were tested for sensitivity to neutralization by a panel of monoclonal antibodies and sCD4, using both CD4-expressing and CD4-negative target cells. Table 1 reports the concentrations of antibodies or sCD4 required to neutralize 50% of the infectivity of viruses pseudotyped with the ADA envelope glycoprotein variants (IC50). Many of the antibodies did not neutralize viruses with the wild-type ADA envelope glycoproteins at the concentrations tested, consistent with the relative degree of neutralization resistance expected for primary HIV-1 isolates (9, 80). The viruses with the wild-type ADA envelope glycoproteins were neutralized by lgG1b12, an unusually potent neutralizing antibody directed against the CD4-binding site of gp120 (9), by the 2F5 anti-gp41 antibody, which also exhibits reasonable potency against primary HIV-1 isolates (63), and by sCD4. Infection of CD4-expressing target cells by viruses with the 190 S/R-197 N/S and 197 N/K envelope glycoproteins was neutralized more efficiently by most of the antibodies tested compared with infection mediated by the wild-type ADA glycoproteins. One exception was the neutralization of viruses with the 190 S/R-197 N/S envelope glycoproteins by the lgG1b12 antibody. This probably results from partial disruption of the lgG1b12 epitope by the addition of carbohydrate to asparagine 195, based on the results of mutagenic analysis. For example, lgG1b12 was able to immunoprecipitate and neutralize envelope glycoproteins with the 197 N/Q change but not the 197 N/S change, regardless of the residue (serine or arginine) at position 190 (data not shown). The effect of V2 loop changes on the integrity of the lgG1b12 epitope has been previously reported (66). Viruses with the 197 N/K envelope glycoproteins were more sensitive to sCD4 than viruses with the wild-type ADA envelope glycoproteins. Viruses with the 190 S/R-197 N/S envelope glycoproteins exhibited only a slight increase in sCD4 sensitivity relative to wild-type viruses for infection of CD4-positive target cells. The anti-CD4 antibody OKT4a inhibited the entry of all three viruses into CD4-positive target cells. This is consistent with the observation that CD4-independent viruses still utilize CD4 when it is available on target cells and are able to infect such cells more efficiently than in the absence of CD4 (43). At the highest concentration of the 2D7 anti-CCR5 antibody used, infection of CD4-expressing cells was not significantly inhibited.
FIG. 1.
Linear sequence map of the HIV-1 ADA envelope glycoproteins used in this study. The gp120 exterior glycoprotein conserved regions (black bars) and variable regions (V1 to V5; white bars) and the signal peptide (S) are depicted. The gp41 transmembrane envelope glycoprotein is in grey, with the transmembrane region (T) shown. The sequence of the wild-type ADA envelope glycoprotein in the V1/V2 stem region is shown, with the sites of N-linked glycosylation ( ) indicated. The amino acid residues altered in the CD4-independent variants are underlined and numbered according to the prototypic HXBc2 sequence (45). In the CD4-independent variants, altered residues are indicated by asterisks.
TABLE 1.
IC50 of ligands required for virus neutralizationa
| Target | Ligand | IC50 (μg/ml)
|
||||
|---|---|---|---|---|---|---|
| CD4-positive target cells
|
CD4-negative
target cells
|
|||||
| Wild type | 190 S/R-197 N/S | 197 N/K | 190 S/R-197 N/S | 197 N/K | ||
| CD4-binding site on gp120 | 15e | >10 | 0.3 | 0.25 | 0.024 | 0.03 |
| F105 | >10 | 0.27 | 0.038 | 0.025 | 0.03 | |
| IgG1b12 | 0.4 | 0.7 | 0.01 | 0.35 | 0.0018 | |
| gp120 V3 loop | 19b | 7.5 | 0.005 | 0.0018 | 0.0007 | 0.001 |
| CD4-induced epitopes on gp120 | 17b | >10 | 0.025 | 0.018 | 0.018 | 0.014 |
| 48d | >10 | 0.01 | 0.25 | 0.0028 | 0.055 | |
| gp41 | 2F5 | 1 | 0.007 | 0.001 | 0.00001 | 0.0014 |
| gp120 | sCD4 | 1 | 0.4 | 0.035 | 0.006 | 0.03 |
| CD4 | OKT4a | 0.01 | 0.04 | 0.004 | >10 | >10 |
| CCR5 | 2D7 | >4.76 | >4.76 | >4.76 | 0.02 | 0.045 |
The target cells were either Cf2Th-CD4/CCR5 or Cf2Th-CCR5 cells.
The sensitivity of viruses with the 190 S/R-197 N/S and 197 N/K envelope glycoproteins to neutralization was also examined in target cells lacking CD4. In general, CD4-independent infection was extremely sensitive to neutralization by antibodies directed against the envelope glycoproteins and against the CCR5 receptor. One exception to this generalization was the observation that the lgG1b12 concentration required to neutralize viruses with the 190 S/R-197 N/S envelope glycoproteins was comparable to that required for neutralization of the wild-type ADA viruses on CD4-expressing target cells. Notably, sCD4 very efficiently inhibited infection of CD4-negative cells by the viruses with the 190 S/R-197 N/S and 197 N/K envelope glycoproteins. As expected, the anti-CD4 antibody OKT4a did not inhibit the infection of CD4-negative cells by these viruses.
In summary, infection of both CD4-expressing and CD4-negative target cells by viruses with envelope glycoproteins capable of CD4-independent entry was neutralized by antibodies directed against gp120 and gp41 more effectively than infection by viruses with the wild-type ADA envelope glycoproteins. Modest or dramatic increases in sensitivity to sCD4 were also observed for the CD4-independent viruses relative to the wild-type viruses. Finally, the CCR5-directed antibody neutralized the infection of CD4-negative target cells by CD4-independent viruses more efficiently than infection of CD4-expressing target cells by the same viruses.
Other gp120 changes associated with CD4 independence result in neutralization sensitivity.
Recently, changes in the V1/V2 stem-loop sequences of the ADA gp120 glycoprotein necessary and sufficient for CD4 independence have been investigated (43a). These studies demonstrated that loss or movement of the N-linked carbohydrate on asparagine 197 resulted in CD4-independent CCR5 binding and infection. Moreover, complete removal of the V1/V2 loops from the ADA gp120 glycoprotein resulted in similar CD4-independent phenotypes. To examine whether CD4 independence per se was associated with sensitivity to neutralization, we tested the abilities of antibodies and sCD4 to inhibit virus infection mediated by the CD4-independent ADA envelope glycoprotein variants created in the aforementioned study (43a). The 197 N/S variant, in which the wild-type glycosylation at asparagine 197 is shifted to asparagine 195 (Fig. 1), supports CD4-independent infection. The results in Tables 2 and 3 demonstrate that viruses with the 197 N/S envelope glycoproteins are more sensitive to neutralization by the F105, 17b, 48d, and 2F5 antibodies than viruses with the wild-type ADA envelope glycoproteins. Likewise, increased sensitivity to antibody neutralization was observed for viruses with the ΔV1/2 envelope glycoproteins, which lack the gp120 V1 and V2 variable loops and support CD4-independent infection (43a). By contrast, viruses with the 190 S/R envelope glycoproteins, which retain the carbohydrate at asparagine 197 (Fig. 1) and are dependent upon CD4 for entry (43a), were resistant to neutralization by the F105 and 17b antibodies (Table 2). Thus, for the panel of ADA gp120 variants examined, there is a strong correlation between the ability to mediate the infection of cells lacking CD4 and neutralization sensitivity.
TABLE 2.
IC50 of ligands required for virus neutralizationa
| Target on gp120 | Ligand | IC50 (μg/ml)
|
|||||
|---|---|---|---|---|---|---|---|
| CD4-positive target cells
|
CD4-negative
target cells
|
||||||
| Wild type | 190 S/R-197 N/S | 190 S/R | 197 N/S | 190 S/R-197 N/S | 197 N/S | ||
| CD4-binding site | F105 | >10 | 0.85 | >10 | 0.15 | 0.95 | 0.06 |
| CD4-induced epitope | 17b | >10 | 0.02 | >10 | 0.25 | 0.03 | 0.025 |
The target cells were either Cf2Th-CD4/CCR5 or Cf2Th-CCR5 cells.
TABLE 3.
IC50 of ligands required for virus neutralizationa
| Target | Ligand | IC50 (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|---|
| CD4-positive target cells
|
CD4-negative
target cells
|
|||||||
| Wild type | 197 N/S | ΔV1/2 | ΔV1/2 197 N/S | 197 N/S | ΔV1/2 | ΔV1/2 197 N/S | ||
| gp120 | F105 | >10 | 0.3 | 0.3 | 0.25 | 0.2 | 0.15 | 0.065 |
| gp120 | 48d | >10 | 0.1 | 0.045 | 0.05 | 0.02 | 0.01 | 0.015 |
| gp41 | 2F5 | 0.4 | 0.07 | 0.025 | 0.007 | 0.005 | 0.01 | 0.03 |
| gp120 | sCD4 | 2.5 | 1 | 0.6 | 0.45 | 0.45 | >10 | 0.14 |
The target cells were either Cf2Th-CD4/CCR5 or Cf2Th-CCR5 cells.
Syncytium inhibition.
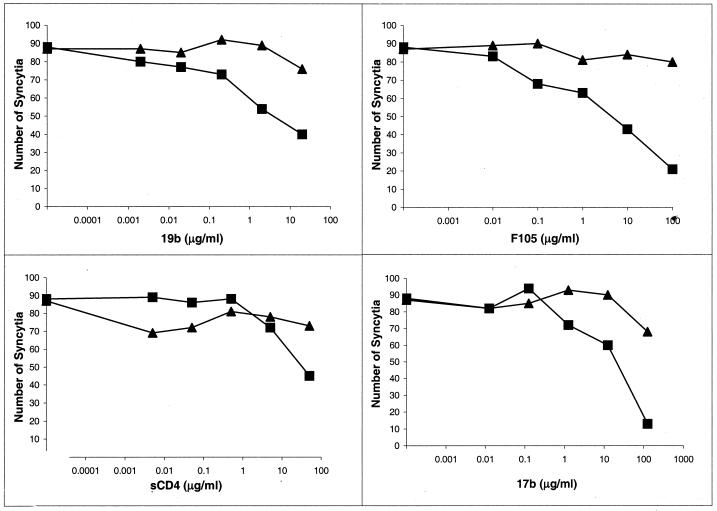
Cells expressing the HIV-1 envelope glycoproteins can fuse with adjacent, receptor-bearing cells to form multinucleated syncytia (50, 76). We examined the sensitivity of syncytium formation mediated by the wild-type ADA and CD4-independent 190 S/R-197 N/S envelope glycoproteins to inhibition by antibodies and sCD4. To increase the cell surface expression of these envelope glycoproteins (72), variant glycoproteins lacking the gp41 cytoplasmic tail were used in the experiments. The ADA and 190 S/R-197 N/S glycoproteins induced comparable levels of syncytia following cocultivation of envelope glycoprotein-expressing cells and Cf2Th cells expressing CD4 and CCR5 (Fig. 2). Syncytium formation mediated by the wild-type ADA envelope glycoproteins was minimally inhibited by sCD4 or the antibodies tested. Syncytium formation mediated by the 190 S/R-197 N/S envelope glycoproteins exhibited greater sensitivity to antibody neutralization and demonstrated some decrease at the highest sCD4 concentration tested. The concentrations of antibody or sCD4 required to achieve 50% inhibition of cell-cell fusion were much greater than that required to inhibit virus entry to a similar degree. These results indicate that the function of the 190 S/R-197 N/S glycoproteins expressed on the cell surface is inhibited by some antibodies more efficiently than that of the wild-type ADA envelope glycoproteins.
FIG. 2.
Inhibition of syncytium formation by gp120 ligands. 293T cells expressing either the wild-type ADA (▴) or 190 S/R-197 N/S (■) envelope glycoproteins were preincubated with the indicated concentrations of sCD4 or the F105, 19b, or 17b antibody and then cocultivated with Cf2Th-CCR5/CD4 cells. Syncytia were scored after 24 h. The leftmost point of each curve indicates the number of syncytia observed in the absence of added ligand. Representative results of two independent experiments are shown.
Affinity of ligands for the envelope glycoproteins.
One explanation for the increased relative neutralization sensitivity observed for the CD4-independent viruses is an increase in the affinity of their envelope glycoproteins for antibodies and sCD4. We measured the relative affinities of these ligands for the monomeric gp120 glycoproteins and for the trimeric envelope glycoprotein complexes of the wild-type ADA and 190 S/R-197 N/S variants.
Radiolabeled ADA and 190 S/R-197 N/S gp120 glycoproteins were precipitated by different concentrations (0.01 to 50 μg/ml) of the F105 and 17b antibodies and by CD4-Ig. The estimated dissociation constants of these ligands for the wild-type ADA and 190 S/R-197 N/S gp120 glycoproteins were similar, in the range of 3 × 10−8 to 5 × 10−8 M (data not shown).
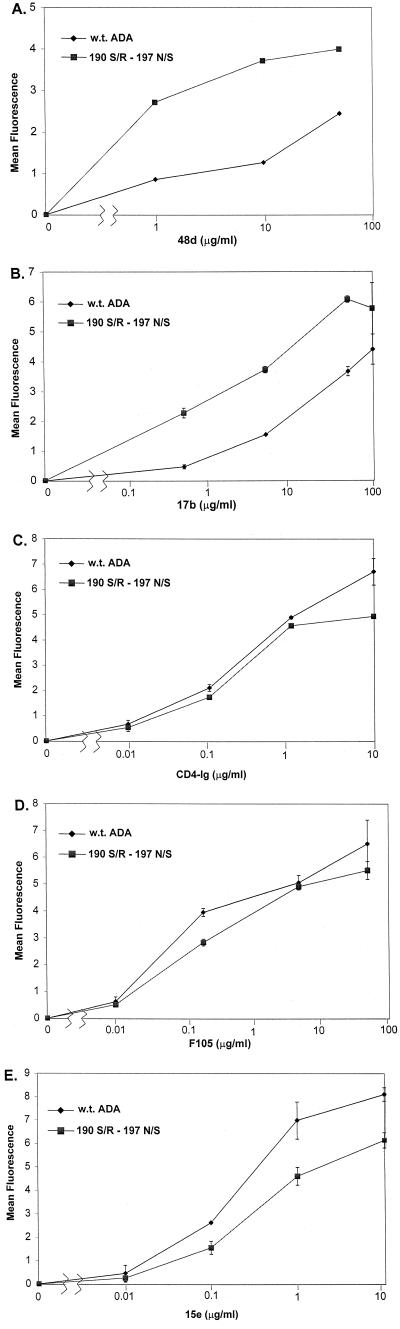
The binding of ligands to functional, trimeric envelope glycoproteins on the surface of cells expressing the wild-type ADA and 190 S/R-197 N/S variants was measured. A range of concentrations of primary antibodies was used to allow differences in antibody affinity for the two envelope glycoproteins to be detected. Comparable levels of surface expression of the wild-type ADA and 190 S/R-197 N/S glycoproteins were verified by staining with the pooled sera from HIV-1-infected individuals (data not shown). Mock-transfected cells were used as negative controls for each antibody, and this background fluorescence was subtracted from the values for cells expressing the envelope glycoproteins. The 17b and 48d antibodies both exhibited a higher affinity for the 190 S/R-197 N/S glycoprotein complexes than for the wild-type ADA glycoproteins (Fig. 3A and B). By contrast, CD4-Ig and the F105 and 15e antibodies bound the wild-type ADA glycoproteins at least as well as the 190 S/R-197 N/S glycoproteins (Fig. 3C to E).
FIG. 3.
Binding of antibodies to envelope glycoproteins on the cell surface. 293T cells expressing cytoplasmic tail-deficient versions of the wild-type (w.t.) ADA or 190 S/R-197 N/S envelope glycoproteins, or mock-transfected control cells, were incubated with the 48d antibody (A), the 17b antibody (B), the CD4-Ig protein (C), the F105 antibody (D), or the 15e antibody (E). In parallel, cells were incubated with a 1:200 dilution of pooled sera from HIV-1-positive individuals to determine the relative abundance of the two envelope glycoproteins on the cell surface. After incubation with a phycoerythrin-conjugated secondary antibody, the cells were washed and sorted by FACS. Background binding for each ligand was determined by using mock-transfected cells. The mean fluorescence intensity for each sample is indicated, after subtraction of background and normalization for relative envelope glycoprotein expression.
The results of the binding assays suggest that increases in ligand affinity may account for the increased sensitivity of the CD4-independent envelope glycoproteins to inhibition by some antibodies, such as those directed against CD4-induced gp120 epitopes, but not others.
Induction of gp120 shedding by ligands.
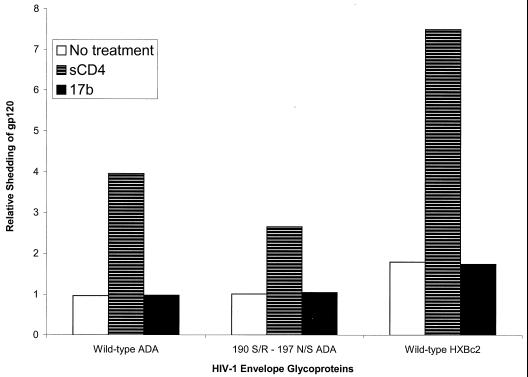
Although the physiological significance is unclear, it is well documented that the gp120 glycoprotein can be induced to shed from the HIV-1 envelope glycoprotein complex by sCD4 binding (8, 33, 35, 41, 58–60, 82, 89). Differential susceptibility of the wild-type ADA and 190 S/R-197 N/S envelope glycoproteins to gp120 shedding after ligand binding could potentially contribute to differences in neutralization sensitivity. To test this hypothesis, cells expressing the two glycoproteins, along with cells expressing an X4 HIV-1 envelope glycoprotein (HXBc2), were radiolabeled, and the amount of gp120 present in the cell supernatants in the absence or presence of ligands was evaluated. The amounts of gp120 spontaneously shed into the supernatants were equivalent for the wild-type ADA and 190 S/R-197 N/S envelope glycoproteins (Fig. 4). Slightly more gp120 glycoprotein was present in the supernatants of untreated cells expressing the HXBc2 envelope glycoproteins. Treatment of the envelope glycoprotein-expressing cells with sCD4 resulted in an increase in the amount of supernatant gp120 for all three envelope glycoproteins. Slightly more gp120 was present in the supernatants of cells expressing the wild-type ADA envelope glycoproteins than was seen for cells expressing the 190 S/R-197 N/S envelope glycoproteins. Treatment of cells with the 17b antibody did not result in higher levels of gp120 in the cell supernatants than were observed in untreated cells. These results argue against an increase in the relative sensitivity of the CD4-independent envelope glycoproteins to gp120 shedding following ligand binding.
FIG. 4.
Ligand-induced shedding of gp120. Radiolabeled cells expressing cytoplasmic tail-deficient versions of the wild-type HXBc2 envelope glycoproteins were incubated in the absence of ligand (No treatment), with sCD4 (30 μg/ml), or with 17b antibody (10 μg/ml). The gp120 glycoproteins present in the supernatants were measured by precipitation with a mixture of sera from HIV-1-infected individuals. The experiment was performed twice with similar results.
Inhibition of CCR5 binding.
We tested whether the 17b antibody could differentially neutralize the ability of wild-type ADA and CD4-independent gp120 glycoproteins to bind CCR5, both in the presence and in the absence of sCD4 (Fig. 5). The gp120 glycoproteins of the wild-type ADA and the two CD4-independent variants (190 S/R-197 N/S and 197 N/K) were preincubated with or without sCD4. Following incubation in the presence of various concentrations of the 17b antibody, the mixtures were added to Cf2Th-CCR5 cells. Binding of all three gp120 glycoproteins to Cf2Th-CCR5 cells, both in the presence and in the absence of sCD4, was inhibited ∼20% by 0.3 μg of 17b per ml relative to control binding in the absence of antibody. At a concentration of 3.0 μg/ml, the 17b antibody almost completely inhibited the binding of the different gp120 molecules to CCR5, regardless of the presence of sCD4. Thus, little difference in the sensitivity of inhibition of CCR5 binding by the 17b antibody was observed among the three envelope glycoproteins.
FIG. 5.
Inhibition of gp120-CCR5 binding. The wild-type ADA or variant gp120 glycoproteins were incubated with the indicated concentrations of the 17b antibody in the absence (−) or presence (+) of sCD4 (10 μg/ml). The mixtures were added to Cf2Th-CCR5 cells, and the bound gp120 was quantitated by precipitation by a mixture of sera from HIV-1-infected individuals. The amount of bound gp120 is indicated, using an arbitrary scale. The experiment was performed twice with comparable results.
DISCUSSION
We have shown that for the HIV-1 ADA envelope glycoproteins, changes that allow CD4-independent virus entry into cells also confer increased sensitivity to neutralization by sCD4 and antibodies. Virus entry into CD4-positive and CD4-negative cells and syncytium formation mediated by CD4-independent envelope glycoproteins were inhibited at concentrations of sCD4 and antibodies significantly lower than those required to inhibit the function of CD4-dependent envelope glycoproteins. The observation that all of the gp120 changes associated with CD4 independence, including single amino acid substitutions and complete deletion of the V1/2 variable loops, also were sufficient to specify an increase in neutralization sensitivity argues that these two properties of the envelope glycoproteins are tightly linked. It may be that an increase in neutralization sensitivity is a necessary consequence of CD4 independence. This predicts that the evolution of CD4-independent HIV-1 variants would not be favored under circumstances, such as in vivo infection, where envelope glycoprotein-directed antibodies are present.
The utilization of the CXCR4 chemokine receptor as the sole receptor for feline immunodeficiency viruses (88) and the ability of naturally occurring SIVs to achieve a measure of CD4-independent infection (27, 53, 59) suggest a model in which chemokine receptors or related molecules represent the primordial receptors for the mammalian immunodeficiency viruses. The evolution of the primate immunodeficiency viruses toward greater dependence on CD4 not only would target virus infection to helper T cells that are critical for antiviral immune responses (69) but would allow the creation of a relatively neutralization-resistant conformation in the viral envelope glycoprotein complex. Primate immunodeficiency virus variants have been identified along this continuum of CD4 independence and neutralization sensitivity. Macrophagetropic SIV strains can utilize low levels of CD4 on the target cell for entry and also exhibit some degree of CD4 independence (5, 27, 74). These SIV strains are typically easier to neutralize than T-cell-tropic SIV isolates such as SIVmac239 (56), which require higher levels of target cell CD4 and exhibit little or no CD4 independence (5, 74). In the absence of specific passage on CD4-negative cells, most HIV-1 isolates remain dependent on CD4 to some extent. However, tissue culture passage of primary HIV-1 isolates often generates viruses that are capable of entering cells expressing lower levels of CD4 (5, 39, 65) and that are neutralized more readily by sCD4 and antibodies (17, 54, 55, 61, 62, 73, 80, 90, 95). In all of these instances, there is a direct relationship between the degree of CD4 independence and neutralization sensitivity.
Virus attachment to target cell CD4 serves as a trigger to change the conformation of the HIV-1 envelope glycoproteins to a state that is competent for chemokine receptor binding. The changes that CD4 induces in free gp120 to achieve the CD4-bound conformation include movement of the V2 variable loop, which has been shown to mask the chemokine receptor-binding site in the native gp120 glycoprotein (93). Recently, the loss of glycosylation of asparagine 197 in the ADA gp120 glycoprotein has been shown to effect CD4 independence by allowing increased flexibility of the V1/2 loops and a spontaneous exposure of the CCR5-binding region (43a). Concomitantly, an increase in the exposure of the CD4-induced epitopes, which are near the CCR5-binding site, occurs (Fig. 3) (43a). Similar phenotypes were associated with deglycosylation of asparagine 197 and complete removal of the V1/2 loops with respect to CD4 independence, exposure of the CD4-induced gp120 epitopes, and neutralization sensitivity. The ability of these mutants to bind CCR5 in the absence of CD4 suggests that they spontaneously sample the gp120 conformation competent for chemokine receptor binding. Our results suggest that this conformation, which presumably resembles that induced in the wild-type gp120 by CD4 binding, is potentially more susceptible to neutralization by envelope glycoprotein-directed ligands. Such ligands during natural infection are predominantly or exclusively antibodies; steric hindrance limits their binding to envelope glycoprotein-CD4 complexes in the virus-cell interface (46, 81, 94). Subneutralizing concentrations of sCD4, when preincubated with primary HIV-1, induce dramatic increases in sensitivity to neutralization by some antibodies against the gp120 glycoprotein (81). This observation is consistent with a model in which the CD4-triggered envelope glycoprotein conformation, which is competent for chemokine receptor binding, is more vulnerable to neutralizing antibodies.
The precise mechanism that leads to increased neutralization sensitivity in the CD4-independent envelope glycoproteins is unclear. Neutralization sensitivity is not merely a consequence of the relative inefficiency of infection of CD4-negative cells, because CD4-independent viruses are also more sensitive to neutralization when infecting CD4-positive target cells, an efficient process. The increased susceptibility of the CD4-independent viruses to the 17b and 48d antibodies could be explained at least in part by the increased exposure of these epitopes on these viruses. Another CD4-independent HIV-1 isolate generated on CXCR4-expressing cells has been shown to exhibit a greater spontaneous exposure of the 17b epitope and a greater sensitivity to neutralization by this antibody (37). Spontaneous exposure of gp120 regions near the chemokine receptor-binding site may be a common feature of CD4-independent HIV-1 variants.
For sCD4 and antibodies other than the CD4-induced antibodies, increased binding of the ligand to oligomeric envelope glycoprotein complexes did not appear to explain the enhanced neutralization sensitivity of the CD4-independent viruses. An alternative explanation is that the wild-type ADA envelope glycoprotein trimers require saturation by antibodies for neutralization (64), whereas the CD4-independent envelope glycoprotein complexes can be neutralized by subsaturating antibody concentrations. The exposure of the chemokine receptor-binding site may result in a metastable state vulnerable to the binding of any ligand, which triggers an irreversible functional inactivation. Some of these conformational transitions may relate to those proposed to occur in the gp41 glycoprotein in response to chemokine receptor binding (10, 86). The observation that sCD4 dramatically inhibits the infection of CD4-negative cells by the 190 S/R-197 N/S and 197 N/K CD4-independent viruses, yet stimulates the binding of the gp120 glycoproteins of these viruses to CCR5, supports the notion that steps in the entry process that occur after chemokine receptor binding may be particularly prone to disruption by ligand binding in these viruses. Such disruption may be time dependent, explaining why the binding of CD4 on the target cell enhances infection by the CD4-independent viruses when sCD4 incubation with these viruses is inhibitory.
The influence of antibodies directed against the CD4 and CCR5 receptors on infection by the wild-type and CD4-independent viruses provides some insight into the relative contribution of receptor density to the entry of these viruses. Infection of CD4-positive target cells by the 190 S/R-197 N/S and 197 N/K viruses is more efficient than infection of CD4-negative target cells, indicating that these viruses still can utilize cell surface CD4 to assist in the virus entry process. For CD4-positive target cells, CD4-independent viruses and the wild-type ADA virus exhibited roughly similar dependence upon CD4, as evidenced by comparable sensitivity to inhibition by the OKT4a anti-CD4 antibody. For CD4-negative target cells, CCR5 concentration is critical to successful infection by the CD4-independent viruses, as suggested by the potent inhibition by the 2D7 anti-CCR5 antibody.
As discussed above, primate immunodeficiency viruses that exhibit decreased dependence on CD4 for infection may share features such as the spontaneous exposure of the chemokine receptor-binding site and an increase in sensitivity to some neutralizing ligands. However, because the structural elements that govern CD4 independence exhibit variability among virus isolates, the details of the structure-function relationships are expected to differ among particular HIV-1 strains. For example, removal of the carbohydrate on asparagine 197 or deletion of the V1/2 variable loops in some HIV-1 isolates other than ADA results in no or only modest increases in CD4-independent chemokine receptor binding and virus infection (10, 66, 91, 92; P. Kolchinsky and J. Sodroski, unpublished observations). The various HIV-1 isolates that have deletions of the V1/2 variable loops exhibit increased sensitivity to different subsets of neutralizing antibodies compared with the wild-type virus counterparts (10, 78). Further investigation of several examples of CD4-independent viruses should allow an improved understanding of the different ways in which the V1/2 and V3 variable loops and associated carbohydrate interact within the envelope glycoprotein oligomers of multiple isolates.
ACKNOWLEDGMENTS
We thank Marshall Posner, James Robinson, Dennis Burton, Herman Katinger, and Lijun Wu for antibodies and Tajib Mirzabekov and Mark Cayabyab for cell lines. We thank Yvette McLaughlin and Sheri Farnum for manuscript preparation.
This work was supported by National Institutes of Health grants AI24755, AI31783, and AI41851 and by Center for AIDS Research grant AI28691. Additional support was provided by the G. Harold and Leila Y. Mathers Foundation, the late William F. McCarty-Cooper, Friends 10, and Douglas and Judith Krupp.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC-CKR5: a RANTES, MIP-1a, MIP-1b receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Allan J, Coligan J, Barin F, McLane M F, Sodroski J, Rosen C, Haseltine W, Lee T H, Essex M. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science. 1985;228:1091–1094. doi: 10.1126/science.2986290. [DOI] [PubMed] [Google Scholar]
- 3.Allan J S. Receptor-mediated activation of immunodeficiency viruses in viral fusion. Science. 1991;252:1322–1323. doi: 10.1126/science.1925547. [DOI] [PubMed] [Google Scholar]
- 4.Allan J S, Strauss J, Buck D W. Enhancement of SIV infection with soluble receptor molecules. Science. 1990;247:1084–1088. doi: 10.1126/science.2309120. [DOI] [PubMed] [Google Scholar]
- 5.Bannert N, Schenten D, Craig S, Sodroski J. The level of CD4 expression limits the infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses. J Virol. 2000;74:10984–10993. doi: 10.1128/jvi.74.23.10984-10993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barre-Sinoussi F, Chermann J C, Rey F, Nugeyre M T, Chamaret S, Gruest J, Daguet C, Axler-Bin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphocyte retrovirus from a patient at risk for acquired immunodeficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 7.Bieniasz P D, Fridell R, Aramori I, Ferguson S, Caron M, Cullen B. HIV-1-induced cell fusion is mediated by multiple regions within both the viral envelope and the CCR5 co-receptor. EMBO J. 1997;16:2599–2609. doi: 10.1093/emboj/16.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bugelski P J, Ellens H, Hart T K, Kirsh R L. Soluble CD4 and dextran sulfate mediate release of gp120 from HIV-1: implications for clinical trials. J Acquir Immune Defic Syndr. 1991;4:923–924. [PubMed] [Google Scholar]
- 9.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W H I, Sawyer L S W, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty I, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F., III Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 10.Cao J, Sullivan N, Desjardins E, Parolin C, Robinson J, Wyatt R, Sodroski J. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1/V2 variable loops of the gp120 envelope glycoprotein. J Virol. 1997;71:9808–9812. doi: 10.1128/jvi.71.12.9808-9812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan D C, Fass D, Berger J M, Kim P. Core structure of gp41 from HIV envelope glycoprotein. Cell. 1997;73:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Zhou P, Ho D D, Landau N, Marx P. Genetically divergent strains of simian immunodeficiency virus use CCR5 as cofactor for entry. J Virol. 1997;71:2705–2714. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 14.Clapham P R, McKnight A, Weiss R A. Human immunodeficiency virus type 2 infection and fusion of CD4-negative human cell lines: induction and enhancement by soluble CD4. J Virol. 1992;66:3531–3537. doi: 10.1128/jvi.66.6.3531-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clavel F. HIV-2, the West African AIDS virus. AIDS. 1987;1:135–140. [PubMed] [Google Scholar]
- 16.Cocchi F, DeVico A, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 17.Daar E S, Li X L, Moudgil T, Ho D D. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc Natl Acad Sci USA. 1990;87:6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalgleish A G, Beverly P C L, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 19.Denisova G, Raviv D, Mondor I, Sattentau Q J, Gershoni J M. Conformational transitions in CD4 due to complexation with HIV-envelope glycoprotein gp120. J Immunol. 1997;158:1157–1164. [PubMed] [Google Scholar]
- 20.Deen K, McDougal J S, Inacker R, Folena-Wasserman G, Arthos J, Rosenberg J, Maddon P, Axel R, Sweet R. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 1988;331:82–84. doi: 10.1038/331082a0. [DOI] [PubMed] [Google Scholar]
- 21.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 22.Desrosiers R C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- 23.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Permentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 24.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 25.Dumonceaux J, Nisole S, Chanel C, Quivet L, Amara A, Baleux F, Briand P, Hazan U. Spontaneous mutations in the envgene of the human immunodeficiency virus type 1 NDK isolate are associated with a CD4-independent phenotype. J Virol. 1998;72:512–519. doi: 10.1128/jvi.72.1.512-519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebenbichler C, Westervelt P, Carrillo A, Henkel T, Johnson D, Ratner L. Structure-function relationships of the HIV-1 envelope V3 loop tropism determinant: evidence for two distinct conformations. AIDS. 1993;7:639–646. [PubMed] [Google Scholar]
- 27.Edinger A L, Mankowski J L, Doranz B J, Margulies B J, Lee B, Rucker J, Sharron M, Hoffman T L, Berson J F, Zink M C, Hirsch V M, Clements J E, Doms R W. CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc Natl Acad Sci USA. 1997;94:14742–14747. doi: 10.1073/pnas.94.26.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 29.Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard N, Gerard C, Sodroski J, Choe H. Tyrosine sulfation of the amino-terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96:667–676. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 30.Fauci A, Macher A, Longo D, Lane H C, Rook A, Masur H, Gelmann E. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984;100:92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- 31.Feng Y, Davey R A, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 32.Fisher R, Bertonis J, Meier W, Johnson V, Costopoulos D, Liu T, Tizard R, Walder B, Hirsch M, Schooley R, Flavell R. HIV infection is blocked in vitroby recombinant soluble CD4. Nature. 1988;331:76–78. doi: 10.1038/331076a0. [DOI] [PubMed] [Google Scholar]
- 33.Fu Y K, Hart T K, Jonak Z L, Bugelski P J. Physicochemical dissociation of CD4-mediated syncytium formation and shedding of human immunodeficiency virus type 1 gp120. J Virol. 1993;67:3818–3825. doi: 10.1128/jvi.67.7.3818-3825.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallo R C, Salahuddin S Z, Popovic M, Shearer G M, Kaplan M, Haynes B F, Palker T J, Redfield R, Oleske J, Safai B, White G, Foster P, Markham P D. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 35.Hart T K, Kirsh R, Ellens H, Sweet R W, Lambert D M, Petteway S R, Jr, Leary J, Bugelski P J. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc Natl Acad Sci USA. 1991;88:2189–2193. doi: 10.1073/pnas.88.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helseth E, Kowalski M, Gabuzda D, Olshevsky U, Haseltine W, Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990;64:2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffman T L, LaBranche C C, Zhang W, Canziani G, Robinson J, Chaiken I, Hoxie J A, Doms R W. Stable exposure of the coreceptor-binding site in a CD4-independent human immunodeficiency virus type 1 envelope protein. J Virol. 1999;96:6359–6364. doi: 10.1073/pnas.96.11.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussey R, Richardson N, Kowalski M, Brown N, Change H, Siliciano R, Dorfman T, Walker B, Sodroski J, Reinherz E. A soluble CD4 protein selectively inhibits HIV replication and syncytium formation. Nature. 1988;331:78–81. doi: 10.1038/331078a0. [DOI] [PubMed] [Google Scholar]
- 39.Kabat D, Kozak S L, Wehrly K, Chesebro B. Differences in CD4 dependence for infectivity of laboratory-adapted and primary isolates of human immunodeficiency virus type 1. J Virol. 1994;68:2570–2577. doi: 10.1128/jvi.68.4.2570-2577.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanki P, McLane M, King N, Essex M. Serological identification and characterization of a macaque T-lymphotropic retrovirus closely related to HTLV-III. Science. 1985;228:1199–1422. doi: 10.1126/science.3873705. [DOI] [PubMed] [Google Scholar]
- 41.Kirsh R, Hart T, Ellens H, Miller J, Petteway S, Lambert D, Leary B, Bugelski P. Morphometric analysis of recombinant soluble CD4-mediated release of the envelope glycoprotein gp120 from HIV-1. AIDS Res Hum Retrovir. 1990;6:1209–1212. doi: 10.1089/aid.1990.6.1209. [DOI] [PubMed] [Google Scholar]
- 42.Klatzmann D, Champagne E, Charmaret S, Gruest J, Guetard D, Hercend T, Glueckman J-C, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 43.Kolchinsky P, Mirzabekov T, Farzan M, Kiprilov E, Cayabyab M, Mooney L J, Choe H, Sodroski J. Adaptation of a CCR5-using, primary human immunodeficiency virus type 1 isolate for CD4-independent replication. J Virol. 1999;73:8120–8126. doi: 10.1128/jvi.73.10.8120-8126.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43a.Kolchinsky, P., E. Kiprilov, P. Bartley, R. Rubinstein, and J. Sodroski. Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 44.Korber B, Foley B, Leitner T, McCutchan F, Hahn B, Mellors J, Myers G, Kuiken C, editors. Human retroviruses and AIDS 1997. Los Alamos, N.Mex: Los Alamos National Laboratory; 1997. [Google Scholar]
- 45.Korber B, Foley B, Kuiken C, Pillai S, Sodroski J. Human retroviruses and AIDS 1998. Los Alamos, N.Mexi: Los Alamos National Laboratory; 1998. [Google Scholar]
- 46.Kwong P D, Wyatt R, Robinson J, Sweet R, Sodroski J, Hendrickson W. Structure of an HIV-1 gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LaBranche C, Hofmann T, Romano J, Haggarty B, Edwards T, Matthews T, Doms R, Hoxie J. Determinants of CD4 independence for a human immunodeficiency virus type 1 variant map outside regions for coreceptor specificity. J Virol. 1999;73:10310–10319. doi: 10.1128/jvi.73.12.10310-10319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leonard C, Spellman M, Riddle L, Harris R, Thomas J, Gregory T. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990;265:10373–10382. [PubMed] [Google Scholar]
- 49.Letvin N L, Daniel M D, Sehgal P K, Desrosiers R C, Hunt R D, Waldron L M, MacKey J J, Schmidt D K, Chalifoux L V, King N W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 50.Lifson J, Feinberg M, Reyes G, Rabin L, Banapour B, Chakrabarti S, Moss B, Wong-Staal F, Steimer K, Engelman E. Induction of CD4-independent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986;323:725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- 51.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 52.Marcon L, Choe H, Martin K A, Farzan M, Ponath P D, Wu L, Newman W, Gerard N, Gerard G, Sodroski J. Utilization of C-C chemokine receptor 5 by the envelope glycoproteins of a pathogenic simian immunodeficiency virus (SIVmac239) J Virol. 1997;71:2522–2527. doi: 10.1128/jvi.71.3.2522-2527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin K A, Wyatt R, Farzan M, Choe H, Marcon L, Desjardins E, Robinson J, Sodroski J, Gerard C, Gerard N P. CD4-independent binding of SIV gp120 to rhesus CCR5. Science. 1997;278:1470–1473. doi: 10.1126/science.278.5342.1470. [DOI] [PubMed] [Google Scholar]
- 54.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 55.Matthews T J. Dilemma of neutralization resistance of HIV-1 field isolates and vaccine development. AIDS Res Hum Retrovir. 1994;10:631–632. doi: 10.1089/aid.1994.10.631. [DOI] [PubMed] [Google Scholar]
- 56.Means R A, Greenough T, Desrosiers R. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mirzabekov T, Bannert N, Farzan M, Hofmann W, Kolchinsky P, Wu L, Wyatt R, Sodroski J. Enhanced expression, native purification, and characterization of CCR5, a principal HIV-1 coreceptor. J Biol Chem. 1999;274:28745–28750. doi: 10.1074/jbc.274.40.28745. [DOI] [PubMed] [Google Scholar]
- 58.Moore J, McKeating J, Weiss R, Sattentau Q. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990;250:1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- 59.Moore J P, Klasse P J. Thermodynamic and kinetic analysis of sCD4 binding to HIV-1 virions and of gp120 dissociation. AIDS Res Hum Retrovir. 1992;8:443–450. doi: 10.1089/aid.1992.8.443. [DOI] [PubMed] [Google Scholar]
- 60.Moore J P, McKeating J A, Norton W A, Sattentau Q J. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J Virol. 1991;65:1133–1140. doi: 10.1128/jvi.65.3.1133-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore J P, McKeating J, Huang Y, Ashkenazi A, Ho D D. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive ones. J Virol. 1992;66:235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas III C F, Burton D R, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parren P W, Mondor L, Naniche D, Ditzel H J, Klasse P J, Burton D R, Sattentau Q J. Neutralization of human immunodeficiency virus type 1 by antibody to gp120 is determined primarily by occupancy of sites on the virion irrespective of epitope specificity. J Virol. 1998;72:3512–3519. doi: 10.1128/jvi.72.5.3512-3519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Platt E J, Kozak S L, Kabat D. Critical role of enhanced CD4 affinity in laboratory adaptation of human immunodeficiency virus type 1. AIDS Res Hum Retrovir. 2000;16:871–882. doi: 10.1089/08892220050042819. [DOI] [PubMed] [Google Scholar]
- 66.Rizzuto C, Wyatt R, Hernádez-Ramos N, Sun Y, Kwong P D, Hendrickson W A, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 67.Roben P, Moore J, Thali M, Sodroski J, Barbas C, Burton D. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J Virol. 1994;68:4821–4828. doi: 10.1128/jvi.68.8.4821-4828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robey W G, Safai B, Oroszlan S, Arthur L, Gonda M, Gallo R, Fischinger P J. Characterization of envelope and core structural gene products of HTLV-III with sera from AIDS patients. Science. 1985;228:593–595. doi: 10.1126/science.2984774. [DOI] [PubMed] [Google Scholar]
- 69.Rosenberg E S, Billingsley J, Caliendo A, Boswell S, Sax P, Kalams S, Walker B. Vigorous HIV-1-specific CD4+T cell responses associated with the control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 70.Sattentau Q J, Moore J P, Vignaux F, Traincard F, Poignard P. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J Virol. 1993;67:7383–7393. doi: 10.1128/jvi.67.12.7383-7393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sattentau Q J, Moore J P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991;174:407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sauter M M, Pelchen-Matthews A, Bron R, Marsh M, LaBranche C C, Vance P J, Romano J, Haggarty B S, Hart T K, Lee W M, Hoxie J A. An internalization signal in the simian immunodeficiency virus transmembrane protein cytoplasmic domain modulates expression of envelope glycoproteins on the cell surface. J Cell Biol. 1996;132:795–811. doi: 10.1083/jcb.132.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sawyer L S, Wrin M T, Crawford-Miksza L, Potts B, Wu Y, Weber P A, Alfonso R D, Hanson C V. Neutralization sensitivity of human immunodeficiency virus type 1 is determined in part by the cell in which the virus is propagated. J Virol. 1994;68:1342–1349. doi: 10.1128/jvi.68.3.1342-1349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schenten D, Marcon L, Karlsson G, Parolin C, Kodama T, Gerard N, Sodroski J. Effects of soluble CD4 on simian immunodeficiency virus infection of CD4-positive and CD4-negative cells. J Virol. 1999;73:5373–5380. doi: 10.1128/jvi.73.7.5373-5380.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith D, Byrn R, Marsters S, Gregory T, Groopman J, Capon D. Blocking of HIV-1 infectivity by a soluble secreted form of the CD4 antigen. Science. 1987;238:1704–1707. doi: 10.1126/science.3500514. [DOI] [PubMed] [Google Scholar]
- 76.Sodroski J, Goh W C, Rosen C, Campbell K, Haseltine W A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. Nature. 1986;322:470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- 77.Speck R, Wehrly K, Platt E, Atchison R, Charo I, Kabat D, Chesebro B, Goldsmith M. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. J Virol. 1997;71:7136–7139. doi: 10.1128/jvi.71.9.7136-7139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stamatatos L, Cheng-Mayer C. An envelope modification that renders a primary, neutralization-resistant clade B human immunodeficiency virus type 1 isolate highly susceptible to neutralization by sera from other clades. J Virol. 1998;72:7840–7845. doi: 10.1128/jvi.72.10.7840-7845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stein B S, Gouda S, Lifson J, Penhallow R, Bensch K, Engelman E. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987;49:659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- 80.Sullivan N, Sun Y, Li J, Hofmann W, Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sullivan N, Sun Y, Sattentau Q, Thali M, Wu D, Denisova G, Gershoni J, Robinson J, Moore J, Sodroski J. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J Virol. 1998;72:4694–4703. doi: 10.1128/jvi.72.6.4694-4703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thali M, Furman C, Helseth E, Repke H, Sodroski J. Lack of correlation between soluble CD4-induced shedding of the human immunodeficiency virus type 1 exterior envelope glycoprotein and subsequent membrane fusion events. J Virol. 1992;66:5516–5524. doi: 10.1128/jvi.66.9.5516-5524.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thali M, Moore J P, Furman C, Charles M, Ho D D, Robinson J, Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Traunecker A, Luke W, Karjalainen K. Soluble CD4 molecules neutralize human immunodeficiency virus type 1. Nature. 1988;331:84–86. doi: 10.1038/331084a0. [DOI] [PubMed] [Google Scholar]
- 85.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its coreceptor CCR5. Nature. 1996;384:184–186. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 86.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 87.Werner W, Levy J. Human immunodeficiency virus type 1 envelope gp120 is cleaved after incubation with recombinant soluble CD4. J Virol. 1993;67:2566–2574. doi: 10.1128/jvi.67.5.2566-2574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Willett B J, Hosie M J, Neil J C, Turner J, Hoxie J A. Common mechanism of infection by lentiviruses. Nature. 1997;385:587. doi: 10.1038/385587a0. [DOI] [PubMed] [Google Scholar]
- 89.Willey R L, Martin M A, Peden K W. Increase in soluble CD4 binding to and CD4-induced dissociation of gp120 from virions correlates with infectivity of human immunodeficiency virus type 1. J Virol. 1994;68:1029–1039. doi: 10.1128/jvi.68.2.1029-1039.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wrin T, Loh T P, Vennari J C, Schuitemaker H, Nunberg J H. Adaptation to persistent growth in the H9 cell line renders a primary isolate of human immunodeficiency virus type 1 sensitive to neutralization by vaccine sera. J Virol. 1995;69:39–48. doi: 10.1128/jvi.69.1.39-48.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 92.Wyatt R, Sullivan N, Thali M, Repke H, Ho D, Robinson J, Posner M, Sodroski J. Functional and immunological characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J Virol. 1993;67:4557–4565. doi: 10.1128/jvi.67.8.4557-4565.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wyatt R, Moore J, Accola M, Desjardins E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol. 1995;69:5723–5733. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wyatt R, Kwong P D, Desjardins E, Sweet R, Robinson J, Hendrickson W, Sodroski J. The antigenic structure of the human immunodeficiency virus gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Fredriksson R, McKeating J, Fenyo E M. Passage of HIV-1 molecular clones into different cell lines confers differential sensitivity to neutralization. Virology. 1997;238:254–264. doi: 10.1006/viro.1997.8812. [DOI] [PubMed] [Google Scholar]