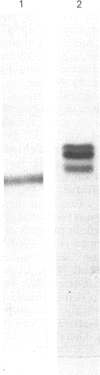
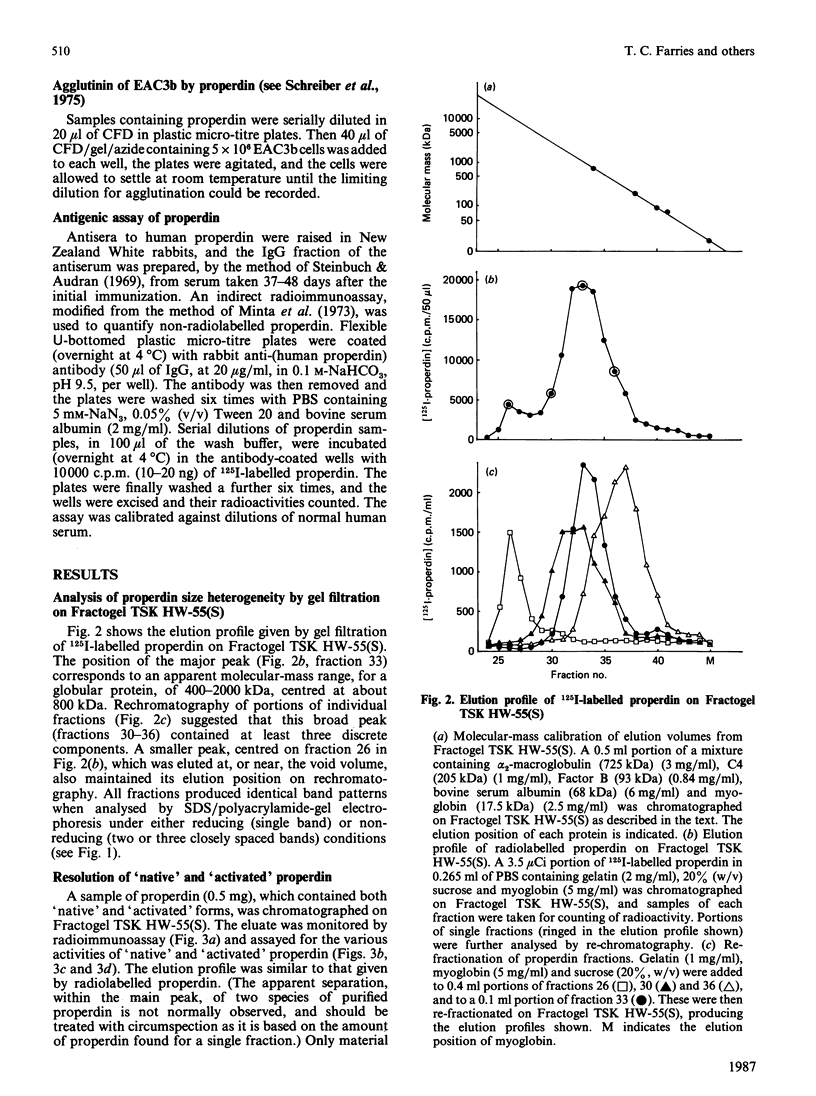
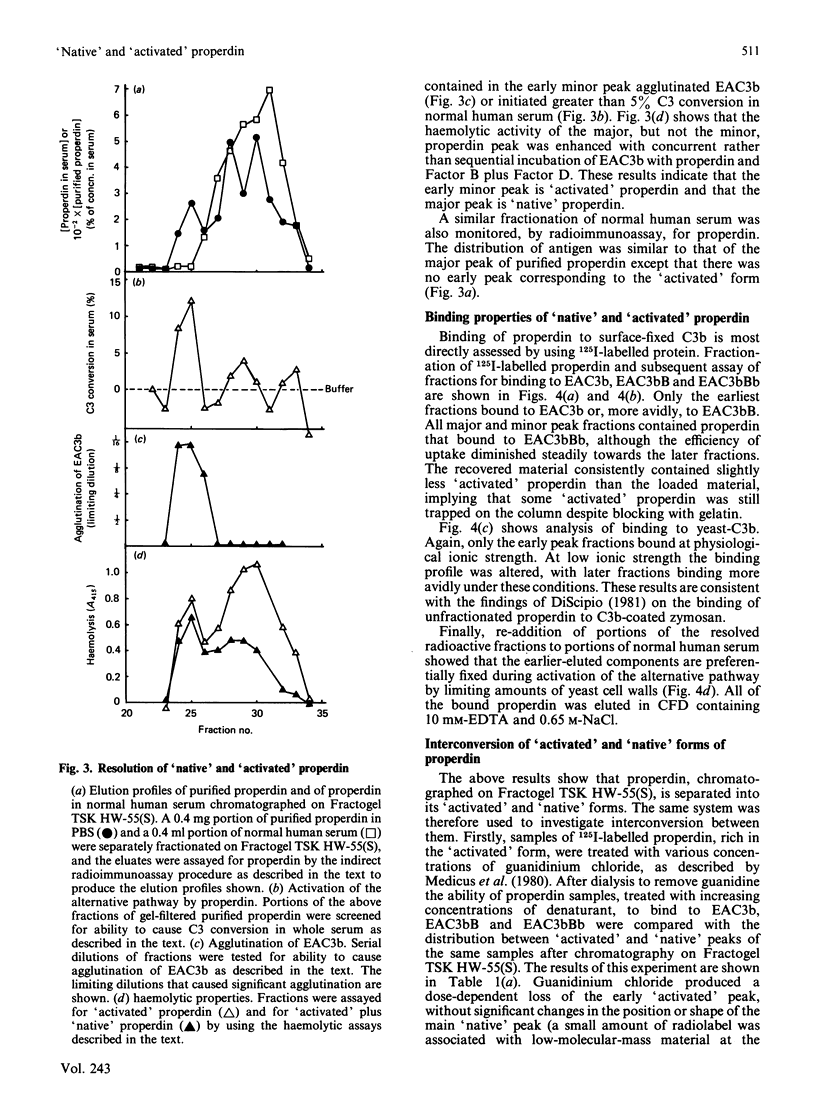
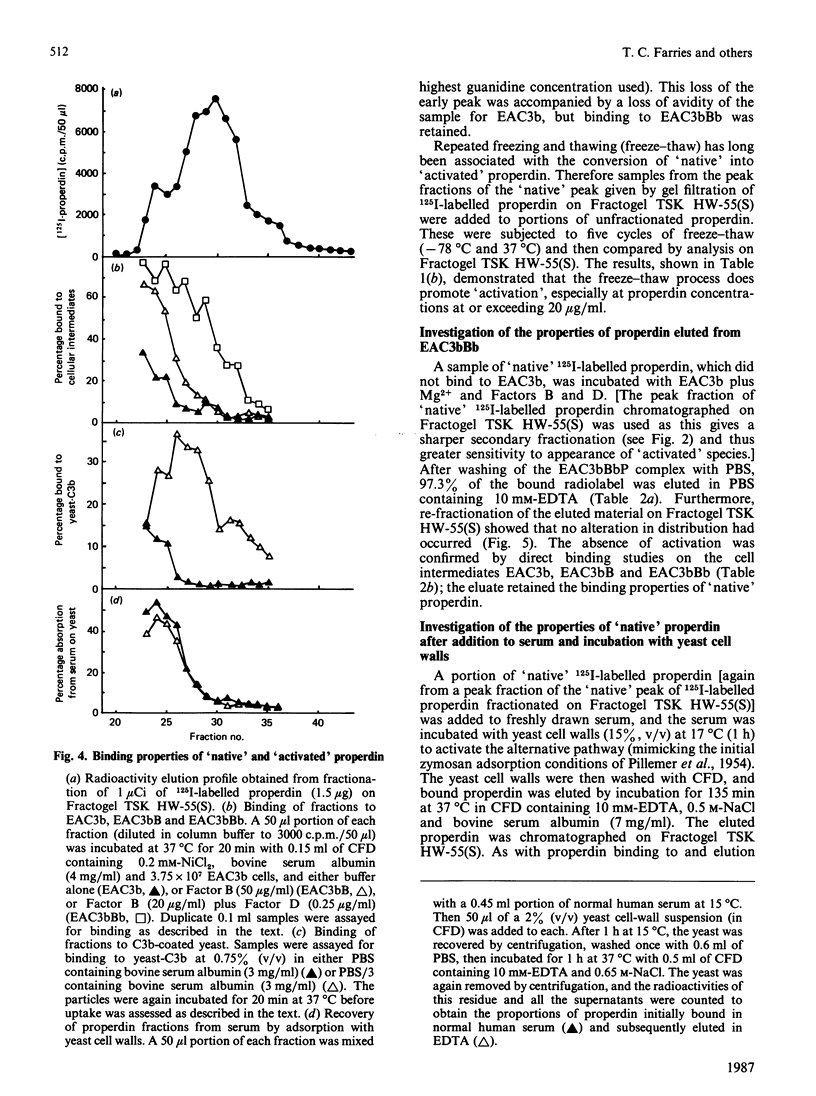
Abstract
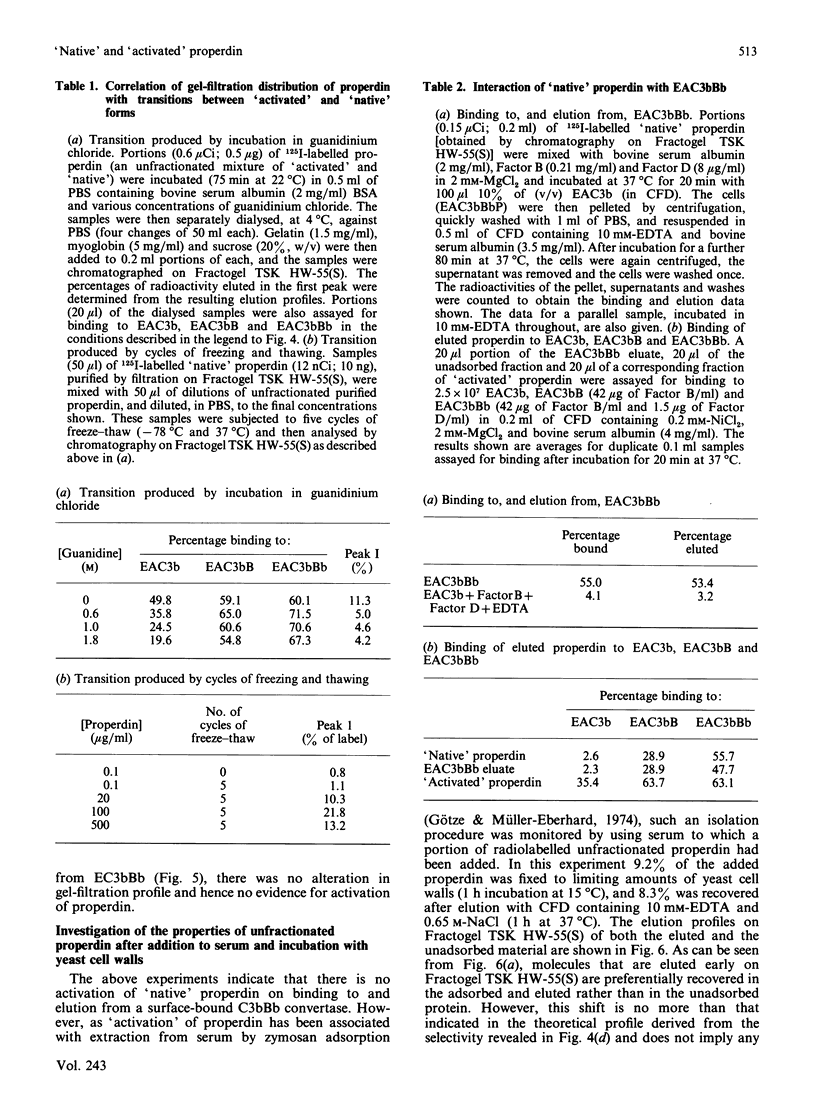
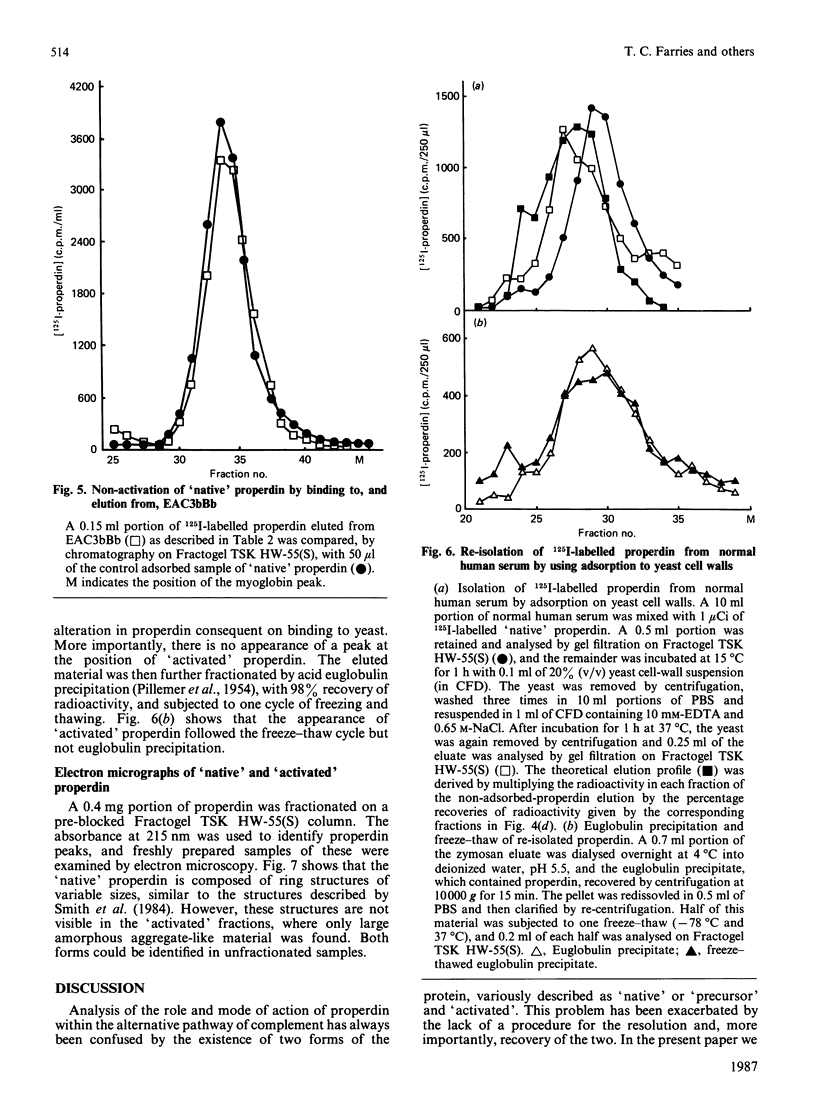
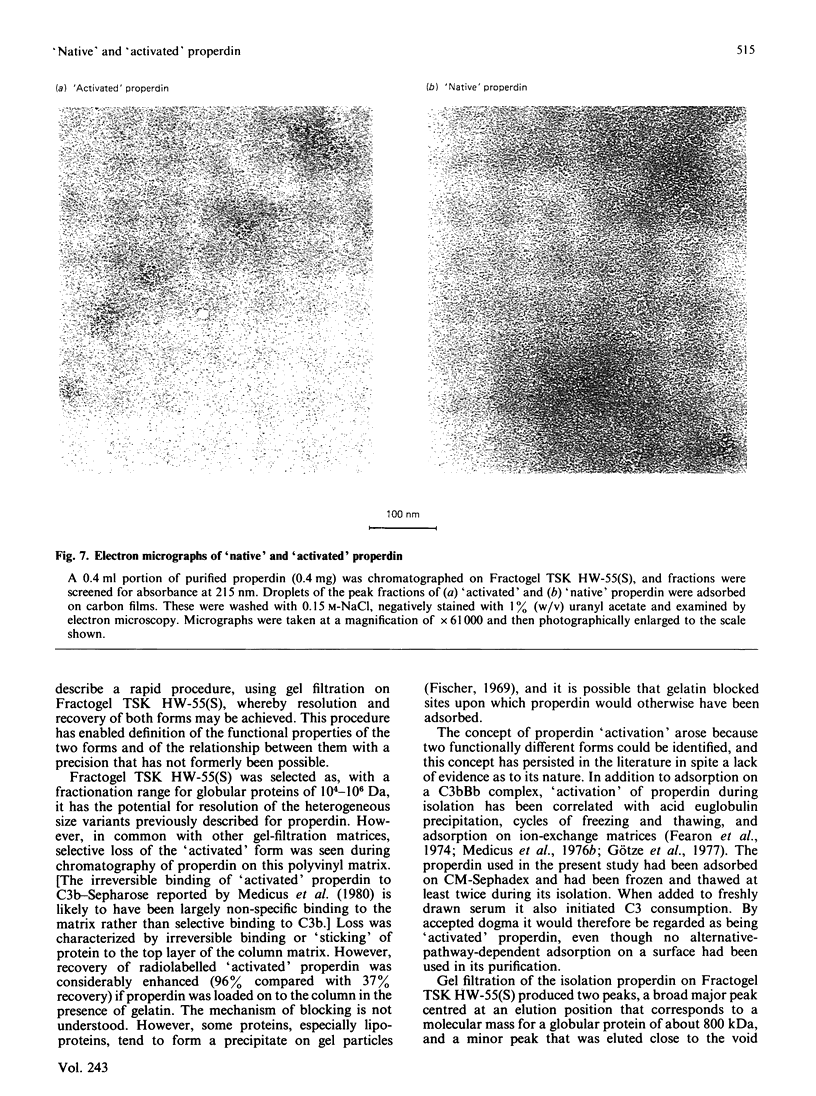
A rapid and reproducible procedure for the resolution of 'native' and 'activated' forms of properdin (a component of the alternative activation pathway of complement), by gel filtration on the polyvinyl matrix Fractogel TSK HW-55(S), is reported. This fractionation permitted effective screening of samples for conditions that cause activation. Only 'native' properdin was detected in serum, even after activation of the alternative pathway by yeast cell walls. Transformation of 'native' into 'activated' properdin in vitro was produced by freeze-thawing of the protein, but not upon binding to and dissociation from the C3 convertase, C3bBb. Electron microscopy showed that only the 'native' population contained the discrete cyclic structures described previously by Smith, Pangburn, Vogel & Müller-Eberhard [(1984) J. Biol. Chem. 259, 4582-4588]. 'Activated' properdin, which was eluted from the gel-filtration column close to the breakthrough peak, was mainly composed of large amorphous aggregates. We therefore conclude that properdin 'activation' is not a physiological event that occurs in serum on complement activation, but is an artifact of isolation. Fractionation of properdin on Fractogel TSK HW-55(S) has, however, enabled detailed analysis of functional heterogeneity within the 'native' population.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam C., Williams D. G., Peters D. K. Mechanisms of activation of the properdin system. Studies on properdin electrophoretic mobility in agarose activation of the alternative pathway. Clin Exp Immunol. 1975 Nov;22(2):240–248. [PMC free article] [PubMed] [Google Scholar]
- Chapitis J., Lepow I. H. Multiple sedimenting species of properdin in human serum and interaction of purified properdin with the third component of complement. J Exp Med. 1976 Feb 1;143(2):241–257. doi: 10.1084/jem.143.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Membrane receptors and hormone action. Adv Protein Chem. 1976;30:251–451. doi: 10.1016/s0065-3233(08)60481-7. [DOI] [PubMed] [Google Scholar]
- DiScipio R. G. The binding of human complement proteins C5, factor B, beta 1H and properdin to complement fragment C3b on zymosan. Biochem J. 1981 Dec 1;199(3):485–496. doi: 10.1042/bj1990485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discipio R. G. Properdin is a trimer. Mol Immunol. 1982 Apr;19(4):631–635. doi: 10.1016/0161-5890(82)90232-2. [DOI] [PubMed] [Google Scholar]
- Ensky J., Hinz C. F., Jr, Todd E. W., Wedgwood R. J., Boyer J. T., Lepow I. H. Properties of highly purified human properdin. J Immunol. 1968 Jan;100(1):142–158. [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Properdin: binding to C3b and stabilization of the C3b-dependent C3 convertase. J Exp Med. 1975 Oct 1;142(4):856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F., Ruddy S. Properdin factor D. II. Activation to D by properdin. J Exp Med. 1974 Aug 1;140(2):426–436. doi: 10.1084/jem.140.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Götze O., Medicus R. G., Müller-Eberhard H. J. Alternative pathway of complement: nonenzymatic, reversible transition of precursor to active properdin. J Immunol. 1977 Feb;118(2):525–532. [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The role of properdin in the alternate pathway of complement activation. J Exp Med. 1974 Jan 1;139(1):44–57. doi: 10.1084/jem.139.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Mornaghi R., Nussenzweig V. Complement receptor (CR1) deficiency in erythrocytes from patients with systemic lupus erythematosus. J Exp Med. 1982 May 1;155(5):1427–1438. doi: 10.1084/jem.155.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEPOW I. H., PILLEMER L., SCHOENBERG M. D., TODD E. W., WEDGWOOD R. J. The properdin system and immunity. X. Characterization of partially purified human properdin. J Immunol. 1959 Oct;83:428–436. [PubMed] [Google Scholar]
- Lachmann P. J., Oldroyd R. G., Milstein C., Wright B. W. Three rat monoclonal antibodies to human C3. Immunology. 1980 Nov;41(3):503–515. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- McLean R. H., Michael A. F. The immunoelectrophoretic pattern of properdin in fresh and aged human serum. Proc Soc Exp Biol Med. 1972 Nov;141(2):403–407. doi: 10.3181/00379727-141-36786. [DOI] [PubMed] [Google Scholar]
- McLean R. H., Townsend K., Michael A. F. The effect of anticomplementary substances on properdin in normal and C2-deficient sera. Clin Exp Immunol. 1975 Mar;19(3):435–444. [PMC free article] [PubMed] [Google Scholar]
- Medicus R. G., Esser A. F., Fernandez H. N., Müller-Eberhard H. J. Native and activated properdin: interconvertibility and identity of amino- and carboxy-terminal sequences. J Immunol. 1980 Feb;124(2):602–606. [PubMed] [Google Scholar]
- Medicus R. G., Götze O., Müller-Eberhard H. J. Alternative pathway of complement: recruitment of precursor properdin by the labile C3/C5 convertase and the potentiation of the pathway. J Exp Med. 1976 Oct 1;144(4):1076–1093. doi: 10.1084/jem.144.4.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicus R. G., Schreiber R. D., Götze O., Müller-Eberhard H. J. A molecular concept of the properdin pathway. Proc Natl Acad Sci U S A. 1976 Feb;73(2):612–616. doi: 10.1073/pnas.73.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minta J. O. Changes in the immunochemical properties of highly purified properdin in human serum. J Immunol. 1975 Apr;114(4):1415–1421. [PubMed] [Google Scholar]
- Minta J. O., Goodkofsky I., Lepow I. H. Solid phase radioimmunoassay of properdin. Immunochemistry. 1973 May;10(5):341–350. doi: 10.1016/0019-2791(73)90082-7. [DOI] [PubMed] [Google Scholar]
- Minta J. O., Kunar E. S. Effect of proteolytic digestion on the structure and function of human properdin. J Immunol. 1976 Apr;116(4):1099–1104. [PubMed] [Google Scholar]
- Minta J. O., Lepow I. H. Studies on the sub-unit structure of human properdin. Immunochemistry. 1974 Jul;11(7):361–368. doi: 10.1016/0019-2791(74)90189-x. [DOI] [PubMed] [Google Scholar]
- Minta J. O., Movat H. Z. Production of antiserum to human properdin and demonstration of antigenic differences between the native and activated protein. Proc Soc Exp Biol Med. 1976 Feb;151(2):411–414. doi: 10.3181/00379727-151-39222. [DOI] [PubMed] [Google Scholar]
- Minta J. O. Purification of native properdin by reversed affinity chromatography and its activation by proteolytic enzymes. J Immunol. 1976 Aug;117(2):405–412. [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Schreiber R. D. Molecular biology and chemistry of the alternative pathway of complement. Adv Immunol. 1980;29:1–53. doi: 10.1016/s0065-2776(08)60042-5. [DOI] [PubMed] [Google Scholar]
- PILLEMER L., BLUM L., LEPOW I. H., ROSS O. A., TODD E. W., WARDLAW A. C. The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954 Aug 20;120(3112):279–285. doi: 10.1126/science.120.3112.279. [DOI] [PubMed] [Google Scholar]
- PONDMAN K. W., van der HART, HOOGENDIJK J., van LOGHEM J. J., Jr The formation of antibodies against a properdin preparation. II. Vox Sang. 1960 Feb;5:142–156. doi: 10.1111/j.1423-0410.1960.tb04671.x. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Gagnon J. Amino acid sequence studies of human properdin--N-terminal sequence analysis and alignment of the fragments produced by limited proteolysis with trypsin and the peptides produced by cyanogen bromide treatment. Mol Immunol. 1981 Nov;18(11):949–959. doi: 10.1016/0161-5890(81)90112-7. [DOI] [PubMed] [Google Scholar]
- Schreiber R. D., Medicus R. G., Gïtze O., Müller-Eberhard H. J. Properdin- and nephritic factor-dependent C3 convertases: requirement of native C3 for enzyme formation and the function of bound C3b as properdin receptor. J Exp Med. 1975 Sep 1;142(3):760–772. doi: 10.1084/jem.142.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Pangburn M. K., Vogel C. W., Müller-Eberhard H. J. Molecular architecture of human properdin, a positive regulator of the alternative pathway of complement. J Biol Chem. 1984 Apr 10;259(7):4582–4588. [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- TODD E. W., PILLEMER L., LEPOW I. H. The properdin system and immunity. IX. Studies on the purification of human properdin. J Immunol. 1959 Oct;83:418–427. [PubMed] [Google Scholar]
- Tack B. F., Harrison R. A., Janatova J., Thomas M. L., Prahl J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5764–5768. doi: 10.1073/pnas.77.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. G., Fearon D. T., Stevens R. L., Seno N., Austen K. F. Inhibition of the function of activated properdin by squid chondroitin sulfate E glycosaminoglycan and murine bone marrow-derived mast cell chondroitin sulfate E proteoglycan. J Immunol. 1984 Jun;132(6):3058–3063. [PubMed] [Google Scholar]