Abstract
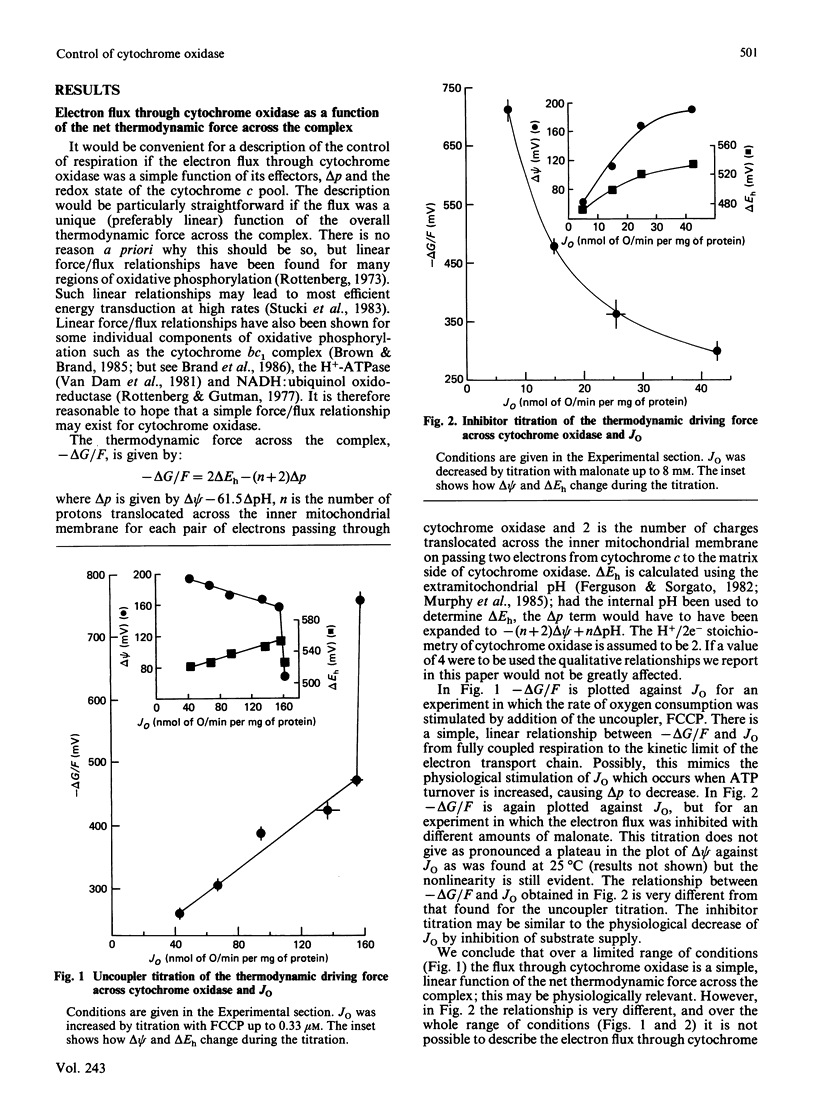
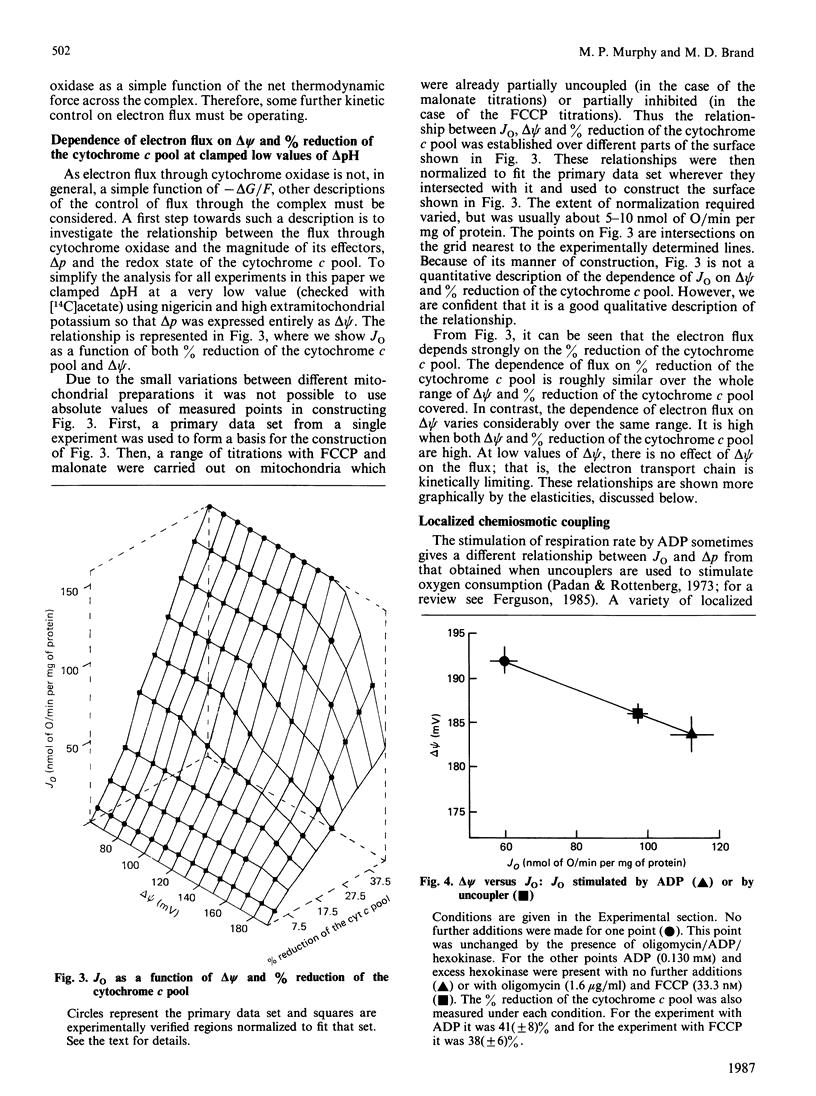
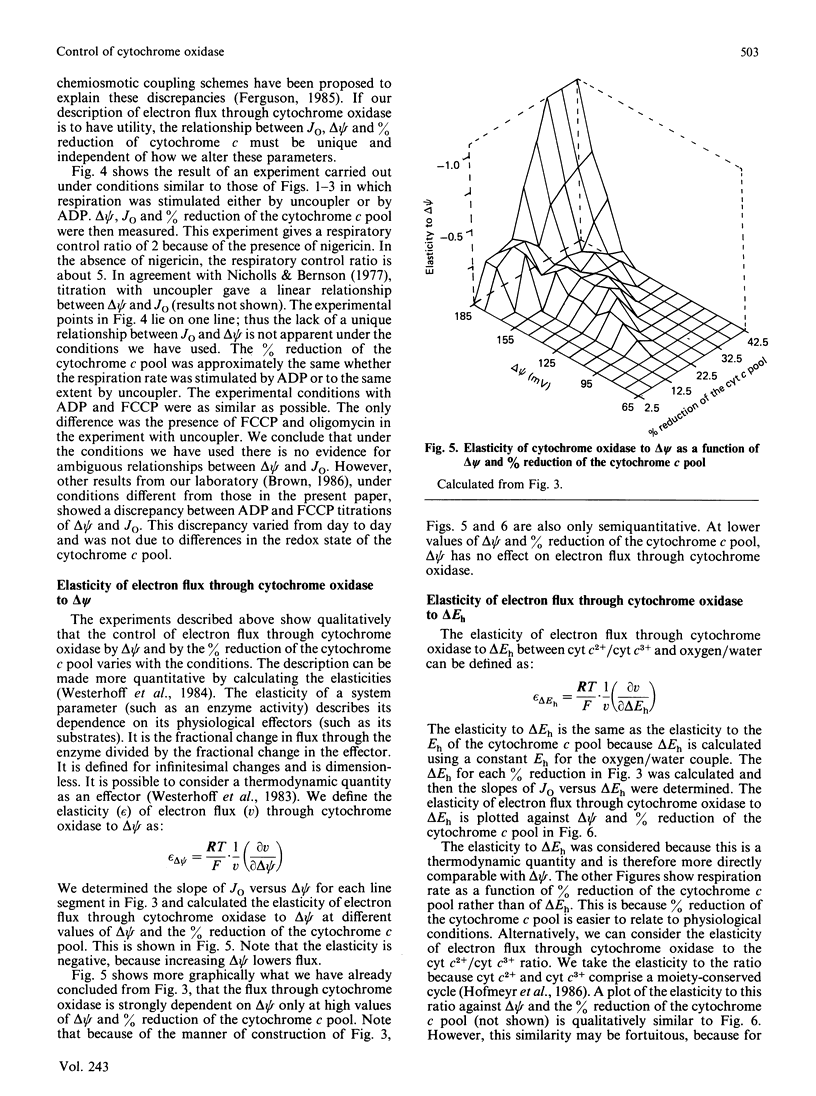
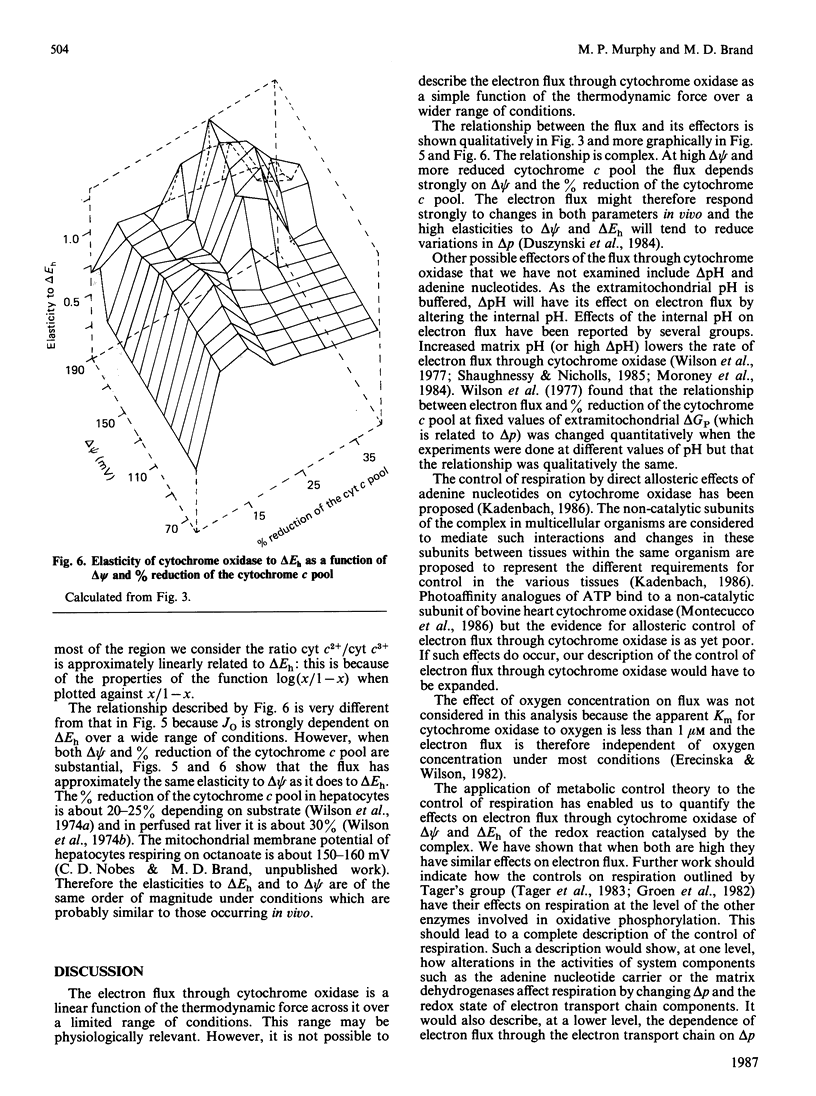
1. The electron flux through cytochrome oxidase is a linear function of the net thermodynamic force across the complex over a limited range of conditions. 2. Over a wide range of conditions the electron flux is a complicated function of the percentage reduction of the cytochrome c pool and of delta psi (at low values of delta pH). 3. We have estimated the elasticities of electron flux through cytochrome oxidase to delta Eh of the redox reaction catalysed by cytochrome oxidase (or to cyt c2+/cyt c3+) and to delta psi. The elasticities varied depending on the values of delta psi and of the percentage reduction of the cytochrome c pool. 4. At intermediate rates (which may correspond to those in vivo) the electron flux through cytochrome oxidase is controlled to about the same extent by delta psi and by delta Eh.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown G. C., Brand M. D. Thermodynamic control of electron flux through mitochondrial cytochrome bc1 complex. Biochem J. 1985 Jan 15;225(2):399–405. doi: 10.1042/bj2250399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- Davis E. J., Davis-Van Thienen W. I. Rate control of phosphorylation-coupled respiration by rat liver mitochondria. Arch Biochem Biophys. 1984 Sep;233(2):573–581. doi: 10.1016/0003-9861(84)90481-8. [DOI] [PubMed] [Google Scholar]
- Duszyński J., Bogucka K., Wojtczak L. Homeostasis of the protonmotive force in phosphorylating mitochondria. Biochim Biophys Acta. 1984 Dec 18;767(3):540–547. doi: 10.1016/0005-2728(84)90053-7. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Wilson D. F., Lee C. P. Oxidation-reduction potentials of cytochromes in mitochondria. Biochemistry. 1970 Dec 22;9(26):5077–5082. doi: 10.1021/bi00828a006. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F. Regulation of cellular energy metabolism. J Membr Biol. 1982;70(1):1–14. doi: 10.1007/BF01871584. [DOI] [PubMed] [Google Scholar]
- Ferguson S. J., Sorgato M. C. Proton electrochemical gradients and energy-transduction processes. Annu Rev Biochem. 1982;51:185–217. doi: 10.1146/annurev.bi.51.070182.001153. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Wanders R. J., Westerhoff H. V., van der Meer R., Tager J. M. Quantification of the contribution of various steps to the control of mitochondrial respiration. J Biol Chem. 1982 Mar 25;257(6):2754–2757. [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- Heinrich R., Rapoport T. A. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur J Biochem. 1974 Feb 15;42(1):89–95. doi: 10.1111/j.1432-1033.1974.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Hofmeyr J. H., Kacser H., van der Merwe K. J. Metabolic control analysis of moiety-conserved cycles. Eur J Biochem. 1986 Mar 17;155(3):631–641. doi: 10.1111/j.1432-1033.1986.tb09534.x. [DOI] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. MOlecular democracy: who shares the controls? Biochem Soc Trans. 1979 Oct;7(5):1149–1160. doi: 10.1042/bst0071149. [DOI] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- Kadenbach B. Regulation of respiration and ATP synthesis in higher organisms: hypothesis. J Bioenerg Biomembr. 1986 Feb;18(1):39–54. doi: 10.1007/BF00743611. [DOI] [PubMed] [Google Scholar]
- Montecucco C., Schiavo G., Bisson R. ATP binding to bovine heart cytochrome c oxidase. A photoaffinity labelling study. Biochem J. 1986 Feb 15;234(1):241–243. doi: 10.1042/bj2340241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney P. M., Scholes T. A., Hinkle P. C. Effect of membrane potential and pH gradient on electron transfer in cytochrome oxidase. Biochemistry. 1984 Oct 9;23(21):4991–4997. doi: 10.1021/bi00316a025. [DOI] [PubMed] [Google Scholar]
- Murphy M. P., Brown G. C., Brand M. D. Thermodynamic limits to the stoichiometry of H+ pumping by mitochondrial cytochrome oxidase. FEBS Lett. 1985 Jul 22;187(1):16–20. doi: 10.1016/0014-5793(85)81204-7. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Bernson V. S. Inter-relationships between proton electrochemical gradient, adenine-nucleotide phosphorylation potential and respiration, during substrate-level and oxidative phosphorylation by mitochondria from brown adipose tissue of cold-adapted guinea-pigs. Eur J Biochem. 1977 May 16;75(2):601–612. doi: 10.1111/j.1432-1033.1977.tb11560.x. [DOI] [PubMed] [Google Scholar]
- Padan E., Rottenberg H. Respiratory control and the proton electrochemical gradient in mitochondria. Eur J Biochem. 1973 Dec 17;40(2):431–437. doi: 10.1111/j.1432-1033.1973.tb03212.x. [DOI] [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Gutman M. Control of the rate of reverse electron transport in submitochondrial particles by the free energy. Biochemistry. 1977 Jul 12;16(14):3220–3227. doi: 10.1021/bi00633a028. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The thermodynamic description of enzyme-catalyzed reactions. The linear relation between the reaction rate and the affinity. Biophys J. 1973 Jun;13(6):503–511. doi: 10.1016/S0006-3495(73)86004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy S., Nicholls P. Control of respiration in sonicated cytochrome oxidase proteoliposomes by gated and ungated ionophores. Biochem Biophys Res Commun. 1985 Apr 30;128(2):1025–1030. doi: 10.1016/0006-291x(85)90150-0. [DOI] [PubMed] [Google Scholar]
- Stucki J. W., Compiani M., Caplan S. R. Efficiency of energy conversion in model biological pumps. Optimization by linear nonequilibrium thermodynamic relations. Biophys Chem. 1983 Sep;18(2):101–109. doi: 10.1016/0301-4622(83)85003-0. [DOI] [PubMed] [Google Scholar]
- Tager J. M., Wanders R. J., Groen A. K., Kunz W., Bohnensack R., Küster U., Letko G., Böhme G., Duszynski J., Wojtczak L. Control of mitochondrial respiration. FEBS Lett. 1983 Jan 10;151(1):1–9. doi: 10.1016/0014-5793(83)80330-5. [DOI] [PubMed] [Google Scholar]
- Westerhoff H. V., Groen A. K., Wanders R. J. Modern theories of metabolic control and their applications (review). Biosci Rep. 1984 Jan;4(1):1–22. doi: 10.1007/BF01120819. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Owen C. S., Holian A. Control of mitochondrial respiration: a quantitative evaluation of the roles of cytochrome c and oxygen. Arch Biochem Biophys. 1977 Aug;182(2):749–762. doi: 10.1016/0003-9861(77)90557-4. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Stubbs M., Oshino N., Erecińska M. Thermodynamic relationships between the mitochondrial oxidation-reduction reactions and cellular ATP levels in ascites tumor cells and perfused rat liver. Biochemistry. 1974 Dec 17;13(26):5305–5311. doi: 10.1021/bi00723a008. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Stubbs M., Veech R. L., Erecińska M., Krebs H. A. Equilibrium relations between the oxidation-reduction reactions and the adenosine triphosphate synthesis in suspensions of isolated liver cells. Biochem J. 1974 Apr;140(1):57–64. doi: 10.1042/bj1400057. [DOI] [PMC free article] [PubMed] [Google Scholar]


