Abstract
Tva is the cellular receptor for subgroup A avian sarcoma and leukosis virus (ASLV-A). The viral receptor function of Tva is determined by a 40-residue cysteine-rich motif called the LDL-A module. In this study, we expressed and purified the wild-type (wt) Tva LDL-A module as well as several mutants and examined their in vitro folding properties. We found that, as for other LDL-A modules, correct folding and structure of the Tva LDL-A module is Ca2+ dependent. When calcium was present during in vitro protein folding, the wt module was eluted as a single peak by reverse-phase high-pressure liquid chromatography. Furthermore, two-dimensional nuclear magnetic resonance (NMR) spectroscopy gave well-dispersed spectra in the presence of calcium. In contrast, the same protein folded in vitro in the absence of calcium was eluted as multiple broad peaks and gave a poorly dispersed NMR spectrum in the presence of calcium. The calcium affinity (Kd) of the Tva LDL-A module, determined by isothermal titration calorimetry, is approximately 40 μM. Characterization of several Tva mutants provided further evidence that calcium is important in protein folding and function of Tva. Mutations of the Ca2+-binding residues (D46A and E47A) completely abrogated the Ca2+-binding ability of Tva, and the proteins were not correctly folded. Interestingly, mutations of two non-calcium-binding residues (W48A and L34A) also exerted adverse effect on Ca2+-dependent folding, albeit to a much less extent. Our results provide new insights regarding the structure and function of Tva in ASLV-A entry.
The cellular receptor for subgroup A avian sarcoma and leukosis virus (ASLV-A) is Tva, a small membrane-associated protein (2, 3, 25). The interaction between Tva and EnvA, the ASLV-A glycoprotein, has been well characterized by several groups. Tva specifically binds EnvA with high affinity (1, 14, 23, 27). Furthermore, the high-affinity binding between Tva and EnvA in vitro can trigger a series of conformational changes on EnvA believed to be essential for postbinding steps during ASLV-A entry (8, 9, 15, 16). Although only avian cells are naturally susceptible to ASLV-A infection, expression of Tva in resistant cells from a wide range of species renders them susceptible to ASLV-A entry. These results appear to support the notion that Tva is the only receptor required for ASLV-A entry in avian cells. Thus, analysis of Tva-EnvA interaction may provide a simple model to study the entry mechanisms of viruses including other retroviruses.
Within the extracellular domain of Tva is a 40-residue, cysteine-rich module that is closely related to the repeat modules of the human low-density lipoprotein receptor (hLDLR). In hLDLR, seven imperfect tandem LDL-A modules form the binding domain for its ligands, lipoproteins apoB and apoE (12, 24). In contrast, the single LDL-A module of Tva appears necessary and sufficient to mediate ASLV-A entry. The LDL-A module of Tva could bind EnvA and mediate efficient ASLV-A infection when it was fused to the membrane-spanning domain of mouse CD8 (20). Many point mutations within the module impaired or abolished the viral receptor function of Tva (21, 26, 27). Furthermore, the LDL-A module of Tva could be functionally replaced by the fourth repeat of hLDLR with minor amino acid substitutions (22). These results demonstrate that a single LDL-A module can independently mediate protein-protein interaction. Since the viral receptor function of Tva is solely determined by this module, it is imperative to characterize the role of this motif in viral infection by an integral approach of molecular, biochemical, and structural techniques in order for us to understand the molecular mechanism of ASLV-A entry.
To date, the role of the LDL-A module of Tva in viral infection has been mainly dissected by mutational analysis. Several residues within this module that appear to be important in mediating ASLV-A infection have been identified (21, 22, 26, 27). However, one drawback of these studies is that it is difficult to distinguish whether a mutation within the motif affects the overall structure of Tva or specifically disrupts the ligand recognition, a common problem in interpreting results with mutational analysis of any protein. Fortunately, structures of several individual LDL-A modules have been recently reported (6, 7, 11, 13, 17, 19). These provide insights regarding the overall folding of the LDL-A module of Tva, since the LDL-A modules reported so far all adopt similar three-dimensional conformations. Each module consists of a short antiparallel β sheet and a one-turn α helix. The structure is stabilized by three pairs of disulfide bonds formed by six invariable cysteines. Furthermore, six amino acids, including four highly conserved acidic residues, form a “calcium cage,” coordinating calcium with high affinity (5, 13). However, the role of calcium in Tva folding and function has not been previously examined.
In this study, using biochemical and biophysical techniques, we examined the role of calcium in protein folding of the LDL-A module of Tva and found that calcium is indeed required for its correct folding and structure. Furthermore, we characterized the role of calcium in protein folding of two classes of Tva mutants: those residues that are directly involved in calcium binding and those residues that are not directly involved in calcium coordination but that, when mutated, result in impaired function of Tva. As expected, mutations of those Ca2+-binding residues are defective in protein folding. Surprisingly, mutations of the non-calcium-binding residues can also affect protein folding. This study provides new insights on structure and function of Tva in ASLV-A entry.
MATERIALS AND METHODS
Cloning and preparation of wt Tva LDL-A module and its mutants.
The LDL-A module of wild-type (wt) Tva and its mutants were amplified by PCR from Tva and Tva mutant expression plasmids. To facilitate the cloning procedure, a BamHI site and a XhoI site were engineered into the upstream and downstream primers, respectively. The PCR products were cloned into pGEX-4T-1 (Pharmacia), and the identity of each construct was confirmed by DNA sequencing.
The unlabeled LDL-A module of Tva and its mutants were expressed in Escherichia coli strain BL21 as GST (glutathione S-transferase) fusion proteins. Expression of the fusion proteins was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Sigma) at an optical density at 600 nm 0.6. The cells were grown for another 4 h at 37°C in 2×YT. The 15N-labeled proteins were expressed in minimal medium containing 0.6% basal medium Eagle vitamin solution (Gibco), 1 g of (15NH4)2SO4/liter and 2 g of unlabeled glucose/liter. Protein expression was induced by IPTG for 6 h. The expressed fusion proteins were then purified by glutathione (GSH)-Sepharose affinity chromatography, cleaved with thrombin (1/4,000 [wt/wt], 25 min at 20°C), and rechromatographed on GSH-Sepharose. The nonbinding fraction was further purified by reverse-phase high-pressure liquid chromatography (HPLC) on a Vydac C18 column operated at a flow rate of 4.00 ml/min, using a linear gradient of 0.1% trifluoroacetic acid (buffer A) and 90% acetonitrile (buffer B) (10 to 60% buffer B) at room temperature. The eluting fraction was collected and lyophilized.
In vitro refolding of the wt Tva LDL-A module and its mutants.
The lyophilized protein samples were dissolved in 6 M guanidinum chloride–50 mM Tris-HCl–1 mM dithiothreitol (pH 8.5). The samples were diluted to approximately 100 μg/ml with folding buffer (50 mM Tris [pH 8.5], 1 mM reduced GSH, 0.5 mM oxidized GSH) and then dialyzed against folding buffer containing either 10 mM CaCl2 or 1 mM EDTA for at least 24 h at room temperature under oxygen-free conditions. Folded Tva LDL-A modules were purified by reverse-phase HPLC on a Vydac C18 column as described above. The unlabeled purified samples were used for calorimetry analysis, and the 15N-labeled purified samples were used for nuclear magnetic resonance (NMR) spectroscopy.
Reverse-phase HPLC.
To examine whether folding of the Tva LDL-A module is Ca2+ dependent, aliquots of the refolding reaction mixtures (either in the presence or in the absence of CaCl2) were analyzed by reversed-phase HPLC on a Vydac C18 column under the same conditions as for protein purification described above.
NMR spectroscopy.
NMR spectra were acquired as described previously (10). Briefly, all NMR spectra were recorded at the University of Illinois at Chicago on a Bruker DRX600 spectrometer equipped with a pulsed-field-gradient accessory and operating at 600.13 MHz for 1H. Spectra were processed and analyzed using Triad version 6.3 software (Tripos, Inc., St. Louis, Mo.). The lyophilized 15N-labeled LDL-A module of Tva and its mutants were dissolved in 50 mM Tris-HCl–100 mM NaCl–10% D2O (pH 7.0). To examine the effect of Ca2+ binding on protein folding and structure of the LDL-A module of Tva, CaCl2 was added either in the folding reaction or in NMR analysis or in both with a final concentration of 10 mM. The final protein concentration was 100 μM. [1H-15N] HSQC (heteronuclear single quantum correlation spectroscopy) spectra were recorded at 25°C. The central frequencies were 4.70 and 118 ppm for 1H and 15N, respectively.
Isothermal titration calorimetry (ITC).
Calcium titrations were performed on a MicroCal MSC isothermal titration calorimeter as specified by the manufacturer (MicroCal Inc., Northampton, Mass.). Experiments were performed at 30°C. Buffers used were 20 mM Tris-HCl, 100 mM NaCl, and 0.02% NaN3, pH 7.4. HPLC-purified LDL-A module of Tva and its mutants were dialyzed overnight in the above pH 7.4 buffer, containing Chelex 100 (Bio-Rad) to remove any metal ion contamination in the protein samples. Then the samples were diluted to 10 μM in buffer and titrated with a 2-μl injection of 2 mM CaCl2 in buffer. Fifty injections were used in each experiment. Data were analyzed with Origin (MicroCal) and were fitted to a simple one-binding-site model.
RESULTS
Expression and purification of the wt Tva LDL-A module and its mutants.
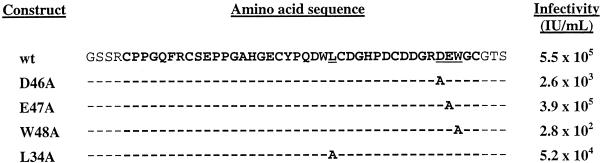
The coding regions of the wt Tva LDL-A module and its mutants (Fig. 1) were amplified by PCR and cloned into an E. coli expression vector, pGEX-4T-1, as GST fusion proteins. We used these mutants in this study because although they are all impaired in the viral receptor function of Tva (Fig. 1), their roles in protein folding and function might differ. Based on structure information for other LDL-A modules, two of these mutants (D46A and E47A) should be defective in calcium binding since the side chains of these residues are predicted to be involved in Ca2+ coordination along with four other amino acids in the module. In contrast, L34 and W48 are not predicted to be directly involved in Ca2+ binding but are likely to be involved in ligand recognition.
FIG. 1.
Amino acid sequences of wt and mutants Tva LDL-A modules in GST fusion constructs. Dashes indicate identical residues; underlines indicate mutated residues. The LDL-A module region is shown as boldface letters. The ability of the wt Tva and Tva mutants to mediate ASLV-A infection was expressed as the number of alkaline phosphatase-positive cells per milliliter of viral stock. The data are from references 21 and 22.
High levels of expression in E. coli strain BL21 were achieved by induction with IPTG, and fusion proteins were purified by one-step affinity chromatography with GSH-Sepharose. We could purify approximately 30 mg of GST fusion proteins from 1 liter of 2×YT medium or 20 mg from supplemented minimal medium. To purify the wt Tva LDL-A module and its mutants, GST fusion proteins were cleaved with thrombin, and the GST portion was removed by GSH-Sepharose chromatography. The cleaved products were further purified by reverse-phase HPLC on a Vydac C18 column as described in Materials and Methods. The predicted proteins contain 47 amino acids including the 40-residue module, four additional residues (GSSR) at the N terminus, and three (GTS) at the C terminus, as shown in Fig. 1. These LDL-A module proteins ran as approximately 14 kDa on sodium dodecyl sulfate-polyacrylamide gel electrophoresis instead of 5 kDa as calculated (data not shown).
Examination of Ca2+-dependent protein folding by reverse-phase HPLC.
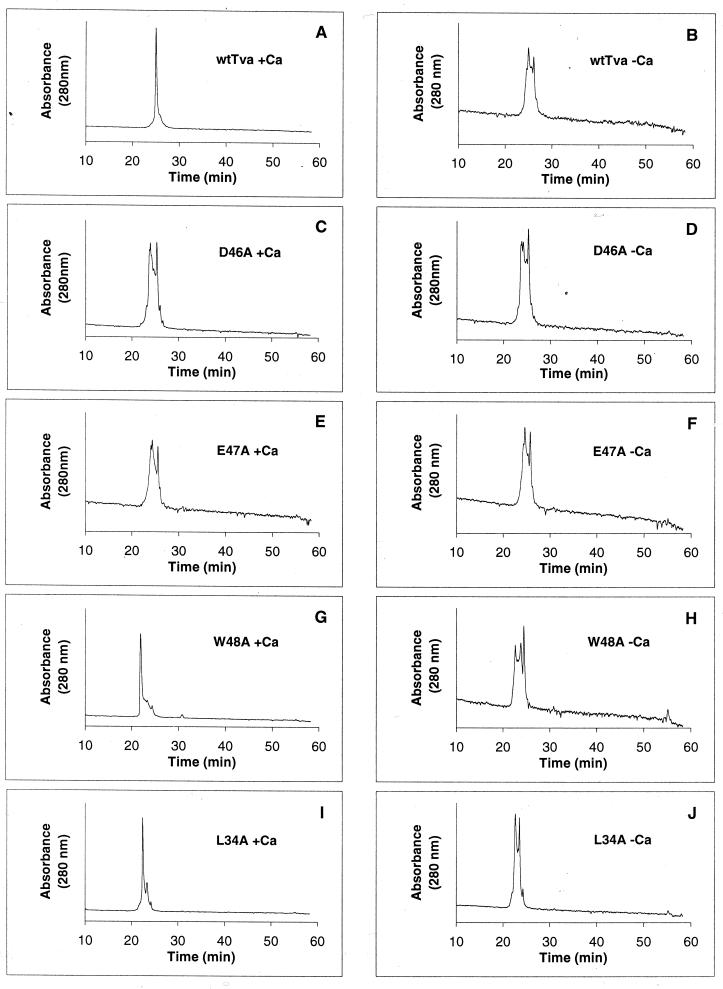
HPLC has been previously used by others to examine in vitro Ca2+-dependent folding of several LDL-A modules (5, 19). It was demonstrated that LDL-A modules were eluted as many broad peaks in the absence of calcium but as a sharp single peak in the presence of calcium. These results suggest that correct folding of an LDL-A module is dependent on calcium and that without it, the module will fold as various isomers. Therefore, we used reverse-phase HPLC to characterize the folding patterns of the wt Tva LDL-A module as well as its mutants either in the presence or absence of calcium.
The purified LDL-A module proteins were first refolded either in the absence or in the presence of calcium, and the samples were eluted by reverse-phase HPLC on a Vydac C18 column as described in Materials and Methods. As expected, in the presence of calcium, the wt Tva LDL-A module was eluted as a single sharp peak, at about 25 min (Fig. 2A). In contrast, in the absence of calcium, the wt Tva module was eluted as several broad peaks (Fig. 2B). These results suggest that the LDL-A module of Tva, like other LDL-A modules, requires calcium for correct folding. D46 and E47 are predicted to be involved in calcium binding, based on the structures of other LDL-A modules. Thus, mutations at these positions would adversely affect calcium binding. Indeed, D46A mutant protein was eluted as several broad peaks, whether folded in the presence (Fig. 2C) or absence (Fig. 2D) of calcium. Similar results were observed for E47A mutant protein (Fig. 2E and F). These results are consistent with the notion that these highly conserved acidic residues are directly involved in calcium coordination.
FIG. 2.
Elution profiles of wt and mutant Tva LDL-A modules by reverse-phase HPLC. The purified proteins were folded in vitro in the presence (A, C, E, G, and I) or absence (B, D, F, H, and J) of calcium and then eluted by reverse-phase HPLC.
L34 and W48 do not appear to participate in Ca2+ binding. Nevertheless, mutations at these positions significantly impaired the viral receptor function of Tva (Fig. 1 and references 21, 22, 26, and 27). Based on this finding, it was hypothesized that these residues may be involved in ligand recognition (22, 27). However, the HPLC elution profiles of L34A and W48A proteins suggest that these mutations may also influence Ca2+-dependent folding of Tva, albeit to a much lesser extent than D46 and E47. In the absence of calcium, multiple broad peaks were detected for both W48A and L34A proteins (Fig. 2H and J). Interestingly, in the presence of calcium, both W48A and L34A proteins were eluted predominately as a sharp peak at about 22 min. However, several minor peaks were also reproducibly observed (Fig. 2G and I). These results seem to suggest that although L34 and W48 are unlikely to be involved in Ca2+ binding, mutations of these residues can also adversely affect Ca2+-dependent protein folding of Tva. It is notable that W48A and L34A proteins were eluted earlier than wt Tva protein or D46A or E47A mutant protein (about 22 min for W48A and L34A proteins, versus 25 min for wt Tva [Fig. 2]). A plausible explanation is that L34A and W48A proteins are less hydrophobic than the wt Tva LDL-A module and therefore were eluted faster on a hydrophobic Vydac C18 column.
Examination of Ca2+-dependent protein folding by NMR spectroscopy.
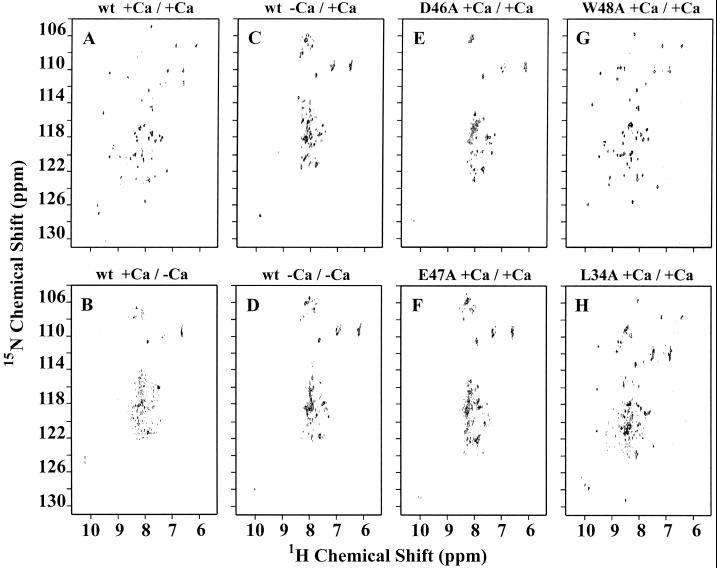
To further characterize Ca2+-dependent protein folding of the wt Tva LDL-A module and its mutants, the 15N-labeled proteins were prepared for acquisition of two-dimensional NMR spectra ([1H-15N] HSQC spectra) after in vitro folding either in the presence or in the absence of calcium. It is known that correctly folded proteins display well-dispersed spectra in both proton and amide 15N dimensions, while unfolded or misfolded proteins give ill-defined and poorly dispersed spectra.
As mentioned above, the wt Tva LDL-A module was eluted as a single sharp peak on reverse-phase HPLC after in vitro folding in the presence of calcium (Fig. 2H). The peak fraction of the 15N-labeled wt Tva LDL-A module was purified and prepared by reverse-phase HPLC, and two-dimensional [1H-15N] HSQC spectra were collected using a Bruker DRX600 spectrometer. Since calcium was released from the peptide during purification by HPLC, the role of calcium in maintaining the structure of the module was also examined by either adding or omitting CaCl2 before NMR spectroscopy. When the protein was folded in the presence of calcium, and calcium was present during acquisition of the NMR spectrum, the spectrum exhibited very good dispersion in both proton and amide 15N dimensions (Fig. 3A). However, the same protein gave poorly dispersed spectra when calcium was omitted during the NMR analysis, even if the protein was folded in the presence of calcium (Fig. 3B). The whole sample of the LDL-A module of Tva after folding in the absence of calcium was used for the NMR data collection, since the protein was eluted as multiple and overlapping peaks and it was difficult to separate and collect individual peaks (Fig. 2B). When calcium was absent from the folding reaction, the Tva module gave highly clustered spectra in both dimensions regardless of whether calcium was present during acquisition of NMR spectra (Fig. 3C and D). This clustering was more pronounced with regard to 1H proton, since the 1H proton spectra were mainly clustered around 8 ppm. These results indicate that calcium is required not only for correct folding of the Tva module but also to maintain the overall structure conformation of the protein.
FIG. 3.
Two-dimensional [1H-15N] HSQC spectra of the LDL-A module of Tva and its mutants. The purified 15N-labeled proteins were folded in vitro in the presence (A, B, E, F, G, and H) or absence (C and D) of calcium, and two-dimensional [1H-15N] HSQC spectra were acquired either with added calcium (A, C, E, F, G, and H) or with no added calcium (B and D) as described in Materials and Methods. For example, wt +Ca/+Ca (A) indicates that calcium was present in the folding reaction (+Ca/) and with added calcium in NMR analysis (/+Ca).
However, whole preparations of D46A or E47A mutant proteins gave similarly poorly dispersed spectra when calcium was present in both the folding reaction and the acquisition of NMR spectra (Fig. 3E and F). These results, which are consistent with the HPLC analysis shown in Fig. 2, provide further evidence that calcium is absolutely required for correct folding and that mutations of the Ca2+-binding residues adversely influence Ca2+ coordination and protein structure of the LDL-A module of Tva.
As shown above, in the presence of calcium, both W48A and L34A proteins were eluted as a predominately single sharp peak at about 22 min (Fig. 2G and I). When the protein from this peak fraction of W48A was subjected to NMR analysis, it gave a well-dispersed spectrum with many similarities to that of wt Tva (Fig. 3G), suggesting that the majority of W48A mutant protein can be correctly folded in the presence of calcium. In contrast, although the peak fraction of L34A protein also gave a better-dispersed spectrum (Fig. 3H) than D46A and E47A mutant proteins, the dispersion was greatly reduced in both proton and amide 15N dimensions compared to that of wt Tva or W48A protein. These results suggest that the predominant single peak eluted by reverse-phase HPLC shown in Fig. 2G may represent a mixture of more than one isomer or else that the protein is not well folded. Thus, the L34A mutation in Tva may exert a greater adverse effect on Tva folding than the W48A mutation. Careful examination of the spectra by superimposition identified 28 peak positions as being identical between W48A and the wt module proteins (Fig. 3G versus A). Although it was more difficult to determine the exact number of identical peak positions between L34A protein and the wt module due to the less-defined dispersion of spectra of L34A protein, only 9 to 11 identical peak positions were identical between them (Fig. 3H versus A). These numbers represent significant structural differences between W48A and L34A mutant proteins.
Measurement of Ca2+-binding affinity by ITC.
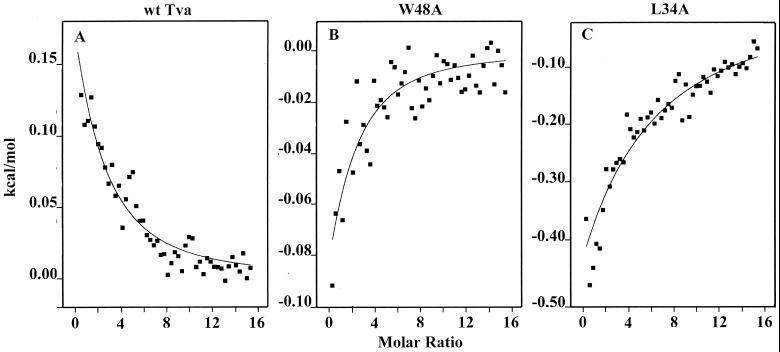
ITC was used to directly measure Ca2+-binding affinity of the wt Tva LDL-A module and its mutants. Calcium titrations were performed on a MicroCal MSC isothermal titration calorimeter. A CaCl2 solution (2 mM) was added in 2-μl increments up to a molar ratio of 15:1 for each protein (10 μM). As shown in Fig. 4A, Ca2+ binding of the wt LDL-A module gave a measureable heat change with an apparent dissociation constant (Kd) of 40 μM at 30°C. The Ca2+ binding of W48A (Fig. 4B) and L34A (Fig. 4C) proteins was also measurable, with apparent dissociation constants of approximately 48 and 100 μM, respectively. These results are in agreement with those for HPLC and NMR spectra, indicating that the wt Tva LDL-A module binds calcium with a relatively high affinity, while W48A and L34A proteins are slightly impaired in Ca2+ binding (wt > W48A > L34A). In contrast, under the same conditions, neither D46A nor E47A mutant protein displayed any detectable Ca2+-binding activity (data not shown), indicating that these mutations greatly reduced the Ca2+-binding affinity of Tva. A major difference between the L34A variant and either wt Tva or the W48A variant was in the thermodynamics of binding. Although wt Tva shows slightly endothermic binding of Ca2+ (ΔH = 0.84 kcal mol−1), while the W48A and L34A variants show slightly exothermic (ΔH = −0.35 kcal mol−1) and strongly exothermic (ΔH = −5.38 kcal mol−1) binding, respectively, the more significant difference is in the relative contributions of entropy to the binding process. For wt and the W48A variant, binding is driven primarily by entropic considerations, with −TΔS° being −7.28 kcal mol−1 (out of ΔG° of −6.44 kcal mol−1) and −5.98 kcal mol−1 (out ofΔG° of −6.33 kcal mol−1), respectively. In marked contrast, calcium binding to the L34A variant is predominantly enthalpic (ΔH = −5.38 kcal mol−1 out of ΔG° of −5.86 kcal mol−1), with only a minor contribution from entropy (−TΔS° = −0.48 kcal mol−1). This is consistent with similar folds and structures of wt and W48A Tva but a major structural difference for the L34A variant, as indicated both by the NMR HSQC spectra and the HPLC elution profiles. It may be that the leucine present in wt Tva becomes buried upon correct folding and plays an important role in such folding. This might be expected to release considerable solvent and so contribute to the favorable entropy of binding and folding. Such favorable entropy would be largely lost upon replacement of the large hydrophobic leucine side chain with the small alanine and might no longer provide the impetus for folding along the correct pathway.
FIG. 4.
Calcium titration of the Tva LDL-A module and its mutants by ITC. The Ca2+-binding affinity and enthalpy of binding of wt and mutant Tva LDL-A modules were measured by ITC. D46A and E47A mutant proteins did not display detectable Ca2+ binding by ITC (data not shown). x axis, Ca2+/protein molar ratio; y axis, heat release or uptake upon calcium binding. Note that the scales differ among the three panels.
DISCUSSION
In this report we demonstrated that the LDL-A module of Tva, like other LDL-A modules, requires calcium for correct folding. This conclusion is based on in vitro folding studies of the wt LDL-A module and the mutants of Tva by several different methods. We have shown that the wt LDL-A module of Tva was eluted as a single sharp peak by reverse-phase HPLC and gave a well-dispersed NMR spectrum when the module was folded in the presence of calcium. In contrast, when the module was folded in the absence of calcium, it was eluted as several broad peaks in HPLC profiles, and the NMR spectrum was ill defined and highly clustered. When two of the Ca2+-binding residues were mutated (D46A and E47A), the LDL-A module of Tva was not correctly folded either in the presence or in the absence of calcium. In addition, we have shown that mutations of the non-calcium-binding residues (W48A and L34A) could also influence Ca2+-dependent folding of Tva. Compared to the wt LDL-A module of Tva, W48A and L34A mutant proteins have only slightly lower Ca2+-binding affinity (Kd = 40 μM for wt versus 48 and 100 μM for W48A and L34A mutant proteins) but show large differences in the importance of entropy in forming the Ca2+-bound conformation. This study, reveals for the first time the critical role of calcium in Tva folding, structure, and function.
As demonstrated by molecular and mutational analysis previously, the viral receptor function of Tva is solely determined by a single LDL-A module within the extracellular domain of Tva (20, 26). Since the LDL-A module of Tva is highly conserved compared to other LDL-A motifs, it is reasonable to assume that the LDL-A module of Tva adopts a three-dimensional conformation similar to other reported LDL-A structures. Thus, it was hypothesized, and supported by mutational analysis, that six invariable cysteines in Tva form three pairs of disulfide bonds as C1-C3, C2-C5, and C4-C6 (4), like other LDL-A modules. However, it appears that the correct pairing of these cysteines is Ca2+ dependent. The facts that the wt Tva LDL-A module was eluted as multiple broad peaks by reverse-phase HPLC and gave highly clustered two-dimensional NMR spectra when folded in the absence of calcium suggest that at least several isomers were formed. Theoretically, there are 15 possible combinations of disulfide bonds with six cysteines in the LDL-A module of Tva. It is obvious, however, that when calcium is present in the folding reaction, the LDL-A module of Tva folds as a single isomer, presumably the biologically active, native form in vivo. When the Ca2+-binding residues such as D46 or E47 in Tva are mutated, Tva cannot coordinate efficient Ca2+ binding. Thus, the protein is misfolded even in the presence of calcium, as we proposed previously (21, 22). This study provides direct biochemical evidence to bolster our hypothesis that the role of the Ca2+-binding residues (such as D46 and E47) of Tva is probably structural in nature. Thus, it is expected that E47A mutant protein should be impaired in EnvA binding and in mediating ASLV-A infection. Indeed, this mutant protein did not display detectable EnvA binding, measured either by an enzyme-linked immunosorbent assay (21) or by flow cytometry (27). One plausible explanation that may reconcile the discrepancy between the folding defect of E47A protein reported in this study and its ability to mediate ALSV-A infection as reported previously by us (21) (Fig. 1) and by others (27) is that although the majority of E47A mutant protein was misfolded, a small fraction of this protein could adopt the native conformation, and this small fraction was sufficient to mediate ALSV-A infection since the mutant protein was overexpressed in our infection assay, as we previously suggested (21). Consistent with this explanation, we found that several Tva mutants, including E47A, displayed a more pronounced defect in mediating ALSV-A infection when they were expressed at lower levels on the cell surface (data not shown), indicating that overexpression of Tva mutant proteins can mask the defective phenotype.
One important finding of this study is that we now have a better understanding of the structure-function relationship of Tva in viral entry. Residues leucine 34 and tryptophan 48 have been found previously to be critical for the viral receptor function of Tva (22, 27). Because these residues are not predicted to be Ca2+ binding, it was hypothesized that these residues might be directly involved in ligand recognition. However, the results presented in this study suggest that this hypothesis is likely too simplistic. Although W48A and L34A mutant proteins behaved more like the wt Tva module than D46A and E47A mutant proteins in Ca2+-dependent folding in vitro, they displayed distinct differences in HPLC elution profiles, [1H-15N] HSQC spectra, and Ca2+-binding affinity. First, both mutant proteins were eluted as a predominant sharp peak corresponding to that of the wt protein, but there were several detectable shoulders with these proteins (Fig. 2G and I), indicating that several different isomers were folded even in the presence of calcium. Furthermore, examination of the NMR spectra of these mutant proteins indicates that the spectra were not as well dispersed as that of wt Tva, particularly for the L34A mutant protein (compare Fig. 3G and H to Fig. 3A). These results indicate that although L34 or W48 of Tva is not directly involved in Ca2+ binding, mutations of these residues nevertheless exert an adverse effect on the structure of Tva to such an extent that they impair Ca2+-mediated folding to the correct conformation. These results are consistent with a report by others showing that a non-Ca2+-binding residue in an LDL-A module can affect Ca2+ coordination (18). Therefore, we now believe that although it is still likely that both L34 and W48 are involved in ligand recognition, the defect of L34A or W48A mutant protein in mediating ASLV-A entry (Fig. 1) is at least partially due to structural alteration in Tva.
Another important aspect of this study is that an integral approach of molecular, biochemical, and structural techniques is needed for us to elucidate how a simple receptor like Tva can mediate efficient ASLV-A infection. Three techniques used in this study, HPLC, two-dimensional NMR, and calorimetry, gave not only internally consistent but also very complementary results. For example, reverse-phase HPLC indicated that L34A or W48A mutant protein did not fold exactly like the wt Tva module in the presence of calcium. The two-dimensional NMR spectra suggested that even the predominant sharp peak of L34A mutant protein may not be a single isomer. Furthermore, ITC data basically confirmed that the wt Tva module binds calcium with a higher affinity than L34A and W48A proteins. Somewhat surprisingly, the wt and W48A Tva modules bound to calcium predominantly as a result of favorable increase in entropy, while the L34A mutant protein relied mainly on enthalpy for binding (Fig. 4 and Results). Again these results suggest that L34A protein is very different from wt Tva, while the W48A variant, though not identical, is more closely similar to wt Tva.
In conclusion, this study firmly established the critical role of calcium in Tva folding and function. In addition, the well-defined two-dimensional NMR spectra of the wt LDL-A module of Tva suggest that it is feasible to use NMR to determine the three-dimensional structure of the viral interaction domain of Tva. Structure-function analysis of this receptor may yield new insights on how a receptor mediates efficient viral infection.
ACKNOWLEDGMENTS
We thank Marty Waterson, Northwestern University, for use of the isothermal titration calorimeter.
This work was partially supported by American Heart Association Midwest Affiliate Grant-In-Aid 9951134Z (L.R.) and National Institutes of Health grant GM 54414 (P.G.W.G.).
REFERENCES
- 1.Balliet J W, Berson J, D'Cruz C M, Huang J, Crane J, Gilbert J M, Bates P. Production and characterization of a soluble, active form of tva, the subgroup A avian sarcoma and leukosis virus receptor. J Virol. 1999;73:3054–3061. doi: 10.1128/jvi.73.4.3054-3061.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates P, Rong L, Varmus H E, Young J A T, Crittenden L B. Genetic mapping of the cloned subgroup A avian sarcoma and leukosis virus receptor gene to the TVA locus. J Virol. 1998;72:2505–2508. doi: 10.1128/jvi.72.3.2505-2508.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates P, Young J A T, Varmus H E. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993;74:1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- 4.Belanger C, Zingler K, Young J A T. Importance of cysteines in the LDLR-related domains of the subgroup A avian leukosis and sarcoma virus receptor for viral entry. J Virol. 1995;69:1019–1024. doi: 10.1128/jvi.69.2.1019-1024.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blacklow S C, Kim P S. Protein folding and calcium binding defects arising from familial hypercholesterolemia mutations of the LDL receptor. Nat Struct Biol. 1996;3:758–762. doi: 10.1038/nsb0996-758. [DOI] [PubMed] [Google Scholar]
- 6.Daly N L, Scanlon M J, Tjordjevic J T, Kroon P A, Smith R. Three-dimensional structure of a cysteine-rich repeat from the low-density lipoprotein receptor. Proc Natl Acad Sci USA. 1995;92:6334–6338. doi: 10.1073/pnas.92.14.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly N L, Djordjevic J T, Kroon P A, Smith R. Three-dimensional structure of the second cysteine-rich repeat from the human low-density lipoprotein receptor. Biochemistry. 1995;34:14474–14481. doi: 10.1021/bi00044a025. [DOI] [PubMed] [Google Scholar]
- 8.Damico R, Bates P. Soluble receptor-induced retroviral infection of receptor-deficient cells. J Virol. 2000;74:6469–6475. doi: 10.1128/jvi.74.14.6469-6475.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damico R L, Crane J, Bates P. Receptor-triggered membrane association of a model retroviral glycoprotein. Proc Natl Acad Sci USA. 1998;95:2580–2585. doi: 10.1073/pnas.95.5.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolmer K, Huang W, Gettins P G. Characterization of the calcium site in two complement-like domains from the low-density lipoprotein receptor-related protein (LRP) and comparison with a repeat from the low-density lipoprotein receptor. Biochemistry. 1998;37:17016–17023. doi: 10.1021/bi982022s. [DOI] [PubMed] [Google Scholar]
- 11.Dolmer K, Huang W, Gettins P G. NMR solution structure of complement-like repeat CR3 from the low density lipoprotein receptor-related protein. Evidence for specific binding to the receptor binding domain of human alpha(2)-macroglobulin. J Biol Chem. 2000;275:3264–3269. doi: 10.1074/jbc.275.5.3264. [DOI] [PubMed] [Google Scholar]
- 12.Esser V, Limbird L E, Brown M S, Goldstein J L, Russell D W. Mutational analysis of the ligand binding domain of the low density lipoprotein receptor. J Biol Chem. 1988;263:13282–13290. [PubMed] [Google Scholar]
- 13.Fass D, Blacklow S, Kim P S, Berger J M. Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature. 1997;388:691–693. doi: 10.1038/41798. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert J M, Bates P, Varmus H E, White J M. The receptor for the subgroup A avian leukosis-sarcoma viruses binds to subgroup A but not to subgroup C envelope protein. J Virol. 1994;68:5623–5628. doi: 10.1128/jvi.68.9.5623-5628.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert J M, Hernandez L D, Balliet J W, Bates P, White J M. Receptor-induced conformational changes in the subgroup A avian leukosis and sarcoma virus envelope glycoprotein. J Virol. 1995;69:7410–7415. doi: 10.1128/jvi.69.12.7410-7415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez L D, Peters R J, Delos S E, Young J A T, Agard D A, White J M. Activation of a retroviral membrane fusion protein: soluble receptor-induced liposome binding of the ALSV envelope glycoprotein. J Cell Biol. 1997;139:1455–1464. doi: 10.1083/jcb.139.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang W, Dolmer K, Gettins P G. NMR solution structure of complement-like repeat CR8 from the low density lipoprotein receptor-related protein. J Biol Chem. 1999;274:14130–14136. doi: 10.1074/jbc.274.20.14130. [DOI] [PubMed] [Google Scholar]
- 18.North C L, Blacklow S C. Solution structure of the sixth LDL-A module of the LDL receptor. Biochemistry. 2000;39:2564–2571. doi: 10.1021/bi992087a. [DOI] [PubMed] [Google Scholar]
- 19.North C L, Blacklow S C. Structural independence of ligand-binding modules five and six of the LDL receptor. Biochemistry. 1999;38:3926–3935. doi: 10.1021/bi9821622. [DOI] [PubMed] [Google Scholar]
- 20.Rong L, Bates P. Analysis of the subgroup A avian sarcoma and leukosis virus receptor: the 40-residue, cysteine-rich, low-density lipoprotein receptor repeat motif of Tva is sufficient to mediate viral entry. J Virol. 1995;69:4847–4853. doi: 10.1128/jvi.69.8.4847-4853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rong L, Gendron K, Strohl B, Shenoy R, Wool-Lewis R J, Bates P. Characterization of determinants for envelope binding and infection in Tva, the subgroup A avian sarcoma and leukosis virus receptor. J Virol. 1998;72:4552–4559. doi: 10.1128/jvi.72.6.4552-4559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rong L, Gendron K, Bates P. Conversion of a human low-density lipoprotein receptor ligand-binding repeat to a virus receptor: identification of residues important for ligand specificity. Proc Natl Acad Sci USA. 1998;95:8467–8472. doi: 10.1073/pnas.95.15.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rong L, Edinger A, Bates P. Role of basic residues in the subgroup-determining region of the subgroup A avian sarcoma and leukosis virus envelope in receptor binding and infection. J Virol. 1997;71:3458–3465. doi: 10.1128/jvi.71.5.3458-3465.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell D W, Brown M S, Goldstein J L. Different combinations of cysteine-rich repeats mediate binding of low density lipoprotein receptor to two different proteins. J Biol Chem. 1989;264:21682–21688. [PubMed] [Google Scholar]
- 25.Young J A T, Bates P, Varmus H E. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J Virol. 1993;67:1811–1816. doi: 10.1128/jvi.67.4.1811-1816.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zingler K, Belanger C, Peters R, Agard D, Young J A T. Identification and characterization of the viral interaction determinants of the subgroup A avian leukosis virus receptor. J Virol. 1995;69:4261–4266. doi: 10.1128/jvi.69.7.4261-4266.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zingler K, Young J A T. Residue Trp-48 of Tva is critical for viral entry but not for high-affinity binding to the SU glycoprotein of subgroup A avian leukosis and sarcoma viruses. J Virol. 1996;70:7510–7516. doi: 10.1128/jvi.70.11.7510-7516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]