Abstract
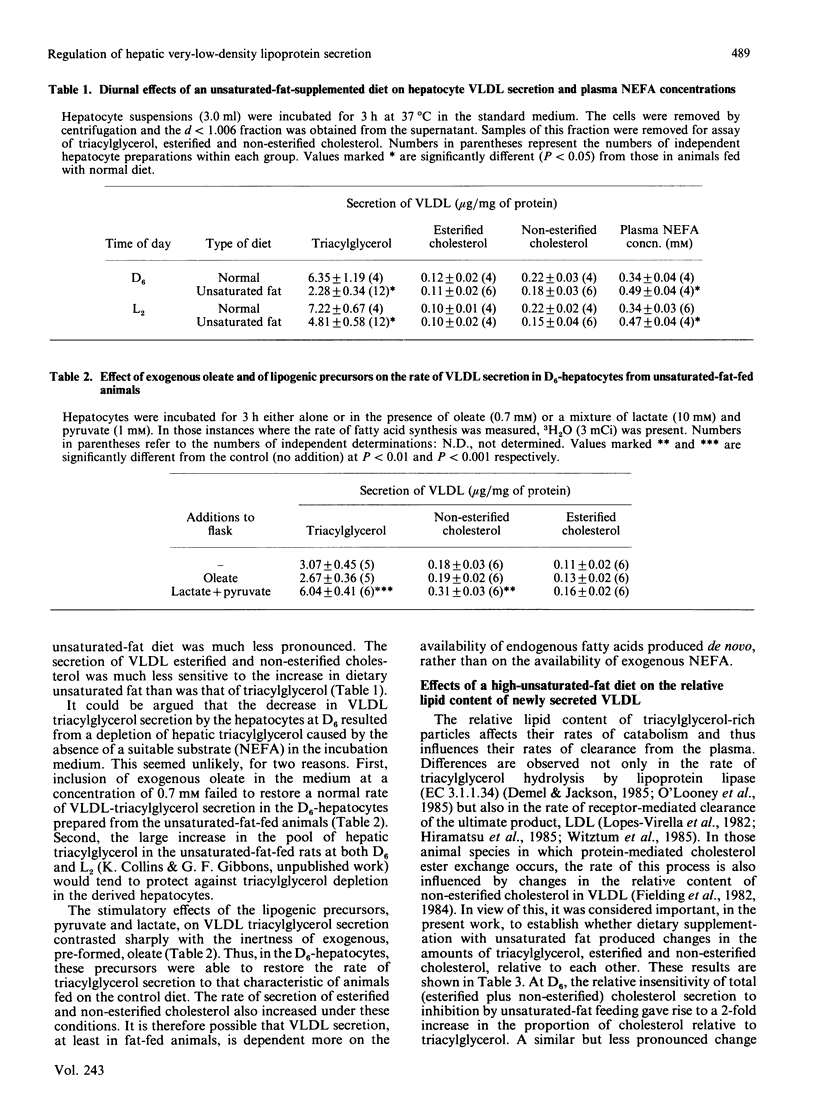
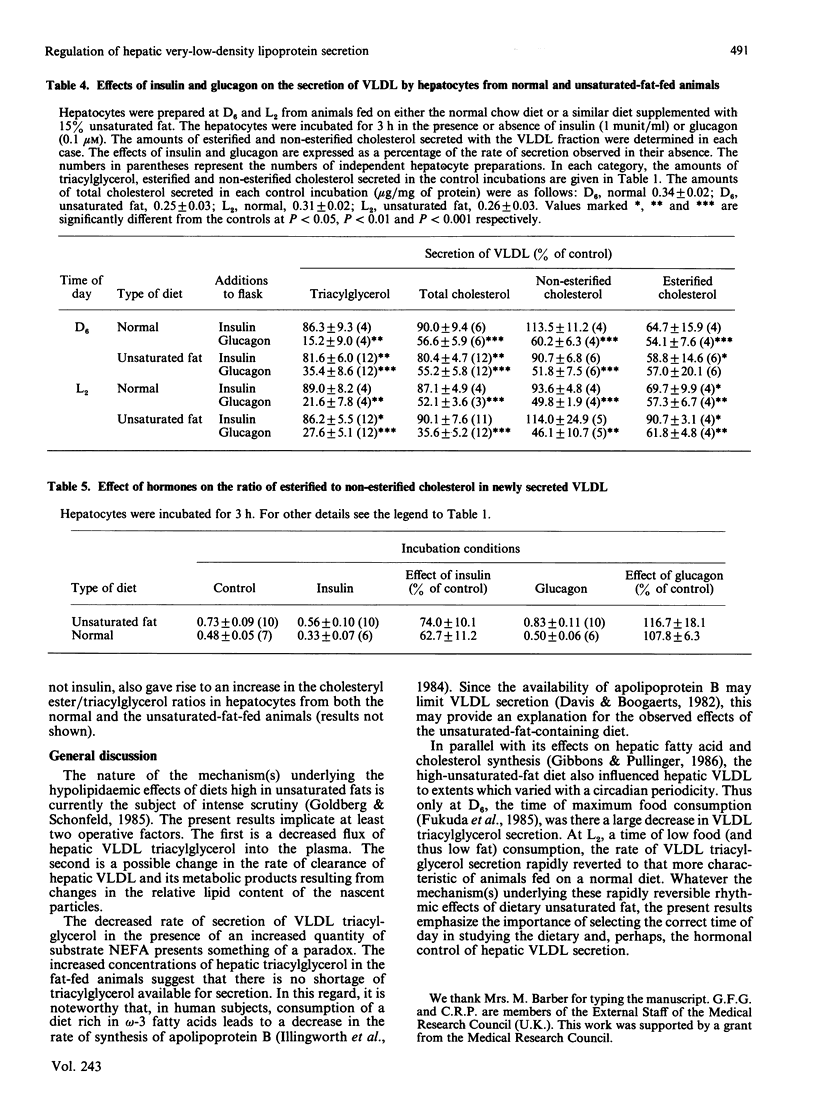
Rats were fed ad libitum on either a standard, high-carbohydrate, chow diet or a similar diet supplemented with 15% unsaturated fat (corn oil). Hepatocytes were prepared either during the dark phase (D6-hepatocytes) or during the light phase (L2-hepatocytes) of the diurnal cycle. In hepatocytes from rats fed on the unsaturated-fat-containing diet, secretion of very-low-density lipoprotein (VLDL) triacylglycerol was inhibited to a greater extent in the D6- than in the L2-hepatocytes. Plasma non-esterified fatty acid concentrations were elevated to the same extent at both D6 and L2 in the unsaturated-fat-fed animals. The secretion of VLDL esterified and non-esterified cholesterol was relatively insensitive to changes in the unsaturated-fat content of the diet. This resulted in proportionate increases in the content of these lipid constituents compared with that of triacylglycerol in the nascent VLDL. There was also an increase in the ratio of esterified to non-esterified cholesterol in the nascent VLDL produced by hepatocytes of the unsaturated-fat-fed animals. In the D6-hepatocytes from the unsaturated-fat-fed animals, the decrease in the secretion of VLDL triacylglycerol could not be reversed by addition of exogenous oleate (0.7 mM) to the incubation medium. In contrast, addition of a mixture of lactate (10 mM) and pyruvate (1 mM) stimulated both fatty acid synthesis de novo and the rate of VLDL triacylglycerol secretion. Secretion of esterified and non-esterified cholesterol also increased under these conditions. Insulin suppressed the secretion of VLDL triacylglycerol and cholesteryl ester under a wide range of conditions in all types of hepatocyte preparations. Non-esterified cholesterol secretion was unaffected. In hepatocytes prepared from the fat-fed animals, these effects of insulin were more pronounced at D6 than at L2. Glucagon also inhibited VLDL lipid secretion in all types of hepatocyte preparations. The decrease in cholesterol secretion was due equally to decreases in the rates of secretion of both esterified and non-esterified cholesterol.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beynen A. C., Vaartjes W. J., Geelen M. J. Opposite effects of insulin and glucagon in acute hormonal control of hepatic lipogenesis. Diabetes. 1979 Sep;28(9):828–835. doi: 10.2337/diab.28.9.828. [DOI] [PubMed] [Google Scholar]
- Boyd M. E., Albright E. B., Foster D. W., McGarry J. D. In vitro reversal of the fasting state of liver metabolism in the rat. Reevaluation of the roles of insulin and glucose. J Clin Invest. 1981 Jul;68(1):142–152. doi: 10.1172/JCI110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. A., Boogaerts J. R. Intrahepatic assembly of very low density lipoproteins. Effect of fatty acids on triacylglycerol and apolipoprotein synthesis. J Biol Chem. 1982 Sep 25;257(18):10908–10913. [PubMed] [Google Scholar]
- Demel R. A., Jackson R. L. Lipoprotein lipase hydrolysis of trioleoylglycerol in a phospholipid interface. Effect of cholesteryl oleate on catalysis. J Biol Chem. 1985 Aug 15;260(17):9589–9592. [PubMed] [Google Scholar]
- Durrington P. N., Newton R. S., Weinstein D. B., Steinberg D. Effects of insulin and glucose on very low density lipoprotein triglyceride secretion by cultured rat hepatocytes. J Clin Invest. 1982 Jul;70(1):63–73. doi: 10.1172/JCI110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East A. G., Louis L. N., Hoffenberg R. Albumin synthesis by isolated rat liver cells. Exp Cell Res. 1973 Jan;76(1):41–46. doi: 10.1016/0014-4827(73)90416-3. [DOI] [PubMed] [Google Scholar]
- Fielding C. J., Reaven G. M., Fielding P. E. Human noninsulin-dependent diabetes: identification of a defect in plasma cholesterol transport normalized in vivo by insulin and in vitro by selective immunoadsorption of apolipoprotein E. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6365–6369. doi: 10.1073/pnas.79.20.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding C. J., Reaven G. M., Liu G., Fielding P. E. Increased free cholesterol in plasma low and very low density lipoproteins in non-insulin-dependent diabetes mellitus: its role in the inhibition of cholesteryl ester transfer. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2512–2516. doi: 10.1073/pnas.81.8.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H., Katsurada A., Iritani N. Diurnal variations of lipogenic enzymes, their substrate and effector levels, and lipogenesis from tritiated water in rat liver. Biochim Biophys Acta. 1985 Jul 9;835(2):163–168. doi: 10.1016/0005-2760(85)90269-3. [DOI] [PubMed] [Google Scholar]
- Geiger P. J., Bessman S. P. Protein determination by Lowry's method in the presence of sulfhydryl reagents. Anal Biochem. 1972 Oct;49(2):467–473. doi: 10.1016/0003-2697(72)90450-2. [DOI] [PubMed] [Google Scholar]
- Gibbons G. F., Attwell Thomas C. P., Pullinger C. R. The metabolic route by which oleate is converted into cholesterol in rat hepatocytes. Biochem J. 1986 Apr 1;235(1):19–24. doi: 10.1042/bj2350019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons G. F. Hyperlipidaemia of diabetes. Clin Sci (Lond) 1986 Nov;71(5):477–486. doi: 10.1042/cs0710477. [DOI] [PubMed] [Google Scholar]
- Gibbons G. F., Pullinger C. R., Björnsson O. G. Changes in the sensitivity of lipogenesis in rat hepatocytes to hormones and precursors over the diurnal cycle and during longer-term starvation of donor animals. J Lipid Res. 1984 Dec 1;25(12):1358–1367. [PubMed] [Google Scholar]
- Gibbons G. F., Pullinger C. R. Diurnal variations in the effects of an unsaturated-fat-containing diet on fatty acid and cholesterol synthesis in rat hepatocytes. Biochem J. 1986 Nov 1;239(3):617–623. doi: 10.1042/bj2390617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons G. F., Pullinger C. R. Utilization of endogenous and exogenous sources of substrate for cholesterol biosynthesis by isolated hepatocytes. Biochem J. 1979 Jan 1;177(1):255–263. doi: 10.1042/bj1770255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh E. H., Heimberg M. Relationship between activity of hepatic 3-hydroxy-3-methylglutaryl-coenzyme A reductase and secretion of very-low-density-lipoprotein cholesterol by the isolated perfused liver and in the intact rat. Biochem J. 1979 Oct 15;184(1):1–6. doi: 10.1042/bj1840001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. C., Schonfeld G. Effects of diet on lipoprotein metabolism. Annu Rev Nutr. 1985;5:195–212. doi: 10.1146/annurev.nu.05.070185.001211. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Progress in understanding the LDL receptor and HMG-CoA reductase, two membrane proteins that regulate the plasma cholesterol. J Lipid Res. 1984 Dec 15;25(13):1450–1461. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg M., Dunn G. D., Wilcox G. The derivation of plasma-free fatty acids from dietary neutral fat in man. J Lab Clin Med. 1974 Mar;83(3):393–402. [PubMed] [Google Scholar]
- Hems D. A., Rath E. A., Verrinder T. R. Fatty acid synthesis in liver and adipose tissue of normal and genetically obese (ob/ob) mice during the 24-hour cycle. Biochem J. 1975 Aug;150(2):167–173. doi: 10.1042/bj1500167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K., Bierman E. L., Chait A. Metabolism of low-density lipoprotein from patients with diabetic hypertriglyceridemia by cultured human skin fibroblasts. Diabetes. 1985 Jan;34(1):8–14. doi: 10.2337/diab.34.1.8. [DOI] [PubMed] [Google Scholar]
- Ide T., Okamatsu H., Sugano M. Regulation by dietary fats of 3-hydroxy-3-methylglutaryl-Coenzyme A reductase in rat liver. J Nutr. 1978 Apr;108(4):601–612. doi: 10.1093/jn/108.4.601. [DOI] [PubMed] [Google Scholar]
- Illingworth D. R., Harris W. S., Connor W. E. Inhibition of low density lipoprotein synthesis by dietary omega-3 fatty acids in humans. Arteriosclerosis. 1984 May-Jun;4(3):270–275. doi: 10.1161/01.atv.4.3.270. [DOI] [PubMed] [Google Scholar]
- Kalopissis A. D., Griglio S., Malewiak M. I., Rozen R., Liepvre X. L. Very-low-density-lipoprotein secretion by isolated hepatocytes of fat-fed rats. Biochem J. 1981 Aug 15;198(2):373–377. doi: 10.1042/bj1980373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-Virella M. F., Sherer G. K., Lees A. M., Wohltmann H., Mayfield R., Sagel J., LeRoy E. C., Colwell J. A. Surface binding, internalization and degradation by cultured human fibroblasts of low density lipoproteins isolated from type 1 (insulin-dependent) diabetic patients: changes with metabolic control. Diabetologia. 1982 Jun;22(6):430–436. doi: 10.1007/BF00282585. [DOI] [PubMed] [Google Scholar]
- Mangiapane E. H., Brindley D. N. Effects of dexamethasone and insulin on the synthesis of triacylglycerols and phosphatidylcholine and the secretion of very-low-density lipoproteins and lysophosphatidylcholine by monolayer cultures of rat hepatocytes. Biochem J. 1986 Jan 1;233(1):151–160. doi: 10.1042/bj2330151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Takabayashi Y., Foster D. W. The role of malonyl-coa in the coordination of fatty acid synthesis and oxidation in isolated rat hepatocytes. J Biol Chem. 1978 Nov 25;253(22):8294–8300. [PubMed] [Google Scholar]
- O'Looney P., Irwin D., Briscoe P., Vahouny G. V. Lipoprotein composition as a component in the lipoprotein clearance defect in experimental diabetes. J Biol Chem. 1985 Jan 10;260(1):428–432. [PubMed] [Google Scholar]
- Parker T. S., McNamara D. J., Brown C., Garrigan O., Kolb R., Batwin H., Ahrens E. H., Jr Mevalonic acid in human plasma: relationship of concentration and circadian rhythm to cholesterol synthesis rates in man. Proc Natl Acad Sci U S A. 1982 May;79(9):3037–3041. doi: 10.1073/pnas.79.9.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch W., Franz S., Schonfeld G. Role of insulin in lipoprotein secretion by cultured rat hepatocytes. J Clin Invest. 1983 May;71(5):1161–1174. doi: 10.1172/JCI110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri A. O., Dunn F. L., Grundy S. M., Raskin P. The effect of continuous subcutaneous insulin infusion on very-low-density lipoprotein triglyceride metabolism in type I diabetes mellitus. Diabetes. 1983 Jan;32(1):75–81. doi: 10.2337/diab.32.1.75. [DOI] [PubMed] [Google Scholar]
- Pullinger C. R., Gibbons G. F. Effects of hormones and pyruvate on the rates of secretion of very-low-density lipoprotein triacylglycerol and cholesterol by rat hepatocytes. Biochim Biophys Acta. 1985 Jan 9;833(1):44–51. doi: 10.1016/0005-2760(85)90251-6. [DOI] [PubMed] [Google Scholar]
- Pullinger C. R., Gibbons G. F. The relationship between the rate of hepatic sterol synthesis and the incorporation of [3H]water. J Lipid Res. 1983 Oct;24(10):1321–1328. [PubMed] [Google Scholar]
- Rabolli D., Martin R. J. Effects of diet composition on serum levels of insulin, thyroxine, triiodothyronine, growth hormone, and corticosterone in rats. J Nutr. 1977 Jun;107(6):1068–1074. doi: 10.1093/jn/107.6.1068. [DOI] [PubMed] [Google Scholar]
- Schlierf G., Dorow E. Diurnal patterns of triglycerides, free fatty acids, blood sugar, and insulin during carbohydrate-induction in man and their modification by nocturnal suppression of lipolysis. J Clin Invest. 1973 Mar;52(3):732–740. doi: 10.1172/JCI107235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Inoue K., Tani Y., Yamada H. Enzymatic microdetermination of serum free fatty acids. Anal Biochem. 1979 Oct 1;98(2):341–345. doi: 10.1016/0003-2697(79)90151-9. [DOI] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. Insulin and non-esterified fatty acids. Acute regulators of lipogenesis in perfused rat liver. Biochem J. 1982 May 15;204(2):433–439. doi: 10.1042/bj2040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harken D. R., Dixon C. W., Heimberg M. Hepatic lipid metabolism in experimental diabetes. V. The effect of concentration of oleate on metabolism of triglycerides and on ketogenesis. J Biol Chem. 1969 May 10;244(9):2278–2285. [PubMed] [Google Scholar]
- Weekes T. E., Wahle K. W., Lebaijuri M. B. Effects of dietary triolein and sunflower oil on insulin release and lipid metabolism in Zucker rats. Lipids. 1986 Mar;21(3):220–225. doi: 10.1007/BF02534825. [DOI] [PubMed] [Google Scholar]
- Witztum J. L., Young S. G., Elam R. L., Carew T. E., Fisher M. Cholestyramine-induced changes in low density lipoprotein composition and metabolism. I. Studies in the guinea pig. J Lipid Res. 1985 Jan;26(1):92–103. [PubMed] [Google Scholar]
- Wong S. H., Nestel P. J., Trimble R. P., Storer G. B., Illman R. J., Topping D. L. The adaptive effects of dietary fish and safflower oil on lipid and lipoprotein metabolism in perfused rat liver. Biochim Biophys Acta. 1984 Feb 9;792(2):103–109. doi: 10.1016/0005-2760(84)90209-1. [DOI] [PubMed] [Google Scholar]


