Abstract
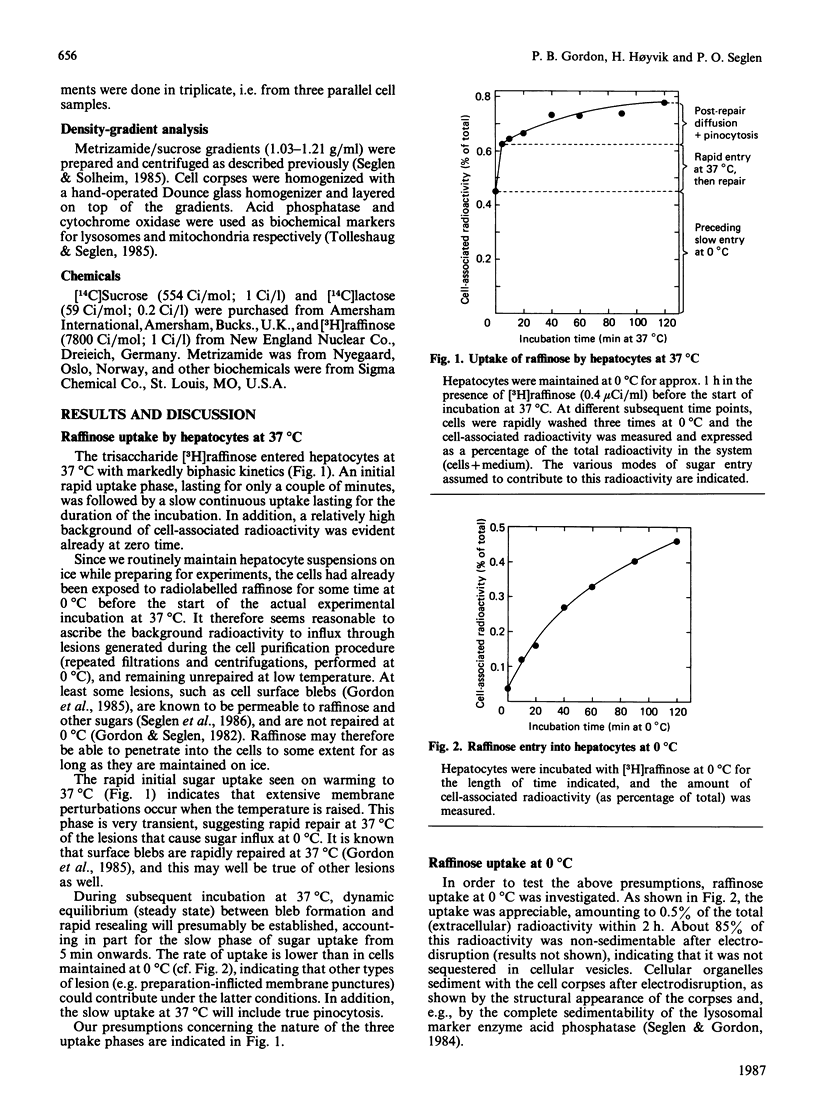
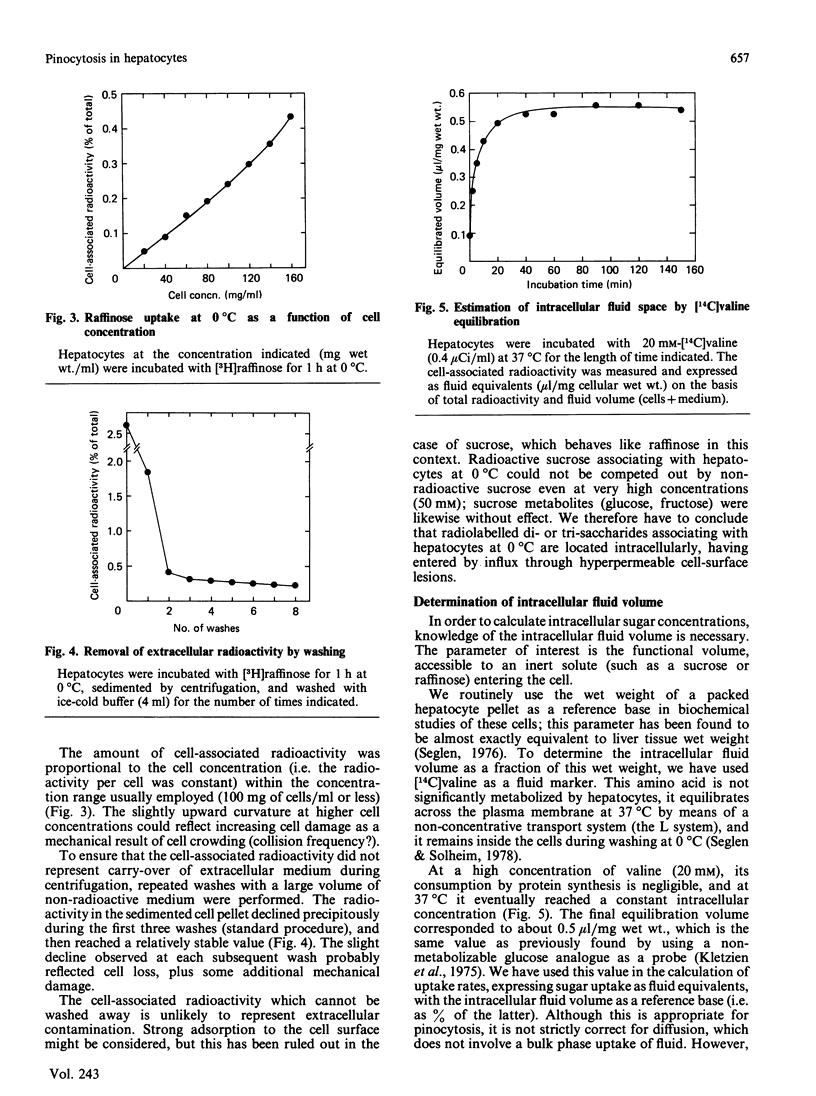
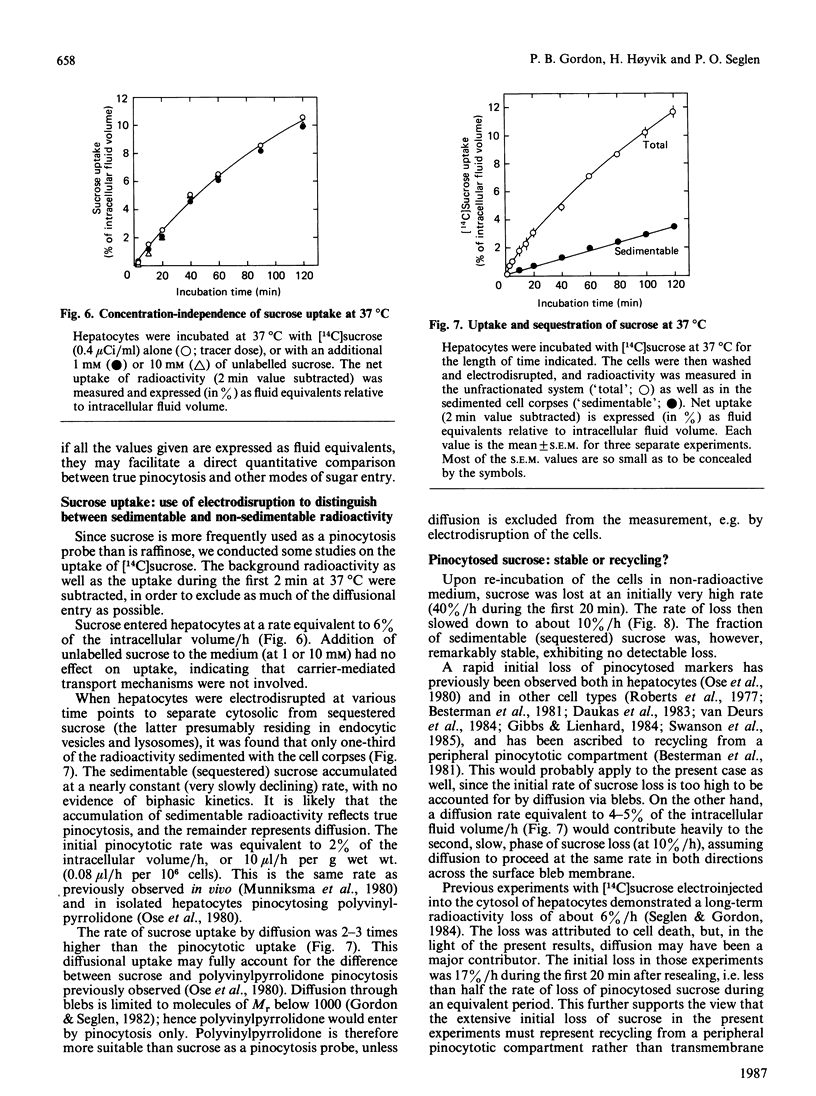
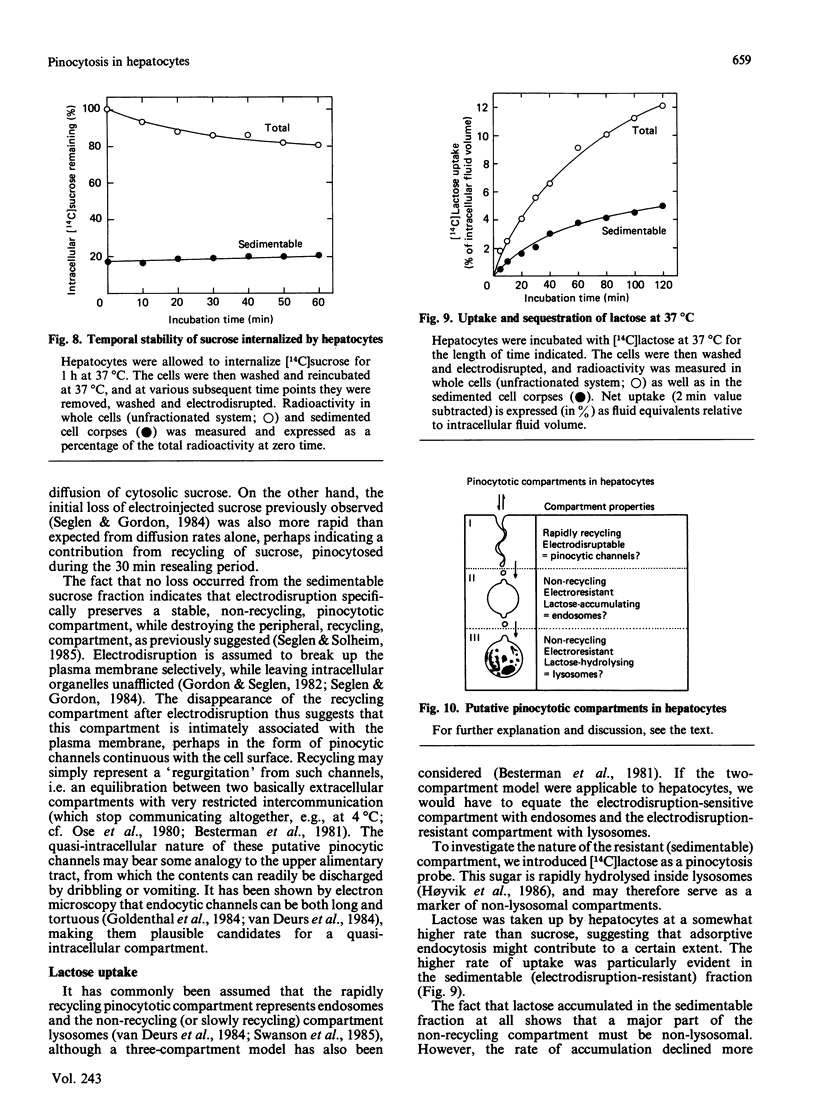
Measurements of sugar pinocytosis (fluid-phase endocytosis of radiolabelled sucrose, lactose and raffinose) in freshly isolated rat hepatocytes are disturbed by sugar diffusing into the cells through plasma-membrane blebs. Non-pinocytic entry may be even more pronounced at 0 degrees C, and is a major contributor to 'background' radioactivity. By electrodisruption of the plasma membrane, a distinction can be made between pinocytotically sequestered sugar and free sugar that has entered the cytosol by diffusion. Pinocytosis proceeds at a rate of 2%/h (relative to the intracellular fluid volume), whereas the rate of sucrose entry by diffusion is more than twice as high. Three pinocytotic compartments are distinguishable in isolated hepatocytes: (1) a rapidly recycling compartment, which is completely destroyed by electrodisruption, and which may represent pinocytic channels continuous with the plasma membrane; (2) a non-recycling (or very slowly recycling) electrodisruption-resistant compartment, which allows accumulation of the lysosomally hydrolysable sugar lactose, and which therefore must represent non-lysosomal vacuoles (endosomes?); (3) a lysosomal compartment (non-recycling, electrodisruption-resistant), which accumulates raffinose and sucrose, but which hydrolyses lactose. The last two compartments can be partially resolved in metrizamide/sucrose density gradients by the use of different sugar probes.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besterman J. M., Airhart J. A., Woodworth R. C., Low R. B. Exocytosis of pinocytosed fluid in cultured cells: kinetic evidence for rapid turnover and compartmentation. J Cell Biol. 1981 Dec;91(3 Pt 1):716–727. doi: 10.1083/jcb.91.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman J. M., Low R. B. Endocytosis: a review of mechanisms and plasma membrane dynamics. Biochem J. 1983 Jan 15;210(1):1–13. doi: 10.1042/bj2100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daukas G., Lauffenburger D. A., Zigmond S. Reversible pinocytosis in polymorphonuclear leukocytes. J Cell Biol. 1983 Jun;96(6):1642–1650. doi: 10.1083/jcb.96.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T. Accelerated fluid endocytosis and re-exocytosis by lysosomal storage disease fibroblasts. Exp Cell Res. 1984 Apr;151(2):563–566. doi: 10.1016/0014-4827(84)90404-x. [DOI] [PubMed] [Google Scholar]
- Forker E. L. Hepatocellular uptake of inulin, sucrose, and mannitol in rats. Am J Physiol. 1970 Dec;219(6):1568–1573. doi: 10.1152/ajplegacy.1970.219.6.1568. [DOI] [PubMed] [Google Scholar]
- Gibbs E. M., Lienhard G. E. Fluid-phase endocytosis by isolated rat adipocytes. J Cell Physiol. 1984 Dec;121(3):569–575. doi: 10.1002/jcp.1041210316. [DOI] [PubMed] [Google Scholar]
- Goldenthal K. L., Pastan I., Willingham M. C. Initial steps in receptor-mediated endocytosis. The influence of temperature on the shape and distribution of plasma membrane clathrin-coated pits in cultured mammalian cells. Exp Cell Res. 1984 Jun;152(2):558–564. doi: 10.1016/0014-4827(84)90658-x. [DOI] [PubMed] [Google Scholar]
- Gordon P. B., Seglen P. O. Autophagic sequestration of [14C]sucrose, introduced into rat hepatocytes by reversible electro-permeabilization. Exp Cell Res. 1982 Nov;142(1):1–14. doi: 10.1016/0014-4827(82)90402-5. [DOI] [PubMed] [Google Scholar]
- Gordon P. B., Tolleshaug H., Seglen P. O. Autophagic sequestration of [14C]sucrose introduced into isolated rat hepatocytes by electrical and non-electrical methods. Exp Cell Res. 1985 Oct;160(2):449–458. doi: 10.1016/0014-4827(85)90192-2. [DOI] [PubMed] [Google Scholar]
- Høyvik H., Gordon P. B., Seglen P. O. Use of a hydrolysable probe, [14C]lactose, to distinguish between pre-lysosomal and lysosomal steps in the autophagic pathway. Exp Cell Res. 1986 Sep;166(1):1–14. doi: 10.1016/0014-4827(86)90503-3. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Pariza M. W., Becker J. E., Potter V. R. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem. 1975 Oct;68(2):537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- Munniksma J., Noteborn M., Kooistra T., Stienstra S., Bouma J. M., Gruber M., Brouwer A., Praaning-van Dalen Dalen D., Knook D. L. Fluid endocytosis by rat liver and spleen. Experiments with 125I-labelled poly(vinylpyrrolidone) in vivo. Biochem J. 1980 Nov 15;192(2):613–621. doi: 10.1042/bj1920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ose L., Ose T., Reinertsen R., Berg T. Fluid endocytosis in isolated rat parenchymal and non-parenchymal liver cells. Exp Cell Res. 1980 Mar;126(1):109–119. doi: 10.1016/0014-4827(80)90475-9. [DOI] [PubMed] [Google Scholar]
- Pauw P. G., Biagi K. G., Stadler J. K. Reduced pinosome formation in a cell-fusion-impaired mutant of mouse lymphocytic cell line L5178Y. J Cell Physiol. 1986 Feb;126(2):243–248. doi: 10.1002/jcp.1041260213. [DOI] [PubMed] [Google Scholar]
- Roberts A. V., Williams K. E., Lloyd J. B. The pinocytosis of 125I-labelled poly(vinylpyrrolidone), [14C]sucrose and colloidal [198Au]gold by rat yolk sac cultured in vitro. Biochem J. 1977 Nov 15;168(2):239–244. doi: 10.1042/bj1680239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O., Gordon P. B. Amino acid control of autophagic sequestration and protein degradation in isolated rat hepatocytes. J Cell Biol. 1984 Aug;99(2):435–444. doi: 10.1083/jcb.99.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O., Gordon P. B., Tolleshaug H., Høyvik H. Pathways of intracellular sequestration and protein degradation in isolated rat hepatocytes. Prog Clin Biol Res. 1985;180:437–446. [PubMed] [Google Scholar]
- Seglen P. O., Gordon P. B., Tolleshaug H., Høyvik H. Use of [3H]raffinose as a specific probe of autophagic sequestration. Exp Cell Res. 1986 Jan;162(1):273–277. doi: 10.1016/0014-4827(86)90446-5. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Solheim A. E. Conversion of dense lysosomes into light lysosomes during hepatocytic autophagy. Exp Cell Res. 1985 Apr;157(2):550–555. doi: 10.1016/0014-4827(85)90141-7. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Solheim A. E. Valine uptake and incorporation into protein in isolated rat hepatocytes. Nature of the precursor pool for protein synthesis. Eur J Biochem. 1978 Apr;85(1):15–25. doi: 10.1111/j.1432-1033.1978.tb12208.x. [DOI] [PubMed] [Google Scholar]
- Swanson J. A., Yirinec B. D., Silverstein S. C. Phorbol esters and horseradish peroxidase stimulate pinocytosis and redirect the flow of pinocytosed fluid in macrophages. J Cell Biol. 1985 Mar;100(3):851–859. doi: 10.1083/jcb.100.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolleshaug H., Seglen P. O. Autophagic-lysosomal and mitochondrial sequestration of [14C]sucrose. Density gradient distribution of sequestered radioactivity. Eur J Biochem. 1985 Dec 2;153(2):223–229. doi: 10.1111/j.1432-1033.1985.tb09290.x. [DOI] [PubMed] [Google Scholar]


