Abstract
Schistosomiasis is the most important helminth disease in the world from a public health perspective. S mansoni and S japonicum account for the majority of global intestinal schistosomiasis cases, and the pathogenesis is widely assumed to be fundamentally similar. However, the majority of research on schistosomiasis has been carried out on S mansoni and comparisons between the two species are rarely made. Here, we will discuss aspects of both older and recent literature where such comparisons have been made, with a particular focus on the pathological agent, the host granulomatous response to the egg. Major differences between the two species are apparent in features such as egg production patterns and cellular infiltration; however, it is also clear that even subtle differences in the cascade of various cytokines and chemokines contribute to the different levels of pathology observed between these two main species of intestinal schistosomiasis. A better understanding of such differences at species level will be vital when it comes to the development of new treatment strategies and vaccines.
Keywords: cytokines, granuloma, helminth, neutrophils, schistosome
1. INTRODUCTION
Schistosomiasis (bilharzia) is a disease that currently afflicts over 200 million people worldwide. Twenty million individuals are characterized as having severe morbidity, and death estimates vary between 24 000 and 200 000 deaths per year. 1 , 2 The disease is endemic in 74 countries across the world and is caused by six species of schistosomes: Schistosoma mansoni (occurring in Africa, South America, the Caribbean and the Middle East), S haematobium (mainly occurring in Africa and the Middle East), S intercalatum and S guineensis (confined to a few countries in Central Africa), S mekongi (Mekong Delta, including Cambodia and Lao) and S japonicum (Asia). 3 Of these species, S haematobium is the principal cause of urinary schistosomiasis, with the remaining giving rise to the intestinal forms of the disease. This heterogeneity of disease outcome is primarily due to the venule location in which the adult worms reside. 3 Although S mansoni is the most common intestinal species across Africa and Latin America, in Asia Schistosoma japonicum is the most prevalent species. While China is making good progress in controlling S japonicum, 4 it remains a major health risk in other parts of Asia, most notably in the Philippines where S japonicum is moderately endemic (5‐25%) in most of the endemic regions. 5 , 6 Complicating this is the fact that S japonicum is a zoonosis and can infect a wide array of animals such as dogs, 7 wild rats, cattle, and in particular, water buffaloes. 8 No vaccine is available against any species of schistosome but considerable progress in the control of schistosomiasis has been made in recent decades, mainly through mass drug administration (MDA) programmes, using Praziquantel administration to entire at‐risk populations without prior diagnosis. However, preventive chemotherapy alone is insufficient to break the transmission cycle and low‐grade chronic infections leading to organ damage, anaemia, growth retardation and cognitive impairment are still prevalent across the world.
The infection is mediated by the freshwater snail intermediate host, with humans acquiring the infection by water contact. Regarding intestinal schistosomiasis, once the parasite is established in the mesenteric veins, the adult female worm produce eggs, half of which exit the host via the intestinal lumen, while half become trapped in the intestinal wall or in organs such as the liver, generating a potent granulomatous inflammatory response leading to hepatosplenomegaly and pipestem fibrosis. The most significant lesion in severe chronic infections is marked periportal fibrosis of the liver (clay pipestem fibrosis or Symmers’ fibrosis) associated with splenomegaly and portal hypertension.
Evolutionarily, S mansoni and S japonicum are genetically distinct, as highlighted by geographic separation and snail host variation. Since the full genome sequencing of both species in 2009, 9 , 10 this differentiation has been further elucidated from combinational sequencing of nuclear and mitochondrial genome sequencing. The prevailing theory is that Central and SE Asian schistosome species (the S japonicum group) are phylogenetically basal and separate from all other animal and human Schistosoma spp., including the S mansoni group. Current estimates place African schistosome colonization 15‐20 million years ago (MYA), S japonicum‐S mansoni divergence ~14 MYA, and S mansoni‐S haematobium divergence ~4 MYA, 11 which together has formed the basis of the ‘out of Asia’ hypothesis regarding the Asian origin of these species. 12
S mansoni and S japonicum account for the majority of global intestinal schistosomiasis cases, and the pathogenesis is generally viewed to be fundamentally similar. However, the majority of research on schistosomiasis has been carried out on S mansoni, and direct comparisons between the two species are rarely made. Here, we will discuss the aspects of both older and recent literature where such comparisons have been made, with a particular focus on the pathological agent, the host granulomatous response to the egg.
2. LARVAL MIGRATION
Already upon initial infection of the schistosomula through the skin, there are marked differences between S mansoni and S japonicum. Experimental comparisons using human skin cultures found that 90% of S mansoni schistosomula were located in the epidermis after 24 hrs, moving on to the dermis after 48 hrs, and by 72 hrs they had reached the dermal vessels. Conversely, 90% of S japonicum schistosomula had reached the dermis within 24 hrs post‐infection (p.i.) with some schistosomula found in the dermal vessels already after 2 hrs, demonstrating a much more rapid migration of S japonicum. 13 The skin cytokine expression during S mansoni schistosomula migration was dominated by interleukin‐1 receptor antagonist (IL‐1ra), IL‐10 and tumour necrosis factor (TNF)‐α at 8 hrs p.i., while expression of skin cytokines during S japonicum migration was seen to be more extensive, consisting of IL‐1β, IL‐1ra, IL‐2, IL‐6, IL‐8, IL‐10, IL‐15, IL‐18 and TNF‐α after 8 hrs, suggesting a broader activation of immune cells during S japonicum migration. 13 In addition, in S japonicum infection, there was a marked increase in IL‐1ra in the skin comparative to S mansoni, indicative of smokescreen activity subduing inflammation around the parasite to evade detection. 13
Both species then use the blood circulation and lymphatic system to reach the lungs where S mansoni numbers peak after 6 days, 14 while S japonicum numbers peak already after 3 days. 14 , 15 The mechanism of S japonicum's swift migration is not fully understood; however, it may be that they possess more potent penetrating enzymes through skin tissue, which may also play a part in explaining why this species can establish in over 40 known mammalian host species, 13 , 16 while S mansoni exhibit a much more limited host specificity.
These differences in immunomodulatory and migratory capabilities between the two species may attribute to variations in the priming of the immune system of naïve individuals and influencing the downstream response to the parasite culminating in acute infection.
3. ADULT WORM LOCATION AND OVIPOSITION PATTERNS
While both S mansoni and S japonicum adult worms primarily occupy the mesenteric veins surrounding the intestines neither species is constrained to one location and appear to move considerable distances through the mesenteries. 17 Adult S japonicum in the portal venous system have been found to aggregate to other worms, forming clusters and creating large areas of focussed pathology around the intestine with adjacent regions relatively free of eggs. 18 S mansoni lesions on the other hand have a more diffuse pattern of distribution, contrasting the clustered, massed or ‘nested’ pattern seen in S japonicum. 19
As the eggs are released from the adult worms, the host response to them dictates the extent of the resulting pathology. As such, any differences between species here will manifest in the attributed diseases. Most notably there are significant differences in egg production rates with S mansoni producing around 800 eggs per day per female worm laid as single eggs, whereas S japonicum females produce up to 3000 eggs per day laid in clusters of as many as 50. 20 , 21 Combined with the clustering of adult S japonicum worms in groups, this worm behaviour contributes greatly to the increased disease severity seen in S japonicum compared to that of S mansoni infections. 19
4. HOST IMMUNE RESPONSE AND LOCAL PATHOLOGY
As with other helminth infections, the host immune response during schistosomiasis infection is characterized by a T helper type 2 (Th2)–skewed response in terms of cytokine profile and activated cell types. There is, however, also a role for Th1 cell activity and cytokines, and the balance between the types of T helper cell responses is strongly regulated over the duration of infection, and a too robust response of either type can result in extensive tissue damage. 22 , 23 , 24 , 25 , 26
The initial host immune response after infection is primarily Th1‐mediated, targeting the schistosomulum or juvenile worm and characterized by the production of cytokines such as IL‐1, IL‐12, TNF‐α and interferon (IFN)‐γ. 27 The host response progressively switches to a Th2‐mediated response around six to eight weeks p.i. as egg deposition begins, characterized by an increase in IL‐4, IL‐5, IL‐13 and the production of immunoglobulin (Ig)E. 28 Over time, this local Th2 environment leads to the formation of cellular infiltrates (granulomas) and the generation of irreversible tissue fibrosis. The egg is therefore the primary pathogenic factor in schistosomiasis as it acts as the initiator of the granuloma formation and is essential for the pattern of events that lead to the pathology associated with chronic infection.
The granulomatous response to schistosome infections varies temporally but consists of the recruitment of T and B cells, macrophages, neutrophils, myofibroblasts/hepatic stellate cells (HSCs), eosinophils and epithelioid cells. 29 The cells surround the eggs, forming a wall, and as the granuloma develops, fibrosis and collagen are deposited from activated cells and remain permanently in the tissue even after the destruction of the egg itself. The formation of fibrosis around the eggs is widely detrimental to the host in that it leads to hepatic and periportal fibrosis, resulting in blockage of the blood flow leading to portal hypertension and portal shunting that may result in fatal oesophageal bleeding. 30 However, the formation of granulomas is also vital in order to limit the egg‐antigen induced inflammation that would otherwise lead to permanent inflammatory tissue damage and extensive necrosis, as seen in the increased mortality in S mansoni‐infected T cell–depleted mice. 31 Thus, although the formation of the schistosome granuloma is the key pathology in schistosomiasis, it is ultimately also beneficial to the host.
The schistosome egg granuloma have been studied extensively since the von Lichtenberg experiments in the 1960s, where sensitization in mouse models decreased egg destruction and accelerated immune responses in terms of relative size of granuloma and percentage of eggs with reactions, which could be transferable by cells and not serum. 32 , 33 , 34 There is a general correlation of granuloma formation with egg maturation over its ~3‐week viable lifespan, and the basic immunopathologic outcomes associated with granuloma formation and fibrosis are generally fairly similar between S mansoni and S japonicum. 19
However, key differences between the two species lie in the severity of the pathology and some of the mechanisms underlying the immunologic reactions. Early experiments determined that in S mansoni infections, a cell‐mediated delayed type hypersensitive reaction (DTH—also termed type IV hypersensitivity reactions) was elicited in the granulomatous reaction, whereas in S japonicum infections DTH responses were actively suppressed, 35 and instead elicited an immediate type hypersensitive immune response forming granulomas akin to those in foreign body responses, not affected by sensitization and consistently smaller than those of S mansoni. 32 This difference suggests there are key alternative pathways that lead to granuloma formation and hepatic fibrosis in the two species. 29
The immune reactions to S japonicum eggs in hamsters showed consistently smaller granulomas around single eggs but larger around aggregated eggs, and liver enlargement was greater in S japonicum chronic infections than in S mansoni‐infected livers, possibly due to higher antigen concentrations. 19 It is argued whether granuloma measurements are completely relevant parameters as they do not account for cell damage or haemorrhaging; however, at all stages of S japonicum infections in these early experiments, there was considerably more parenchymal liver damage than in S mansoni‐infected tissue pathology. 19 Moreover, it has generally been found that at comparable egg loads and time spans, the liver pathology of S japonicum is the more severe of the two. This is not due to the size of the granulomas formed, but the tendency for S japonicum to produce more exudation and necrosis in early granuloma formation, and encroach on adjacent liver tissue with greater diffuse inflammation. 19 Early experiments found that in S japonicum rabbit infections, cirrhosis was out of proportion to the number of eggs deposited. 36 Moreover, S japonicum hamster hepatic pathology displayed abundant dying liver cells or eosinophil‐central necrotic areas in the exudative granulomas or pseudoabscesses and that the epithelioid cell layer demarcating the granulomas was completely absent or poorly developed. 19 At 1 week post‐patency, S japonicum granulomas were surrounded by inflammatory structures, focal haemorrhaging, oedema or stellate fibroblasts and capillary buds which encroached upon neighbouring liver tissue that often exhibited necrosis. Zonal infarct‐like eosinophilic liver necrosis and Councilman bodies (acidophilic aggregates of cells composed of dying hepatocytes often surrounded by normal parenchyma) were frequent. At 11 weeks post‐patency, however, granulomas were delimited by fibroblastic rims and were less encroaching. But at all stages, S japonicum had substantially more parenchymal liver disturbance than S mansoni. Thus, although single S japonicum eggs and granulomas were smaller than those of S mansoni, greater liver enlargement was observed and the ratio of hepatic inflammatory tissue to parenchymal liver tissue was higher in individuals infected with S japonicum. 19 Similar S japonicum destructive effects have been described in several animal models. 36 , 37 , 38 , 39 Hence, it is clear that the egg‐induced tissue inflammatory responses and resulting damage is greater in S japonicum than in S mansoni infections, even at comparable levels of egg deposition. 36 , 37 , 38 , 39
5. COMPOSITION OF THE GRANULOMA
CD4+ Th1 cells are involved in the initial granuloma development in S mansoni and S japonicum infections. 40 Excessive Th1 polarization, however, is detrimental to the host, as seen in IL‐4 and IL‐4/IL‐10 double–deficient mice which had a higher mortality than wild‐type mice infected with S mansoni. 23 , 28 The shift to the chronic Th2 granulomatous response in S mansoni infections is considered to be driven by toxic elements of the soluble egg antigens (SEA), 41 and only recently has an analogous S japonicum SEA compound been identified. 42 Cells recruited to the granuloma primarily include neutrophils, eosinophils, monocytes, lymphocytes and epithelioid cells. 29 The role of individual Th2 cytokines in granuloma formation and fibrosis have been shown to be different in that IL‐4 or IL‐13 can both generate granuloma formation, while IL‐13 alone is the dominant pro‐fibrotic cytokine in this disease. 43 , 44 The Th2‐dominated granulomatous response is in turn regulated by the lesser Th1 response as demonstrated by the fact that in vivo neutralization of IFN‐γ or IL‐12 results in larger granulomas and more extensive fibrosis. 45 , 46 In addition, T regulatory cells (Tregs) have been shown to modulate and downregulate granuloma size over the course of chronic infections in mice. 47 , 48
Looking more in detail, it is clear that schistosome granuloma composition varies temporally and between species. Early experiments of S mansoni hepatic granuloma composition seem to conflict depending on host species. 19 , 32 These studies agree, however, that at the granuloma's peak and over the duration of development, the predominant cell type is eosinophils. At 16 days post‐intravenous injection of S mansoni eggs in mice, eosinophils account for 50% of the granuloma, with the remainder constituting mostly of macrophages and neutrophils. 32 In S japonicum on the other hand, during the early stages of granuloma formation, the cells in the abscess‐like lesions are predominantly neutrophils in the majority of host species—mice, hamsters, rhesus monkeys and Aotus monkeys. 19 , 38 , 49 As S japonicum infection progresses, early experiments showed that late‐stage granulomas in mice are characterized by a decrease in macrophages and neutrophils and an increase in lymphocyte‐like cells and plasma cells on the periphery of periportal inflammation. 32 The presence of plasma cells suggests local antibody production, and necrotic lesions with polymorphonuclear cells suggest antibody‐antigen complex‐mediated reactions. 50 Fundamentally, neutrophils were the most predominant cell type in the advanced S japonicum granulomas, and they were often degranulated when in contact with the egg shell, with occasional evidence for intraovular immune reactions. 19
Taken together, these early experiments demonstrate how granuloma formation in S japonicum infection contain a significantly higher ration of neutrophils and acute inflammation, compared to those induced by S mansoni which are predominantly composed of eosinophils. 19 , 36 , 49
5.1. Neutrophils
As neutrophils are the predominant cell type in the initial inflammatory infiltrate in lesions of primary infections, 51 and more so in S japonicum granulomas compared to S mansoni, it is important to understand their function. Neutrophils are the first responders during inflammation and are important for functional innate immunity. 52 However, they can also trigger host tissue damage when producing proteases and cytotoxins such as reactive oxygen species. 53 Neutrophils respond to pathogens through a variety of mechanisms, including degranulation, phagocytosis, or via the production of neutrophil extracellular traps (NETs). 54 NETs have been found to be involved in a variety of responses, involving infection, autoimmunity and cancer, 55 , 56 , 57 and represent a component of neutrophil activity in schistosomiasis infections 58 (Figure 1).
FIGURE 1.
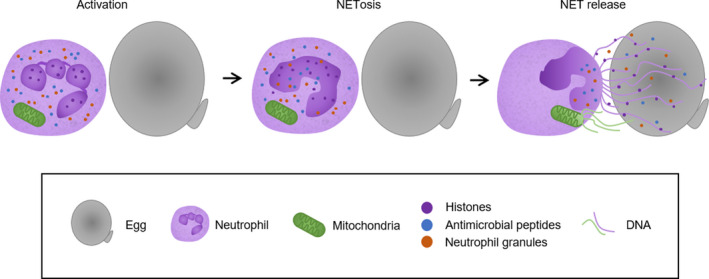
Neutrophil extracellular traps (NETs) in response to Schistosoma japonicum eggs. Neutrophils become activated when in contact with S japonicum eggs and undergo NETosis—a form of cell death that can leave the neutrophil still viable due to the release of mitochondrial DNA instead of nuclear DNA. NET structures are released, comprised of a DNA backbone with attached histones, antimicrobial peptides and neutrophil granules and enzymes including neutrophil elastase (NE), myeloperoxidase (MPO), lactoferrin and gelatinase among others
Neutrophils have been demonstrated to promote damage during schistosomiasis, acting as a major cause of hepatic necrosis and liver damage in the acute stage of S japonicum infection as well as progenitors for hepatic fibrosis 2 weeks after implantation of S japonicum eggs in mice. 59 Furthermore, neutrophils were shown to localize to the core of S japonicum granulomas through the expression of the neutrophil chemoattractant S100A8, resulting in the production of proinflammatory cytokines and chemokines, including IL‐1α, IL‐1β, IL‐6, TNF, CCL3 and CXCL1, and contributing to overall local tissue damage. 58 Interestingly, while neutrophils in the granuloma core enhanced inflammation, those on the periphery of the granulomas were found to degrade collagen, the principle component of fibrotic granulomas, introducing a modulatory role for neutrophils in the pathology. 60
The importance of neutrophils in granuloma formation is evidenced in neutrophil‐depleted S japonicum‐infected mice, where hepatic granulomas were augmented over the course of infection 59 suggestive of a regulatory capacity of neutrophils. Moreover, an S japonicum egg protein, SjE16.7, was identified to specifically recruit neutrophils to the site of deposition in vivo, 61 therefore implicating parasite‐derived factors in modulating neutrophil activity. Such activity includes the employment of NETs after just 4 hours of incubation with S japonicum eggs in vitro, suggestive of NET stimulated cell lysis and not vesicular‐mediated responses. 58 Furthermore, NETs were not produced after interactions with excretory/secretory (ES) proteins from the eggs, suggesting that neutrophil interactions with egg surface or an insoluble factor are required for NET stimulation. Still, NETs were found to not directly kill the egg, but it is suggested that they may restrict further passage. 58 Taken together, the available data suggest that neutrophils have a crucial role in S japonicum granuloma formation and associated pathology.
With regard to S mansoni granulomas, neutrophils are not the predominant cell type although they are present at low numbers. Additionally, no NETs are formed by S mansoni stimulated neutrophils 60 despite the presence of egg shell epitopes such as Lewis X carbohydrate epitope 62 and the ability of the host plasma glycoprotein von Willebrand factor (vWF) to bind to the egg shell surface. 63 S mansoni eggs have been found to secrete a chemokine binding protein (smCKBP), also synonymous with the SEA glycoprotein interleukin‐4‐inducing factor from schistosome eggs (IPSE/α‐1), that interacts with host chemokines and their activity. 64 Interestingly, smCKBP was found to bind to the neutrophil chemokine CXCL8, inhibiting chemotaxis and neutrophil infiltration in vivo, while not altering the activity of the eosinophil chemoattractant CCL11, which may in part explain the differences in neutrophil/eosinophil granuloma composition in these infections. 64
5.2. Eosinophils
While the eosinophil response to S mansoni eggs is much more pronounced than that of neutrophils, the principal role of eosinophils during schistosomiasis is still not fully understood. It remains uncertain whether they act as major effector cells against the worms, or mediators in tissue homeostasis favouring the establishment of the parasites, or merely as facilitators of tissue remodelling and debris clearance during infection. 65 However, in vitro experiments clearly demonstrated that eosinophil activation can occur through antibody‐dependent cytotoxicity to exert damage on S mansoni parasites 66 , 67 and that degranulation products such as Major Basic Protein‐1 (MBP‐1) are primary eosinophil mediators in this activity. 65
Eosinophils constitute around 70% of S mansoni egg granulomas in mice 16 days post‐injection, with neutrophils accounting for approximately 10%. 68 Among the number of chemokines that facilitate eosinophil migration, CCL3‐deficient mice had reduced hepatic granuloma size, fibrosis and eosinophil peroxidase activity, and antibody neutralization of CCL17 and CCL22 both reduced granuloma size and eosinophil recruitment in S mansoni‐infected mice. 69 , 70 , 71
Although eosinophils are present in the granulomas of S mansoni and S japonicum in different proportions, removal of the cell type by administration of anti–IL‐5 antibodies have little effect on granuloma size and fibrosis, 72 and eosinophil depletion in mice infected with either S mansoni or S japonicum resulted in slightly smaller granulomas, but had no overall effect on hepatic fibrosis or histology. 72 , 73 And although evidence suggests eosinophilic activity was associated with limiting pathology in human S mansoni infections, 74 experiments using S mansoni in two different eosinophil ablation mouse models showed no effect on hepatic fibrosis, granuloma size or number. 75 It should be noted that the absence of the high‐affinity IgE receptor, FcεRI, on mouse eosinophils 76 may explain the lack of eosinophil‐related effects in murine models. 77 On the whole, the involvement of eosinophils in S mansoni (and S japonicum to an extent), granuloma formation and pathology progression is evident but not fully clear, and further investigations are required in order to fully understand their role in pathology.
5.3. T cells
T cells are essential for both the generation of granulomas and for the cellular composition of the granuloma, with some differences apparent between the schistosome species. 41 , 77 During early experiments, it was observed that granulomas in S mansoni‐infected hamsters showed a lymphoid periphery in addition to the initial eosinophil rich infiltration and epithelioid cell centre. 19 Similar findings were observed in S japonicum hepatic granulomas in late‐stage mouse models, with lymphoid cells on the periphery of lesions and periportal inflammation. 32 In vivo and in vitro studies of S mansoni‐infected mice demonstrated the recruitment of SEA‐sensitive splenic CD4+ T lymphocytes that were retained in the granuloma and subsequently migrated into the circulation as the infection developed. 78 Comparisons between athymic mice with S mansoni or S japonicum infections showed a complete lack of granulomas in S mansoni infections and reduced morbidity judged by organ weight, portal pressures and reticuloendothelial activity, 79 whereas athymic mice with S japonicum infections had granulomas present, but they were smaller than controls and had less fibrosis. 80 , 81
CD4+ Th cells have important roles in many parasitic infections, and as previously discussed, Th1 and Th2 subsets have important roles in schistosomiasis. However, to date, several other types and subsets of T cells have also been identified and classified into several distinct phenotypic classifications, including Th9, Th17 and Tregs.
IL‐9 was initially considered a Th2 cytokine; however, IL‐9 and IL‐4 are rarely produced by the same T cell and Th9 cells are now deemed a unique IL‐9 producing subset of CD4+ T cells, characterized by the expression of the transcription factors PU.1 and IRF‐4. 82 , 83 , 84 Not only has it been demonstrated that the level of IL‐9 expression in the liver is significantly increased in chronically infected S japonicum mice compared to uninfected controls, but changes in the levels of PU.1, Th9 cells and IL‐9 production all correlate with egg granuloma inflammation. 85 Li et al 86 found that although anti‐IL‐9 monoclonal antibody (mAb) neutralization had limited effect on liver inflammation in S japonicum‐infected mice, it did reduce total collagen deposition. In addition, levels of IL‐9 increased quicker than IL‐4 in S japonicum‐infected mice, indicating a potential role in regulating early stage fibrosis and suggesting that IL‐9 may be a possible target in S japonicum hepatic fibrosis modulation. 85 Similarly, S mansoni‐infected C57BL/6 mice showed elevated IL‐9 levels in liver and spleen. 87 Acute S mansoni infections in transgenic mice constitutively expressing IL‐9 were markedly normal with respect to host response; however, in chronic infections, an increased Th2 phenotype was observed, leading to a mortality of 86% at 10 to 12 weeks p.i. in transgenic mice compared to just 7% in wild‐type mice. 88 Nevertheless, in human S mansoni infections, there was no observed correlation of IL‐9 levels with the level of pathology. 89 These findings suggest that Th9 cells may be involved in the immunopathology of murine schistosomiasis; however, in human infections, the picture is less clear and further studies are required to clarify its role.
Th17 cells are a subset of CD4+ T lymphocytes characterized by production of IL‐17. Neutralization of IL‐17 resulted in the inhibition of hepatic inflammation in S mansoni‐infected C57BL/6 mice, 90 while infections in high pathology‐associated CBA mice displayed high levels of IL‐17 and severe hepatic necrosis, which was ameliorated with anti‐IL‐17 mAbs. 90 Similar results were identified in S japonicum‐infected C57BL/6 mice, where neutralizing IL‐17 decreased inflammatory cytokines and reduced recruitment of neutrophils, resulting in improved clinical outcomes. 91 It, therefore, appears that IL‐17 has a notable contribution in disease severity and clinical resolution in both S mansoni and S japonicum infections.
Last, the regulation of schistosomiasis infection is carried out by a number of cell types, most notably T regulatory cells which can be categorized into inducible Tregs (iTregs) that increase during infections, and natural Tregs (nTregs) that are an endogenous form of these cells. 92 The full role Tregs play in Schistosoma spp. infections is controversial, partly due to the ambiguities of reliable markers and functional assays. 93 , 94 Increased frequencies of human Tregs have been described in both S mansoni and S japonicum infections. 95 , 96 , 97 Murine studies have demonstrated that after infection with S mansoni, the percentage of nTregs (CD4+ CD25+ Foxp3+) increased during the chronic stage of disease 47 but did not seem to regulate CD4+ T cells in vivo, 98 contrasting with the role of iTregs which were involved in regulation via the production of IL‐10. 99 The adoptive transfer of CD25‐ CD4+ T cells to B cell–deficient and T cell–deficient mice (RAG‐deficient) resulted in an increase in liver pathology and mortality during S mansoni infection, suggesting an important regulatory role for Tregs during murine infection. 100 Moreover, Treg involvement in murine colonic granulomas near the site of infection has been shown to be dynamic over the course of the disease. 101 During chronic stages of infection, there was an approximate fourfold increase in CD4+ CD25+ Foxp3+ Tregs in colonic granulomas, evidenced by an increase in the intestinal homing markers CD103+ and CCR5+. These colon‐recruited CD4+ CD25+ Tregs were found to be strong modulators of Th2 responses including granuloma size and eosinophilia, suggesting a role in intestinal schistosomiasis modulation. The enteric environment was also more favourable towards the development of CD4+ Foxp3+ Tregs over the hepatic environment, which may be why colonic granulomas were modulated faster than those in the liver after the acute phase, and why hepatic granulomas retained their size and cellularity through the chronic phase. 101
Studies investigating the role of Tregs in S japonicum infection found that the CD4+ CD25+ Tregs recruited in murine models had decreased activity when treated with anti‐CD25 mAbs. 102 Mice treated with anti‐CD25 mAbs had decreased IL‐10, increased IFN‐γ and a lower worm burden, suggesting that S japonicum induces an altered Treg‐modulated immune response that would otherwise elicit a skewed response to target the parasites. Beside CD25, CD4+ CD25+ Tregs can express markers such as cytotoxic T lymphocyte–associated protein 4 (CTLA‐4), 103 a key molecule expressed on the surface and in the cytoplasm of peripheral CD4+ CD25+ Tregs and instrumental in their development and activation. 103 , 104 Mice treated with mAbs against CD25 and/or CTLA‐4 showed substantial reductions in worm burdens and egg production 105 via the impairment of Treg function, allowing for an overactive immune response—demonstrated by increased IFN‐γ, IL‐4, IL‐5 and IL‐10—targeting the worms and eggs, but at the cost of greater pathology shown by larger hepatic egg granulomas.
As discussed previously, S japonicum infections are associated with dampened DTH responses, which is in part due to Treg activity. The S japonicum HSP‐60–derived immunomodulatory molecule SJMHE1 was shown to inhibit DTH reactions in a mouse model through the induction of CD4+ CD25+ Tregs. 106 The lessened inflammation in the DTH reaction by SJMHE1 treatment was associated with a decrease in the inflammatory cytokines IL‐12 and TNF‐α and an increase in the anti‐inflammatory cytokines IL‐10 and TGF‐β1. 35 Taken together, these findings demonstrate a range of roles that Tregs appear to play in murine S japonicum infections.
Human studies have reported that circulating Tregs are increased during S mansoni infection, with approximately 40% of patients exhibiting high or very high percentages of circulating Tregs, and treatment with Praziquantel resulting in a reduction of circulating Tregs as well as their expression of CD45RO, a marker associated with T‐cell memory and suppressive activity. 95 Interestingly, in a study of human S japonicum infection, blood and spleen Treg levels were found to be higher in individuals with severe hepatosplenic disease; however, Treg migration to tissues was reduced via impaired CXCR3 expression, suggesting that splenic Tregs unable to migrate to the inflamed liver tissue leads to aggravation of liver disease. 96 As such, T regulatory cells are clearly involved in controlling pathology in both experimental and human schistosomiasis.
5.4. Hepatic Stellate Cells (HSCs)
As discussed above, fibrosis during schistosomiasis infections is largely driven by IL‐13, with IL‐13 knockout mice demonstrating reduced fibrosis. 107 Hepatic stellate cells (HSCs) are liver resident cells that primarily store vitamin A when quiescent, but when activated by liver damage transdifferentiate into myofibroblasts. These cells are a predominant contributor to collagen production in both S mansoni and S japonicum infections. 108 , 109 , 110 Characteristics associated with quiescent HSCs are lipid droplet retention and increased expression of peroxisome proliferator‐activated receptor gamma (PPAR‐γ), 111 , 112 while activated cells are associated with decreased lipid retention, increased fibrogenesis gene expression, and the increase of stress fibre expression including α smooth muscle actin (α‐SMA) and collagen (Col1A1). 113
During schistosomiasis, IL‐13 typically activates HSCs to transdifferentiate into myofibroblasts 109 ; however both S mansoni and S japonicum can inhibit this process. S mansoni eggs were able to reverse HSC transdifferentiation to return them back to their quiescent state, characterized by HSCs displaying decreased expression of α‐SMA and Col1A1, decreased stress fibre staining, and increased lipid retention when compared to cells cultured without eggs, 113 supporting the theory that fibrosis is host driven.
Similarly, HSCs cultured with eggs of S japonicum reduce their expression of α‐SMA and Col1A1, but in contrast they do not display lipid droplet storage nor have an increased PPAR‐γ expression. 114 The results were the same for HSCs that had direct contact with the S japonicum eggs and those at a distance, suggesting an egg excreted factor causing the effect. Moreover, HSCs in this setting have increased proinflammatory cytokine expression such as CCL2, IL‐6 and MMP9, 114 proposing a role for HSCs in granuloma development and that both host‐ and parasite‐derived factors contribute to pathology. The variations observed in HSC activation in S mansoni and S japonicum infections may therefore contribute to the differences seen in overall pathological outcome.
5.5. Macrophages
Macrophages (also known as Kupffer cells when liver resident) make up 30% of cells in S mansoni liver granulomas. They arise from recruited monocyte‐derived cells and play a role as both mediators and effectors of granuloma formation. 115 , 116 , 117 However, although information on macrophage population involvement in S japonicum granulomas is limited, 77 some known key differences are discussed here.
Antigen‐presenting cells such as macrophages carry out the presentation of egg antigens to CD4+ T lymphocytes, leading to the production of various cytokines and chemokines involved in mediating the host response, as well as recruiting additional inflammatory cells. 115 , 118 Moreover, macrophages facilitate collagen synthesis through various mechanisms, contributing to the fibrosis‐related pathologies discussed above. 119 , 120 Depending on the surrounding cytokine environment macrophages can be activated to become either classically activated macrophages (CAM or M1) or alternatively activated macrophages (AAM or M2), with CAMs stimulated by IFN‐γ, IL‐12 and IL‐18, whereas AAMs are dependent on stimulation from cytokines such as IL‐4 and IL‐13 inducing expression of arginase‐1 (Arg‐1) and Fizz‐I. 121
During experimental schistosomiasis infections, too extreme polarization to either Th1 or Th2 result in severe pathology, morbidity and death. 112 In strongly Th1‐polarized IL‐4/IL‐13–deficient mice, Arg‐1 fails to be expressed 122 , 123 and such Th1‐polarized mice display increased inducible nitric oxide synthase (iNOS) activity and increased mortality. Furthermore, S mansoni‐infected Arg‐1–deficient mice displayed increased hepatic fibrosis and mortality. As such it appears that Arg‐1 expressing AAMs have a role in controlling pro‐inflammatory pathology, while promoting the generation of fibrotic pathology. 123 , 124 , 125 Macrophage activation to CAMs is seen in the acute stage of S japonicum infections, transitioning to the development of AAMs during the chronic stage, 126 the latter of which is regulated in part by IL‐33 stimulating IL‐5 and IL‐13 secretion. 127 It was observed that egg ES products polarized macrophages to M2b—a subset of AAMs—in a Toll‐like receptor (TLR)‐2–dependent manner, and TLR‐2‐/‐ mice displayed altered cytokine profiles and smaller granulomas with less fibrosis compared to wild type mice. 128 These findings illustrate some of the similarities of responses that can be found to infections of both species, despite more research carried out with S mansoni parasites.
In addition to the role of AAMs in fibrogenesis, they have also been demonstrated to be involved in fibrotic resolution and are a main source of anti‐fibrotic MMP13. 77 , 129 , 130 Macrophages in a cholestatic rat liver model were shown to recruit neutrophils as additional collagenase‐producing cells, during the resolution of extracellular matrices. 131 Gene expression markers of AAM, such as Chi3l3 and Retnlg, were upregulated in the core and periphery of S japonicum‐induced hepatic granulomas, akin to neutrophil infiltration in granulomatous lesions, 60 suggesting a role for macrophages at granuloma peripheries in inducing matrix degradation and stimulating a neutrophil inflammatory response, resulting in both hepatic fibrotic resolution and pathology. Furthermore, the S japonicum egg‐specific protein SjE16.7 was described to recruit neutrophils to the site of egg deposition, however, it was also found to be a potent macrophage activator through both chemotaxis and cytokine production and pathology was greatly reduced after in vivo blockade. 132 As such there appears to be multiple roles for macrophages in granuloma formation and fibrogenesis related to specific cell infiltrates that may affect the variations seen in Schistosoma spp. pathology.
6. CONCLUDING REMARKS
Schistosomiasis is a debilitating and global disease that imposes approximately 3 309 000 disability‐adjusted life years (DALYs), a fundamental measure of disease burden. 133 S mansoni and S japonicum are the main aetiologies for the intestinal forms of the disease and are crucial to target if the disease is to be attenuated. As there is currently just one treatment available (Praziquantel), which does not protect against reinfection, the likelihood of parasite drug resistance is high and mechanisms of pathology must be fully understood in order to generate new treatments to reduce disease burden. 134
The principal cause of chronic pathology is the egg‐induced host granulomatous response, and as discussed here, there are both clear differences and similarities in the mechanisms and regulations of the host response between S mansoni and S japonicum (Figure 2). Despite some features pointing towards S mansoni being the more pathological disease—such as eliciting generally larger single‐egg granulomas and displaying the ability to decrease gut stromal integrity and remodel the vasculature of Peyer's patches 135 there is now much scientific evidence to suggest that the overall pathology induced by S japonicum is the more severe of the two species at all stages of infection and in multiple host species. 19 , 36 , 37 , 38 As we have discussed here, even subtle differences in the initiation of various cytokines and chemokines affect downstream effectors with regard to cellular recruitment, thus contributing to the different levels of pathology seen between S japonicum and S mansoni infections. Some of the mechanisms behind the increased pathogenesis of S japonicum are discussed here, from the role of neutrophils in host‐mediated pathology, to the parasite‐derived compounds modulating the host response. Together, this difference between the two primary causes of intestinal schistosomiasis highlights the need to examine all elements, as well as consolidate where there are similarities, in order to fully understand this disease.
FIGURE 2.
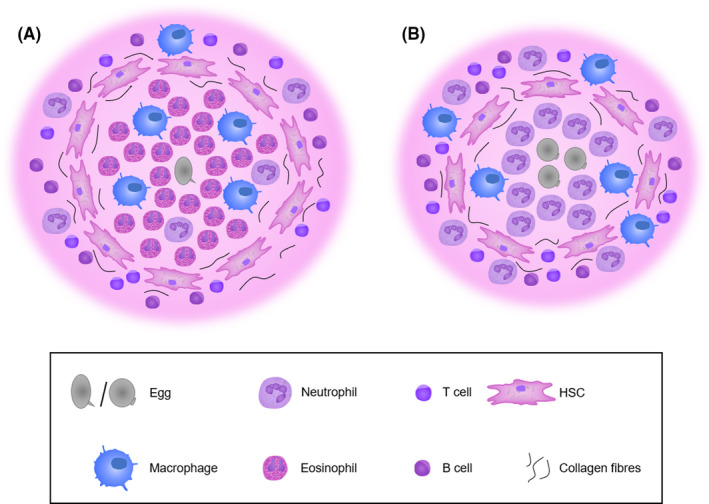
Illustration of granuloma composition in S mansoni (A) and S japonicum (B) and the predominant cell types. S mansoni granulomas are typically larger, induced by a single egg and have an eosinophil dominant core. S japonicum granulomas may be formed around a cluster of eggs and are dominated primarily of neutrophils. HSC, hepatic stellate cell
CONFLICT OF INTEREST
The authors explicitly state that they have no conflict of interest to disclose.
ACKNOWLEDGEMENTS
The authors are grateful for funding for their schistosome research from MRC Newton Fund (MR/R025525/1).
Llanwarne F, Helmby H. Granuloma formation and tissue pathology in Schistosoma japonicum versus Schistosoma mansoni infections. Parasite Immunol.2021;43:e12778. 10.1111/pim.12778
REFERENCES
- 1. World Health Organization . WHO Technical Report Series 912. Prevention and control of schistosomiasis and soil‐transmitted helminthiasis. Published online 2002. [PubMed]
- 2. World Health Organization . Global health estimates 2016: Disease burden by cause, age, sex, by country and by region, 2000–2016. Published 2018. http://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html. Accessed April 1, 2020
- 3. Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383(9936):2253‐2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gray DJ, Li Y‐S, Williams GM, et al. A multi‐component integrated approach for the elimination of schistosomiasis in the People’s Republic of China: design and baseline results of a 4‐year cluster‐randomised intervention trial. Int J Parasitol. 2014;44(9):659‐668. [DOI] [PubMed] [Google Scholar]
- 5. Olveda DU, Inobaya MT, McManus D, et al. Biennial versus annual treatment for schistosomiasis and its impact on liver morbidity. Int J Infect Dis. 2017;54:145‐149. [DOI] [PubMed] [Google Scholar]
- 6. Gordon CA, Acosta LP, Gobert GN, et al. Real‐time PCR demonstrates high prevalence of Schistosoma japonicum in the Philippines: implications for surveillance and control. PLoS Negl Trop Dis. 2015;9(1):e0003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rudge JW, Carabin H, Balolong E, et al. Population genetics of Schistosoma japonicum within the Philippines suggest high levels of transmission between humans and dogs. PLoS Negl Trop Dis. 2008;2(11):e340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu HW, Qin YF, Chu K, et al. High prevalence of Schistosoma japonicum infection in water buffaloes in the Philippines assessed by real‐time polymerase chain reaction. Am J Trop Med Hyg. 2010;82(4):646‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berriman M, Haas BJ, Loverde PT, et al. The genome of the blood fluke Schistosoma mansoni . Nature. 2009;460(7253):352‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou Y, Zheng H, Chen Y, et al. The Schistosoma japonicum genome reveals features of host‐parasite interplay. Nature. 2009;460(7253):345‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo F, Yin M, Mo X, et al. An improved genome assembly of the fluke Schistosoma japonicum . PLoS Negl Trop Dis. 2019;13(8):e0007612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lawton SP, Hirai H, Ironside JE, Johnston DA, Rollinson D. Genomes and geography: genomic insights into the evolution and phylogeography of the genus Schistosoma. Parasit Vectors. 2011;4(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He YX, Chen L, Ramaswamy K Schistosoma mansoni, S haematobium, and S japonicum: early events associated with penetration and migration of schistosomula through human skin. Exp Parasitol. 2002;102(2):99‐108. [DOI] [PubMed] [Google Scholar]
- 14. Rheinberg CE, Moné H, Caffrey CR, Imbert‐Establet D, Jourdane J, Ruppel A Schistosoma haematobium, S intercalatum, S japonicum, S mansoni, and S rodhaini in mice: relationship between patterns of lung migration by schistosomula and perfusion recovery of adult worms. Parasitol Res. 1998;84(4):338‐342. [DOI] [PubMed] [Google Scholar]
- 15. Gui M, Kusel JR, Shi YE, Ruppel A Schistosoma japonicum and S mansoni: comparison of larval migration patterns in mice. J Helminthol. 1995;69(1):19‐25. [DOI] [PubMed] [Google Scholar]
- 16. He YX, Salafsky B, Ramaswamy K. Host‐parasite relationships of Schistosoma japonicum in mammalian hosts. Trends Parasitol. 2001;17(7):320‐324. [DOI] [PubMed] [Google Scholar]
- 17. Cheever AW, Duvall RH Schistosoma japonicum: migration of adult worm pairs within the mesenteric veins of mice. Trans R Soc Trop Med Hyg. 1982;76(5):641‐645. [DOI] [PubMed] [Google Scholar]
- 18. Cheever AW, Duvall RH, Minker RG. Extrahepatic pathology in rabbits infected with Japanese and Philippine strains of Schistosoma japonicum, and the relation of intestinal lesions to passage of eggs in the feces. Am J Trop Med Hyg. 1980;29(6):1316‐1326. [DOI] [PubMed] [Google Scholar]
- 19. von Lichtenberg F, Erickson D, Sadun E. Comparative histopathology of schistosome granulomas in the hamster. Am J Pathol. 1973;72(2):149‐177. [PMC free article] [PubMed] [Google Scholar]
- 20. Cheever AW. Comparison of pathologic changes in mammalian hosts infected with Schistosoma mansoni, S japonicum and S haematobium . Mem Inst Oswaldo Cruz. 1987;82(Suppl 4):39‐45. [DOI] [PubMed] [Google Scholar]
- 21. Cheever AW, Macedonia JG, Mosimann JE, Cheever EA. Kinetics of egg production and egg excretion by Schistosoma mansoni and S japonicum in mice infected with a single pair of worms. Am J Trop Med Hyg. 1994;50(3):281‐295. [DOI] [PubMed] [Google Scholar]
- 22. Chen Y, Boros DL. Polarization of the immune response to the single immunodominant epitope of p38, a major Schistosoma mansoni egg antigen, generates Th1‐ or Th2‐type cytokines and granulomas. Infect Immun. 1999;67(9):4570‐4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoffmann KF, Cheever AW, Wynn TA. IL‐10 and the dangers of immune polarization: excessive Type 1 and Type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164(12):6406‐6416. [DOI] [PubMed] [Google Scholar]
- 24. Rutitzky LI, Hernandez HJ, Stadecker MJ. Th1‐polarizing immunization with egg antigens correlates with severe exacerbation of immunopathology and death in schistosome infection. Proc Natl Acad Sci USA. 2001;98(23):13243‐13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Farwa A, He C, Xia L, Zhou H. Immune modulation of Th1, Th2, and T‐reg transcriptional factors differing from cytokine levels in Schistosoma japonicum infection. Parasitol Res. 2018;117(1):115‐126. [DOI] [PubMed] [Google Scholar]
- 26. Tebeje BM, Harvie M, You H, Rivera V, McManus DP. T cell‐mediated immunity in CBA mice during Schistosoma japonicum infection. Exp Parasitol. 2019;204:107725. [DOI] [PubMed] [Google Scholar]
- 27. Wilson MS, Mentink‐Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85(2):148‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2(7):499‐511. [DOI] [PubMed] [Google Scholar]
- 29. Burke M, McManus D, Ramm GA, et al. Temporal expression of chemokines dictates the hepatic inflammatory infiltrate in a murine model of schistosomiasis. PLoS Negl Trop Dis. 2010;4(2):e598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368(9541):1106‐1118. [DOI] [PubMed] [Google Scholar]
- 31. Fallon PG, Richardson EJ, Smith P, Dunne DW. Elevated type 1, diminished type 2 cytokines and impaired antibody response are associated with hepatotoxicity and mortalities during Schistosoma mansoni infection of CD4‐depleted mice. Eur J Immunol. 2000;30(2):470‐480. [DOI] [PubMed] [Google Scholar]
- 32. Warren KS, Domingo EO. Granuloma formation around Schistosoma mansoni, S haematobium, and S japonicum eggs. Size and rate of development, cellular composition, cross‐sensitivity, and rate of egg destruction. Am J Trop Med Hyg. 1970;19(2):292‐304. [DOI] [PubMed] [Google Scholar]
- 33. Warren K, Domingo E, Cowan R. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am Soc Clin Investig. 1967;51(5):735‐756. [PMC free article] [PubMed] [Google Scholar]
- 34. von Lichtenberg F, Smith JH, Cheever AW. The Hoeppli phenomenon in schistosomiasis. Comparative pathology and immunopathology. Am J Trop Med Hyg. 1966;15(6):886‐895. [DOI] [PubMed] [Google Scholar]
- 35. Wang X, Wang J, Liang Y, et al. Schistosoma japonicum HSP60‐derived peptide SJMHE1 suppresses delayed‐type hypersensitivity in a murine model. Parasites & Vectors. 2016;9(1). 10.1186/s13071-016-1434-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meleney HE, Sandground JH, Moore D, Most H, Carney BH. The histopathology of experimental schistosomiasis. II. Bisexual infections with S mansoni, S japonicum, and S haematobium . Am J Trop Med Hyg. 1953;2(5):883‐913. [PubMed] [Google Scholar]
- 37. Hsü H, Davis J, Hsü S. Histopathological lesions of Rhesus monkeys and chimpanzees infected with Schistosoma japonicum . Z Tropenmed Parasitol. 1969;20(2):184‐205. [PubMed] [Google Scholar]
- 38. Erickson DG, von Lichtenberg F, Sadun EH, Lucia HL, Hickman RL. Comparison of Schistosoma haematobium, S mansoni, and S japonicum infections in the owl monkey, Aotus trivirgatus . J Parasitol. 1971;57(3):543‐558. [PubMed] [Google Scholar]
- 39. Faust EC, Meleny HE. Studies on schistosomiasis japonica. J Parasitol. 1924;11(1):55‐56. [Google Scholar]
- 40. Bogen SA, Villanueva POF, McCusker ME, et al. In situ analysis of cytokine responses in experimental murine schistosomiasis. Lab Investig. 1995;73(2):252‐258. [PubMed] [Google Scholar]
- 41. Zheng B, Zhang J, Chen H, et al. T lymphocyte‐mediated liver immunopathology of schistosomiasis. Front Immunol. 2020;11. 10.3389/fimmu.2020.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ke XD, Shen S, Song LJ, et al. Characterization of Schistosoma japonicum CP1412 protein as a novel member of the ribonuclease T2 molecule family with immune regulatory function. Parasit Vectors. 2017;10(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fallon PG, Richardson EJ, McKenzie GJ, McKenzie ANJ. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL‐4 and IL‐13: IL‐13 is a profibrotic agent. J Immunol. 2000;164(5):2585‐2591. [DOI] [PubMed] [Google Scholar]
- 44. Coutinho HM, Acosta LP, Wu HW, et al. Th2 cytokines are associated with persistent hepatic fibrosis in human Schistosoma japonicum Infection. J Infect Dis. 2007;195(2):288‐295. [DOI] [PubMed] [Google Scholar]
- 45. Wynn TA, Eltoum I, Oswald IP, Cheever AW, Sher A. Endogenous interleukin 12 (IL‐12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL‐12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med. 1994;179(5):1551‐1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang J‐Q, Tasaka K, Chuang C‐K, Yoshikawa H, Nakajima Y. Dynamic analysis of T‐lymphocyte function in relation to hepatopathologic changes and effect of interleukin‐12 treatment in mice infected with Schistosoma japonicum . J Parasitol. 1999;85(2):262. [PubMed] [Google Scholar]
- 47. Singh KP, Gerard HC, Hudson AP, Reddy TR, Boros DL. Retroviral Foxp3 gene transfer ameliorates liver granuloma pathology in Schistosoma mansoni infected mice. Immunology. 2005;114(3):410‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhou S, Jin X, Chen X, et al. Heat shock protein 60 in eggs specifically induces Tregs and reduces liver immunopathology in mice with Schistosomiasis japonica . PLoS One. 2015;10(9):e0139133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hsü L, Hsü H, Davis J, Lust G. Comparative studies on the lesions caused by eggs of Schistosoma japonicum and Schistosoma mansoni in livers of albino mice and rhesus monkeys. Ann Trop Med Parasitol. 1972;66(1):89‐97. [DOI] [PubMed] [Google Scholar]
- 50. Warren K, Boros D, Hang L, Mahmoud AA. The Schistosoma japonicum egg granuloma. Am J Pathol. 1975;80(2):279‐293. [PMC free article] [PubMed] [Google Scholar]
- 51. Bentley AG, Carlisle AS, Phillips SM. Ultrastructural analysis of the cellular response to Schistosoma mansoni. II. Inflammatory responses in rodent skin. Am J Trop Med Hyg. 1981;30(4):815‐824. [DOI] [PubMed] [Google Scholar]
- 52. Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173‐182. [DOI] [PubMed] [Google Scholar]
- 53. Cascão R, Rosário HS, Souto‐Carneiro MM, Fonseca JE. Neutrophils in rheumatoid arthritis: more than simple final effectors. Autoimmun Rev. 2010;9(8):531‐535. [DOI] [PubMed] [Google Scholar]
- 54. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159‐175. [DOI] [PubMed] [Google Scholar]
- 55. Baker VS, Imade GE, Molta NB, et al. Cytokine‐associated neutrophil extracellular traps and antinuclear antibodies in Plasmodium falciparum infected children under six years of age. Malar J. 2008;7(1). 10.1186/1475-2875-7-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hakkim A, Fürnrohr BG, Amann K, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA. 2010;107(21):9813‐9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Teijeira Á, Garasa S, Gato M, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. 2020;52:856‐871.e8. [DOI] [PubMed] [Google Scholar]
- 58. Chuah C, Jones M, Burke M, Mcmanus D, Owen H, Gobert G. Defining a pro‐inflammatory neutrophil phenotype in response to schistosome eggs. Cell Microbiol. 2014;16(11):1666‐1677. [DOI] [PubMed] [Google Scholar]
- 59. Hirata M, Hara T, Kage M, Fukuma T, Sendo F. Neutropenia augments experimentally induced Schistosoma japonicum egg granuloma formation in CBA mice, but not in C57BL/6 mice. Parasite Immunol. 2002;24(9–10):479‐488. [DOI] [PubMed] [Google Scholar]
- 60. Chuah C, Jones M, Burke M, et al. Spatial and temporal transcriptomics of Schistosoma japonicum‐induced hepatic granuloma formation reveals novel roles for neutrophils. J Leukoc Biol. 2013;94(2):353‐365. [DOI] [PubMed] [Google Scholar]
- 61. Wu C, Chen Q, Fang Y, et al. Schistosoma japonicum egg specific protein SjE16.7 recruits neutrophils and induces inflammatory hepatic granuloma initiation. PLoS Negl Trop Dis. 2014;8(2):e2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lejoly‐Boisseau H, Appriou M, Seigneur M, Pruvost A, Tribouley‐Duret J, Tribouley J Schistosoma mansoni: in vitro adhesion of parasite eggs to the vascular endothelium. Subsequent inhibition by a monoclonal antibody directed to a carbohydrate epitope. Exp Parasitol. 1999;91(1):20‐29. [DOI] [PubMed] [Google Scholar]
- 63. Dewalick S, Hensbergen PJ, Bexkens ML, et al. Binding of von Willebrand factor and plasma proteins to the eggshell of Schistosoma mansoni . Int J Parasitol. 2014;44(5):263‐268. [DOI] [PubMed] [Google Scholar]
- 64. Smith P, Fallon RE, Mangan NE, et al. Schistosoma mansoni secretes a chemokine binding protein with antiinflammatory activity. J Exp Med. 2005;202(10):1319‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dias FF, Amaral KB, Malta KK, et al. Identification of piecemeal degranulation and vesicular transport of MBP‐1 in liver‐infiltrating mouse eosinophils during acute experimental Schistosoma mansoni infection. Front Immunol. 2018;9. 10.3389/fimmu.2018.03019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Glauert AM, Butterworth AE, Sturrock RF, Houba V. The mechanism of antibody‐dependent, eosinophil‐mediated damage to schistosomula of Schistosoma mansoni in vitro: a study by phase‐contrast and electron microscopy. J Cell Sci. 1978;34:173‐192. [DOI] [PubMed] [Google Scholar]
- 67. Capron M, Torpier G, Capron A In vitro killing of S mansoni schistosomula by eosinophils from infected rats: role of cytophilic antibodies. J Immunol. 1979;123(5):2220‐2230. [PubMed] [Google Scholar]
- 68. Moore DL, Grove DI, Warren KS. The Schistosoma mansoni egg granuloma: quantitation of cell populations. J Pathol. 1977;121(1):41‐50. [DOI] [PubMed] [Google Scholar]
- 69. Souza ALS, Roffê E, Pinho V, et al. Potential role of the chemokine macrophage inflammatory protein 1α in human and experimental schistosomiasis. Infect Immun. 2005;73(4):2515‐2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jakubzick C, Wen H, Matsukawa A, Keller M, Kunkel SL, Hogaboam CM. Role of CCR4 ligands, CCL17 and CCL22, during Schistosoma mansoni egg‐induced pulmonary granuloma formation in mice. Am J Pathol. 2004;165(4):1211‐1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shang XZ, Chiu BC, Stolberg V, et al. Eosinophil recruitment in type‐2 hypersensitivity pulmonary granulomas: source and contribution of monocyte chemotactic protein‐3 (CCL7). Am J Pathol. 2002;161(1):257‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sher A, Coffman RL, Hieny S, Cheever AW. Ablation of eosinophil and IgE responses with anti‐IL‐5 or anti‐IL‐4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J Immunol. 1990;145(11):3911‐3916. [PubMed] [Google Scholar]
- 73. Cheever AW, Xu YH, Sher A, Macedonia JG. Analysis of egg granuloma formation in Schistosoma japonicum‐infected mice treated with antibodies to interleukin‐5 and gamma interferon. Infect Immun. 1991;59(11):4071‐4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Eriksson J, Reimert CM, Kabatereine NB, et al. The 434(G>C) polymorphism within the coding sequence of Eosinophil Cationic Protein (ECP) correlates with the natural course of Schistosoma mansoni infection. Int J Parasitol. 2007;37(12):1359‐1366. [DOI] [PubMed] [Google Scholar]
- 75. Swartz JM, Dyer KD, Cheever AW, et al. Schistosoma mansoni infection in eosinophil lineage‐ablated mice. Blood. 2006;108(7):2420‐2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. de Andres B, Rakasz E, Hagen M, et al. Lack of Fc‐ε receptors on murine eosinophils: implications for the functional significance of elevated IgE and eosinophils in parasitic infections. Blood. 1997;89(10):3826‐3836. [PubMed] [Google Scholar]
- 77. Chuah C, Jones M, Burke M, Mcmanus D, Gobert G. Cellular and chemokine‐mediated regulation in schistosome‐induced hepatic pathology. Trends Parasitol. 2014;30(3):141‐150. [DOI] [PubMed] [Google Scholar]
- 78. Rumbley CA, Zekavat SA, Sugaya H, Perrin PJ, Ramadan MA, Phillips SM. The schistosome granuloma: characterization of lymphocyte migration, activation, and cytokine production. J Immunol. 1998;161(8):4129‐4137. [PubMed] [Google Scholar]
- 79. Phillips SM, DiConza JJ, Gold JA, Reid WA. Schistosomiasis in the congenitally athymic (nude) mouse. I. Thymic dependency of eosinophilia, granuloma formation, and host morbidity. J Immunol. 1977;118(2):594‐599. [PubMed] [Google Scholar]
- 80. Byram JE, von Lichtenberg F. Altered schistosome granuloma formation in nude mice. Am J Trop Med Hyg. 1977;26(5 Part I):944‐956. [DOI] [PubMed] [Google Scholar]
- 81. Cheever AW. A review. Schistosoma japonicum: the pathology of experimental infection. Exp Parasitol. 1985;59(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 82. Kaplan MH. Th9 cells: differentiation and disease. Immunol Rev. 2013;252(1):104‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhao P, Xiao X, Ghobrial RM, Li XC. IL‐9 and Th9 cells: progress and challenges. Int Immunol. 2013;25(10):547‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor‐β “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9‐producing subset. Nat Immunol. 2008;9(12):1341‐1346. [DOI] [PubMed] [Google Scholar]
- 85. Zhan T, Zhang T, Wang Y, et al. Dynamics of Th9 cells and their potential role in immunopathogenesis of murine schistosomiasis. Parasites & Vectors. 2017;10(1). 10.1186/s13071-017-2242-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li L, Xie H, Wang M, et al. Characteristics of IL‐9 induced by Schistosoma japonicum infection in C57BL/6 mouse liver. Sci Rep. 2017;7(1):2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Khalil RMA, Luz A, Mailhammer R, et al. Schistosoma mansoni infection in mice augments the capacity for interleukin 3 (IL‐3) and IL‐9 production and concurrently enlarges progenitor pools for mast cells and granulocytes‐macrophages. Infect Immun. 1996;64(12):4960‐4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fallon PG, Smith P, Richardson EJ, et al. Expression of interleukin‐9 leads to Th2 cytokine‐dominated responses and fatal enteropathy in mice with chronic Schistosoma mansoni infections. Infect Immun. 2000;68(10):6005‐6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Barreto AVMS, Lacerda GAN, Figueiredo ALC, et al. Evaluation of serum levels of IL‐9 and IL‐17 in human Schistosoma mansoni infection and their relationship with periportal fibrosis. Immunobiology. 2016;221(12):1351‐1354. [DOI] [PubMed] [Google Scholar]
- 90. Rutitzky LI, Lopes da Rosa JR, Stadecker MJ. Severe CD4 T cell‐mediated immunopathology in murine schistosomiasis is dependent on IL‐12p40 and correlates with high levels of IL‐17. J Immunol. 2005;175(6):3920‐3926. [DOI] [PubMed] [Google Scholar]
- 91. Zhang Y, Chen L, Gao W, et al. IL‐17 neutralization significantly ameliorates hepatic granulomatous inflammation and liver damage in Schistosoma japonicum infected mice. Eur J Immunol. 2012;42(6):1523‐1535. [DOI] [PubMed] [Google Scholar]
- 92. Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003;171(12):6323‐6327. [DOI] [PubMed] [Google Scholar]
- 93. Roncarlo MG, Gregori S. Is FOXP3 a bona fide marker for human regulatory T cells? Eur J Immunol. 2008;38(4):925‐927. [DOI] [PubMed] [Google Scholar]
- 94. Hartigan‐O’Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL‐7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319(1–2):41‐52. [DOI] [PubMed] [Google Scholar]
- 95. Watanabe K, Mwinzi PNM, Black CL, et al. T regulatory cell levels decrease in people infected with Schistosoma mansoni on effective treatment. Am J Trop Med Hyg. 2007;77(4):676‐682. [PMC free article] [PubMed] [Google Scholar]
- 96. Romano A, Hou X, Sertorio M, et al. FOXP3+ regulatory T cells in hepatic fibrosis and splenomegaly caused by Schistosoma japonicum: the spleen may be a major source of Tregs in subjects with splenomegaly. PLoS Negl Trop Dis. 2016;10(1):e0004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ondigo BN, Ndombi EM, Nicholson SC, et al. Functional studies of T regulatory lymphocytes in human schistosomiasis in western Kenya. Am J Trop Med Hyg. 2018;98(6):1770‐1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Baumgart M, Tompkins F, Leng J, Hesse M. Naturally occurring CD4+ Foxp3+ regulatory T cells are an essential, IL‐10‐independent part of the immunoregulatory network in Schistosoma mansoni egg‐induced inflammation. J Immunol. 2006;176(9):5374‐5387. [DOI] [PubMed] [Google Scholar]
- 99. McKee AS, Pearce EJ. CD25+ CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173(2):1224‐1231. [DOI] [PubMed] [Google Scholar]
- 100. Hesse M, Piccirillo CA, Belkaid Y, et al. The pathogenesis of schistosomiasis is controlled by cooperating IL‐10‐producing innate effector and regulatory T cells. J Immunol. 2004;172(5):3157‐3166. [DOI] [PubMed] [Google Scholar]
- 101. Turner JD, Jenkins GR, Hogg KG, et al. CD4+CD25+ regulatory cells contribute to the regulation of colonic Th2 granulomatous pathology caused by schistosome infection. PLoS Negl Trop Dis. 2011;5(8):e1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tang CL, Lei JH, Wang T, et al. Effect of CD4+CD25+ regulatory T cells on the immune evasion of Schistosoma japonicum . Parasitol Res. 2011;108(2):477‐480. [DOI] [PubMed] [Google Scholar]
- 103. Mariano FS, Gutierrez FRS, Pavanelli WR, et al. The involvement of CD4+CD25+ T cells in the acute phase of Trypanosoma cruzi infection. Microbes Infect. 2008;10(7):825‐833. [DOI] [PubMed] [Google Scholar]
- 104. Verhagen J, Sabatos CA, Wraith DC. The role of CTLA‐4 in immune regulation. Immunol Lett. 2008;115(1):73‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tang CL, Lei JH, Guan F, et al. Effect of cytotoxic T‐lymphocyte‐associated protein 4 on CD4+CD25+ regulatory T cells in murine Schistosomiasis japonica . Exp Parasitol. 2014;136(1):74‐78. [DOI] [PubMed] [Google Scholar]
- 106. Wang X, Zhou S, Chi Y, et al. CD4+CD25+ Treg induction by an HSP60‐derived peptide SJMHE1 from Schistosoma japonicum is TLR2 dependent. Eur J Immunol. 2009;39(11):3052‐3065. [DOI] [PubMed] [Google Scholar]
- 107. Wynn TA, Thompson RW, Cheever AW, Mentink‐Kane MM. Immunopathogenesis of schistosomiasis. Immunol Rev. 2004;201:156‐167. [DOI] [PubMed] [Google Scholar]
- 108. Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134(6):1655‐1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chang D, Ramalho LNZ, Ramalho FS, Martinelli ALC, Zucoloto S. Hepatic stellate cells in human schistosomiasis mansoni: a comparative immunohistochemical study with liver cirrhosis. Acta Trop. 2006;97(3):318‐323. [DOI] [PubMed] [Google Scholar]
- 110. Bartley PB, Ramm GA, Jones M, Ruddell RG, Li Y, McManus D. A contributory role for activated hepatic stellate cells in the dynamics of Schistosoma japonicum egg‐induced fibrosis. Int J Parasitol. 2006;36(9):993‐1001. [DOI] [PubMed] [Google Scholar]
- 111. She H, Xiong S, Hazra S, Tsukamoto H. Adipogenic transcriptional regulation of hepatic stellate cells. J Biol Chem. 2005;280(6):4959‐4967. [DOI] [PubMed] [Google Scholar]
- 112. Anthony BJ, Allen JT, Li YS, McManus D. A role for peroxisome proliferator‐activated receptors in the immunopathology of schistosomiasis? PPAR Res. 2012;128068:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Anthony BJ, Mathieson W, de Castro‐Borges W, Allen J Schistosoma mansoni: egg‐induced downregulation of hepatic stellate cell activation and fibrogenesis. Exp Parasitol. 2010;124(4):409‐420. [DOI] [PubMed] [Google Scholar]
- 114. Anthony BJ, James KR, Gobert GN, Ramm GA, McManus D Schistosoma japonicum eggs induce a proinflammatory, anti‐fibrogenic phenotype in hepatic stellate cells. PLoS One. 2013;8(6):e68479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Burke M, Jones M, Gobert G, Li YS, Ellis MK, McManus D. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009;31(4):163‐176. [DOI] [PubMed] [Google Scholar]
- 116. Hernandez HJ, Wang Y, Tzellas N, Stadecker MJ. Expression of class II, but not class I, major histocompatibility complex molecules is required for granuloma formation in infection with Schistosoma mansoni . Eur J Immunol. 1997;27(5):1170‐1176. [DOI] [PubMed] [Google Scholar]
- 117. Girgis NM, Gundra UM, Ward LN, Cabrera M, Frevert U, Loke P. Ly6Chigh monocytes become alternatively activated macrophages in schistosome granulomas with help from CD4+ Cells. PLoS Pathog. 2014;10(6):e1004080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Anthony BJ, Ramm GA, McManus D. Role of resident liver cells in the pathogenesis of schistosomiasis. Trends Parasitol. 2012;28(12):572‐579. [DOI] [PubMed] [Google Scholar]
- 119. Barron L, Wynn TA. Macrophage activation governs schistosomiasis‐induced inflammation and fibrosis. Eur J Immunol. 2011;41(9):2509‐2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pradere JP, Kluwe J, De Minicis S, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58(4):1461‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23‐35. [DOI] [PubMed] [Google Scholar]
- 122. Hesse M, Cheever AW, Jankovic D, Wynn TA. NOS‐2 mediates the protective anti‐inflammatory and antifibrotic effects of the Th1‐inducing adjuvant, IL‐12, in a Th2 model of granulomatous disease. Am J Pathol. 2000;157(3):945‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Herbert DR, Hölscher C, Mohrs M, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20(5):623‐635. [DOI] [PubMed] [Google Scholar]
- 124. Hesse M, Modolell M, La Flamme AC, et al. Differential regulation of Nitric Oxide Synthase‐2 and Arginase‐1 by Type 1/Type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of l‐Arginine metabolism. J Immunol. 2001;167(11):6533‐6544. [DOI] [PubMed] [Google Scholar]
- 125. Fairfax KC, Amiel E, King IL, Freitas TC, Mohrs M, Pearce EJ. IL‐10R blockade during chronic Schistosomiasis mansoni results in the loss of B cells from the liver and the development of severe pulmonary disease. PLoS Pathog. 2012;8(1):e1002490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zhu J, Xu Z, Chen X, et al. Parasitic antigens alter macrophage polarization during Schistosoma japonicum infection in mice. Parasites & Vectors. 2014;7(1):122. 10.1186/1756-3305-7-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Peng H, Zhang Q, Li X, et al. IL‐33 Contributes to Schistosoma japonicum‐induced hepatic pathology through induction of M2 macrophages. Sci Rep. 2016;6(29844):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gong W, Huang F, Sun L, et al. Toll‐like receptor‐2 regulates macrophage polarization induced by excretory‐secretory antigens from Schistosoma japonicum eggs and promotes liver pathology in murine schistosomiasis. PLoS Negl Trop Dis. 2018;12(12):e0007000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Fallowfield JA, Mizuno M, Kendall TJ, et al. Scar‐associated macrophages are a major source of hepatic matrix metalloproteinase‐13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178(8):5288‐5295. [DOI] [PubMed] [Google Scholar]
- 130. Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30(3):245‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Harty MW, Papa EF, Huddleston HM, et al. Hepatic macrophages promote the neutrophil‐dependent resolution of fibrosis in repairing cholestatic rat livers. Surgery. 2008;143(5):667‐678. [DOI] [PubMed] [Google Scholar]
- 132. Fang Y, Wu C, Chen Q, et al. SjE16.7 activates macrophages and promotes Schistosoma japonicum egg‐induced granuloma development. Acta Trop. 2015;149:49‐58. [DOI] [PubMed] [Google Scholar]
- 133. Murray CJL, Vos T, Lozano R, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197‐2223. [DOI] [PubMed] [Google Scholar]
- 134. Cioli D, Pica‐Mattoccia L, Basso A, Guidi A. Schistosomiasis control: praziquantel forever? Mol Biochem Parasitol. 2014;195(1):23‐29. [DOI] [PubMed] [Google Scholar]
- 135. Turner JD, Narang P, Coles MC, Mountford AP. Blood flukes exploit Peyer’s patch lymphoid tissue to facilitate transmission from the Mammalian host. PLoS Pathog. 2012;8(12):e1003063. [DOI] [PMC free article] [PubMed] [Google Scholar]


