Abstract
The various alphaherpesviruses, including Marek's disease virus (MDV), have both common and unique features of gene content and expression. The entire MDV Us region has been sequenced in our laboratory (P. Brunovskis and L. F. Velicar, Virology 206:324–338, 1995). Genes encoding the MDV glycoprotein D (gD), glycoprotein I (gI), and glycoprotein E (gE) homologs have been found in this region, although no gG homolog was found. In this work, transcription of the tandem MDV gD, gI, and gE genes was studied and found to have both unique characteristics and also features in common with other alphaherpesviruses. MDV gD could not be immunoprecipitated from MDV GA-infected duck embryo fibroblast cells by antisera reactive to its TrpE fusion proteins, while gI and gE could be. When the gD gene was subjected to in vitro-coupled transcription-translation, the precursor polypeptide was produced and could be immunoprecipitated by anti-gD. Northern blot, reverse transcriptase PCR, and RNase protection analyses have shown that (i) no mRNA initiating directly from the gD gene could be detected; (ii) a large but low-abundance 7.5-kb transcript spanning five genes, including the one encoding gD, was seen on longer exposure; and (iii) transcription of the gI and gE genes formed an abundant bicistronic 3.5-kb mRNA, as well as an abundant 2.0-kb gE-specific mRNA. Therefore, the MDV gD gene expression is down-regulated at the transcription level in MDV-infected cell culture, which may be related to the cell-associated nature of MDV in fibroblast cells. Compared to the highly gD-dependent herpes simplex virus and the other extreme of the varicella-zoster virus which lacks the gD gene, MDV is an intermediate type of alphaherpesvirus.
Marek's disease virus (MDV) is a highly infectious herpesvirus which induces lymphomas in chickens. The nonpathogenic and antigenically related herpesvirus of turkey (HVT) is usually effective as a vaccine against Marek's disease and is the first successful vaccine against a naturally occurring tumor of any species. While being a very interesting and valuable natural host animal model for oncogenesis, this cell-associated herpesvirus system is somewhat complex. Fully enveloped infectious virions are produced only in feather follicle epithelium (FFE) of the skin; they then detach with feather dander, contaminate dust, are spread by the airborne route, and infect new hosts via the respiratory tract. Four phases of infection in vivo can be delineated: (i) early productive-restrictive virus infection causing primarily degenerative changes, (ii) latent infection, (iii) a second phase of cytolytic infection coincident with permanent immunosuppression, and (iv) a proliferative phase involving nonproductively infected lymphoid cells that may progress to the point of lymphoma formation (5).
MDV and HVT have genome structures closely resembling those of alphaherpesviruses such as herpes simplex virus type 1 (HSV-1), the prototype alphaherpesvirus, varicella-zoster virus (VZV), pseudorabies virus (PRV), bovine herpesvirus 1 (BHV-1), and equine herpesvirus 1. The alphaherpesvirus genome structure consists of covalently joined long (L) and short (S) components. The S component comprises a unique short (Us) segment flanked by a pair of inverted repeat regions. There are four glycoprotein genes in the HSV-1 Us region, encoding glycoproteins G (gG), D (gD), I (gI), and E (gE) (10).
HSV-1 gD is a virion envelope component which plays an essential role in HSV-1 entry into susceptible mammalian cells (15). HSV-1 gD has been implicated in receptor binding, cell fusion, and neuroinvasiveness (11). Immunization of animals with HSV-1 gD stimulates the production of virus-neutralizing antibodies and protects them from both lethal challenge with HSV-1 and the establishment of latency (4). Homologs of HSV-1 gD have been identified in the genomes of PRV and BHV-1, among other alphaherpesviruses. The gDs of HSV-1, PRV, and BHV-1 cause viral interference (7, 16, 27). Although the gD homolog of PRV is essential for penetration, its production is not required for cell-to-cell spread (26).
The gI and gE homologs of HSV-1, PRV, and VZV are found to form complexes. HSV-1 gE and VZV gE act as immunoglobulin G Fc receptors that may utilize an antibody bipolar bridging mechanism to protect virus-infected cells from antibody-dependent cellular cytotoxicity (14, 20). HSV-1 and PRV gE are involved in neurotropism and virulence during virus infection of animals (6, 23).
The entire MDV Us region has been sequenced in our laboratory (3). Genes encoding the MDV gD, gI, and gE homologs have been found in this region, although no gG homolog was found. Antisera to their TrpE fusion proteins have been produced (see Fig. 1). MDV gI and gE have been identified in MDV-infected cells by immunoprecipitation with their respective antisera (2). In contrast, no gD was found in MDV-infected cells with antisera to its TrpE fusion proteins (2).
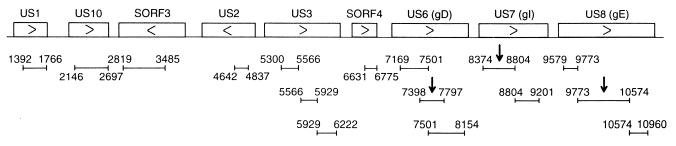
FIG. 1.
Schematic representation of the MDV US region DNA fragments used for antibody production and Northern blot analysis. The nucleotide position numbers are as previously published (3). Each bar represents an MDV Us region fused to TrpE protein for antibody production. Arrows indicate fragments also used as DNA probes specific for the gD, gI, and gE regions of the transcripts in Northern blot analysis.
In this study, antiserum to TrpE-gD was used in a further attempt to detect MDV gD expression in MDV-infected cells, but no protein of the predicted size was found. When Northern blot, reverse transcriptase PCR (RT-PCR) and RNase protection analyses were performed, an abundant 3.5-kb bicistronic mRNA including the MDV gI and gE genes was detected, together with an abundant 2.0-kb monocistronic transcript specific for the gE gene. In contrast, there was no specific mRNA initiating from the gD gene promoter, although a low-abundance polycistronic mRNA including the MDV US3, SORF4, gD, gI, and gE genes was found.
(Preliminary results of this work were presented at the 1996 International Symposium on Marek's Disease.)
MATERIALS AND METHODS
Cells and viruses.
The preparation, propagation, and infection of duck embryo fibroblast (DEF) and chicken embryo fibroblast (CEF) cells with cell-associated MDV were performed as described previously (8). The virulent MDV GA strain used in this study was at cell culture passage level 6 following isolation of cell-free virus from feather tips obtained from infected birds with symptoms of Marek's disease.
Generation of TrpE fusion proteins and antibodies to fusion proteins.
The vector system used to express the MDV genes in Escherichia coli consists of a group of plasmids (pATH vectors) encoding approximately 37 kDa of the bacterial TrpE open reading frame (ORF) product under control of the inducible trp operon promoter. A polylinker with multiple cloning sites at the 3′ end of the trpE ORF allows in-frame insertion of foreign ORFs. The TrpE fusion proteins were generated as described previously (8). The fusion proteins were purified from preparative sodium dodecyl sulfate (SDS)–7.5% polyacrylamide gels and used to prepare antisera by injecting rabbits as described previously (8).
In vitro transcription and translation.
MDV SORF4, gD, gI, and gE genes were cloned downstream of T3 or T7 promoters. In vitro-coupled transcription-translation was performed with Promega's TNT coupled reticulocyte lysate system.
Radiolabeling of proteins and immunoprecipitation analysis.
MDV-infected and mock-infected control DEF cells were labeled with [35S]methionine at 72 h postinfection for 4 h. After labeling was complete, culture medium samples and cell lysates were prepared and immunoprecipitation analysis was carried out as previously described (8). Briefly, 400 μl of 35S-labeled cell lysates were pretreated with normal rabbit serum for 2 h, protein A was added, and the mixture was incubated for 1 h and centrifuged. After centrifugation, the supernatants were mixed with the respective antisera for 2 h. Then protein A-Sepharose was added, and the suspension was incubated with gentle mixing for 2 h, followed by centrifugation. Immunoprecipitates were resuspended in sample buffer, recentrifuged, and subjected to SDS-polyacrylamide gel electrophoresis (PAGE). Protein markers (GIBCO) were used as molecular weight standards.
RNA preparation, isolation, and Northern blot analysis.
RNAs were prepared from MDV-infected and mock-infected control cells at 72 h postinfection by the guanidinium isothiocyanate-phenol extraction procedure. Poly(A)+ RNAs were prepared by oligo(dT) cellulose spin columns. Northern blot analysis was performed as described previously (29). DNA probes were labeled by the random-priming method. Riboprobes were prepared as described below. Transcript size determinations were based on a comparison with a GIBCO RNA ladder run in parallel.
Riboprobe and RNA marker preparation.
Antisense riboprobes and RNA marker were generated by in vitro transcription off linearized plasmid templates with RNA polymerase T3 or T7 (Promega). For riboprobes and RNA markers used in RNase protection, the 20-μl reaction solution included 5 mM each GTP, ATP, and CTP, in addition to 4.5 μl of [α-32P]UTP (800 μCi/mmol). For riboprobes used in Northern blot analysis, 50 μM unlabeled UTP was added to the reaction mixture.
To determine the size of the protected fragments, a set of RNA markers was generated in the proper range. The pBCSK(+) plasmid (Stratagene) was cut by BamHI, XhoI, HinfI, or PvuII, and RNA fragments of 51, 102, 139 and 245 bases were produced, respectively. The RNA marker was a mixture of the four radiolabeled RNA fragments.
RNase protection analysis.
A 500,000-cpm portion of a riboprobe was hybridized with 40 μg of total RNA in 20 μl of hybridization buffer (40 mM PIPES [pH6.4], 1 mM EDTA, 0.4 M NaCl, 80% formamide) and incubated at 42°C overnight. The hybridization solution was diluted with 300 μl of digestion buffer (300 mM NaCl, 10 mM Tris-HCl [pH7.5], 5 mM EDTA, 40 μg of RNase A per ml, 800 U of RNase T1 per ml) and incubated at 30°C for 60 min. After digestion, the samples were extracted with phenol-chloroform and the RNAs were precipitated in ethanol. The pellets were then resuspended in loading buffer and subjected to electrophoresis on a polyacrylamide-urea sequencing gel.
RT-PCR assays.
Total RNA preparations were treated with RNase-free DNase I (10 U per mg of total RNA) for 30 min at 37°C. Reverse transcription was performed with Superscript II RT (GIBCO) using the oligo(dT) primer. This reaction mixture (1/10 aliquot) was subjected to 30 cycles of PCR consisting of 94°C for 1 min, 50°C for 2 min, and 72°C for 3 min. The product (1/10 aliquot) was loaded onto agarose gels for electrophoresis, and DNA was visualized with ethidium bromide. Nucleotide positions of the primers are shown in Fig. 5A. Primer D sense (DS) (5′ TACGTGAATATGCCAACTGC 3′) corresponded to nucleotide positions 7340 to 7359. Primer D antisense (DA) (5′ AGTGAGTCCAGTGTAACCATCC 3′) corresponded to nucleotide positions 7875 to 7896. Primer I sense (IS) (5′ TGGTATATGCTCAACCTCATGG 3′) corresponded to nucleotide positions 8574 to 8595. Primer I antisense (IA) (5′ ACCGATGTATATCCTACGATGG 3′) corresponded to nucleotide positions 8574 to 8595. Primer E sense (ES) (5′ GAATCGCTAAGTCTGAATGG 3′) corresponded to nucleotide positions 9791 to 9800. Primer E antisense (EA) (5′ CAGAATGTCAATGTTGGATGCG 3′) corresponded to nucleotide positions 10249 to 10270.
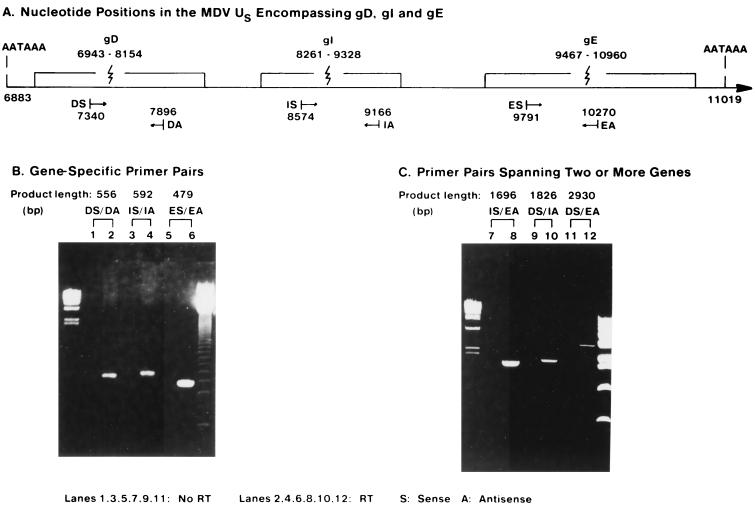
FIG. 5.
RT-PCR analysis of MDV gD transcription in MDV-infected cells. (A) Nucleotide positions in the MDV Us region encompassing gD, gI, and gE. DS, primer gD sense, DA, primer gD antisense; IS, primer gI sense; IA, primer gI antisense; ES, primer gE sense; EA, primer gE antisense. The nucleotide position numbers are as previously published (3). (B) Gene-specific primer pairs. Lanes: 1, 3, and 5, reaction in the absence of RT; 2, 4, and 6, reaction in the presence of RT Left marker: λ/HindIII marker. Right marker: 123-bp ladder. (C) Primer pairs spanning two or more genes. Lanes: 7, 9, and 11, Reaction in the absence of RT; 8, 10, and 12, Reaction in the presence of RT. Left marker: λ/HindIII marker. Right marker: 1-kb ladder.
RESULTS
Immunoprecipitation analysis fails to detect MDV gD in MDV-infected cell culture.
Of the 11 MDV Us genes, 7 are HSV-1 homologs: US1, US10, US2, US3, US6, (gD), US7 (gI), and US8 (gE). Fragments from each gene have been cloned in-frame with trpE to make TrpE fusion proteins (Fig. 1). Antisera to each TrpE fusion protein have been produced. With these antisera, the proteins encoded by MDV US1, US10, US2, US3, US7 (gI), and US8 (gE) have been identified in extracts from MDV-infected DEF cells, but no gD (US6) protein was found (2).
In further attempts to identify possible low levels of MDV gD expression, immunoprecipitation and SDS-PAGE analysis were performed with rabbit anti-gD antibody and radiolabeled proteins from MDV GA-infected DEF cells. Anti-gI, anti-gE, and anti-gB antibodies were used as positive controls. Normal rabbit serum (NRS) was used as a negative control. Anti-gE immunoprecipitated the two glycosylated forms of MDV gE (gp62 and gp72) (Fig. 2, lane 8), and anti-gI immunoprecipitated both MDV gI and gE (lane 6), as reported previously (2). Anti-gB immunoprecipitated MDV gB (lane 10) (8).
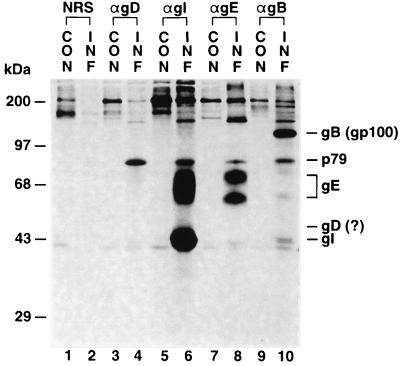
FIG. 2.
MDV gD is not expressed in MDV-infected DEF cells. Lanes: 1, 3, 5, 7, and 9, mock-infected DEF cells; 2, 4, 6, 8, and 10: MDV GA-infected DEF cells; 1 and 2, immunoprecipitation analysis with NRS; 3, and 4, immunoprecipitation analysis with anti-gD; 5 and 6, immunoprecipitation analysis with anti-gI; 7 and 8, immunoprecipitation analysis with anti-gE; 9 and 10, immunoprecipitation analysis with anti-gB. The illustration shows a 4-day exposure.
The MDV gD precursor polypeptide was predicted to be about 43 kDa (3). Based on its predicted amino acid sequence, gD has four potential N-linked glycosylation sites, resulting in a mature glycoprotein predicted to be about 53 kDa. However, no protein in the range of 40 to 60 kDa was immunoprecipitated with anti-gD (Fig. 2 lane 4). A fourfold-longer exposure of the same gel still did not show any MDV-specific protein in that range (data not shown). When the same experiment was done in MDV GA-infected CEF cells instead of DEF cells, the same result was observed (data not shown).
An MDV-specific protein of about 79 kDa was nonspecifically trapped by anti-gD, anti-gI, anti-gE, and anti-gB antibodies (Fig. 2, lanes 4, 6, 8, and 10). A fourfold-longer exposure showed that it also could be trapped by NRS (data not shown). Thus, the 79-kDa protein seen in this gel is most probably the p79 reported by this laboratory previously (13). It was recognized as a sticky protein which could be nonspecifically trapped by either NRS or specific-pathogen-free chicken serum from MDV- or HVT-infected cells but not from mock-infected cells. The gene encoding p79 has been sequenced (9), and it is not the gD gene.
Similar experiments have been done with extracts from MDV Md5-infected, Md11-infected, and JM/102w-infected DEF cells, and no gD expression was detected (unpublished data). Therefore, the absence of a detectable level of gD expression in MDV-infected cell culture is not GA strain specific.
The MDV gD gene encodes a functional protein.
Failure to detect gD expression could result from a defective ORF; the presumed gD ORF may not encode an authentic protein. To address this question, the MDV gD gene, as well as the SORF4, gI, and gE genes as controls, was cloned downstream of T3 or T7 promoters, and in vitro coupled transcription-translation was performed with T3 or T7 RNA polymerases and rabbit reticulocyte lysate (Promega). Each of the SORF4, gD, gI, and gE genes was transcribed and translated into a precursor polypeptide (Fig. 3A). The gD precursor polypeptide was about 43 kDa, close to the mass predicted from its amino acid sequence.
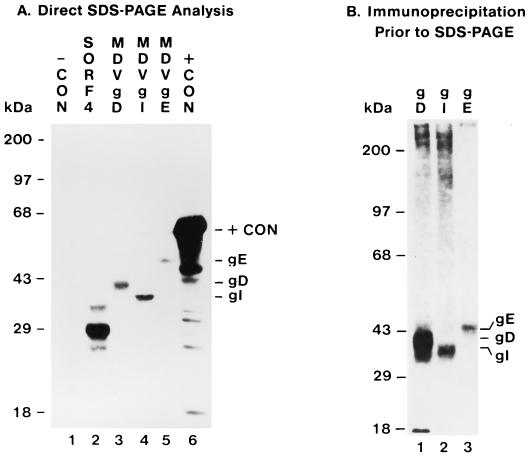
FIG. 3.
Expression of MDV gD and other MDV Us genes by in vitro -coupled transcription-translation. (A) Direct SDS-PAGE analysis, 12-h exposure. Lanes 1, without DNA template; 2, MDV SORF4 gene template; 3, MDV gD gene template; MDV gI gene template; 5, MDV gE gene template; 6, luciferase gene template. (B) Immunoprecipitation analysis prior to SDS-PAGE; 3-day exposure. Lanes 1, precursor polypeptide of glycoprotein gD immunoprecipitated by anti-gD; 2, precursor polypeptide of glycoprotein gI immunoprecipitated by anti-gI; 3, precursor polypeptide of glycoprotein gE immunoprecipitated by anti-gE.
Failure to detect gD expression in MDV-infected cells by immunoprecipitation could result from failure of the antibody to recognize gD. To confirm that the anti-gD antibody is reactive with gD protein, an immunoprecipitation analysis was conducted with the gD, gI, and gE precursor polypeptides produced by in vitro-coupled transcription-translation and their respective antisera. While three antisera to different gD fragments were used (Fig. 1), only antibody against the gD fragment encoded by nucleotides 7501 to 8154 was able to react with the gD precursor polypeptide (Fig. 3B, negative data not shown). This antibody also recognizes the glycosylated form of gD (unpublished data). This anti-gD antiserum had been used in the previous immunoprecipitation analysis (Fig. 2).
Successful detection of the gD precursor polypeptide produced by in vitro-coupled transcription-translation demonstrated that the MDV gD gene encodes a complete gD protein. Therefore, the absence of detectable MDV gD expression in cell culture may be due to inefficient gD transcription, ineffective translation, or both.
Northern blot analysis of mRNA from MDV-infected cells using DNA probes.
Like other alphaherpesvirus homologs, the MDV gD (US6), gI (US7), and gE (US8) genes are clustered in that order within the unique short region of the viral genome, spanning nucleotides 6943 to 10960 (Fig. 1) (3). One poly(A) terminator sequence, AATAAA, is located upstream of US6 (gD), and another AATAAA sequence is located downstream of US8 (gE). There is no such sequence downstream of US6 (gD) or US7 (gI). In eucaryotic systems, the protein is most probably translated from the first favorable ATG of an mRNA. Therefore, for efficient expression of the MDV gD or gI genes, mRNA which starts just upstream of the gD gene or the gI gene should exist. However, if MDV gD expression is down-regulated at the transcription level, the gD-specific transcript may not be detectable.
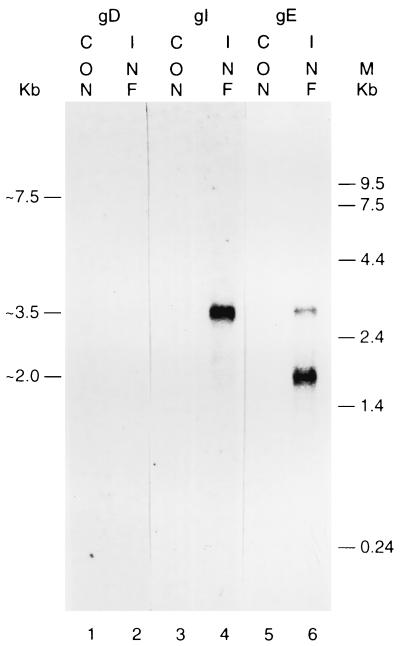
To characterize transcripts of the MDV gD, gI, and gE genes, Northern blot analysis was done with mRNA from MDV GA-infected DEF cells, with RNA from mock-infected DEF cells used as a negative control. Fragments of each of the genes, which had previously been used to make the TrpE fusion protein, were used to make double-stranded DNA probes for Northern blot analysis. The gD probe included nucleotides 7398 to 7797 from US6 (gD), the gI probe included nucleotides 8374 to 8804 from US7 (gI), and the gE probe included nucleotides 9773 to 10574 from US8 (gE) (Fig. 1).
The gD probe did not hybridize with any abundant mRNA (Fig. 4, lane 2), which may account for the failure to detect the MDV gD protein by immunoprecipitation. The gI probe hybridized with an abundant 3.5-kb mRNA that was also detected by the gE probe. In addition, the gE probe hybridized with another 2.0-kb transcript, which may be 3′-coterminal with the 3.5-kb transcript. When the blots were subjected to overexposure, a low-abundance large fragment of about 7.5 kb was barely detected by the gD, gI, and gE probes (data not shown). Thus, an explanation of the 7.5-kb fragment requires further study.
FIG. 4.
Northern blot analysis of mRNA from MDV-infected cells with DNA probes; 3-day exposure. Lanes 1, 3, and 5, uninfected DEF; 2, 4, and 6, MDV GA-infected DEF; 1 and 2; hybridized with an MDV gD-specific probe; 3 and 4, hybridized with an MDV gI-specific probe; 5 and 6, hybridized with an MDV gE-specific probe. The 7.5- to 8-kb band was of very low abundance and was seen only after a 10- to 12-day exposure (data not shown).
RT-PCR analysis of the MDV gD, gI, and gE transcripts.
To confirm the existence of a low-abundance polycistronic transcript that includes the gD gene, RT-PCR was performed with DNase I-treated total RNA from MDV-infected DEF cells. First-strand cDNA was synthesized in the reverse transcription reaction, and this was followed by PCR. In the control experiment, the same procedure was performed, except that no RT was added in the cDNA synthesis step. The primers from the gD gene are designated DS (D sense) and DA (D antisense), the primers from the gI gene are designated IS and IA, and the primers from the gE gene are designated ES and EA (Fig. 5A).
When gene-specific pairs of primers were used in the RT-PCR amplification, all of the gD, gI, and gE cDNA fragments were amplified to the sizes expected from their DNA sequences (Fig. 5B). Reaction with primers DS/DA produced a DNA fragment of about 550 bp; reaction with primers IS/IA produced a fragment of about 600 bp; and reaction with primers ES/EA produced a fragment of about 480 bp. Since no product was found in each control experiment (Fig. 5B, lanes (1, 3 and 5), the positive RT-PCR data did not come from carryover of viral DNA. This result indicated that there is a low-abundance transcript which includes the gD gene.
Instead of gene-specific primer pairs, primers from two different genes were used in pairs in the following RT-PCR amplification. When primer pair IS/EA, which spans the gI and gE genes, was used, a DNA fragment of about 1,700 bp was produced (Fig. 5C, lane 8). This result was consistent with a gI-gE bicistronic transcript. When primer pair DS/IA, spanning the gD and gI genes, was used, a DNA fragment of about 1,800 bp was produced. Further, when the DS/EA primer pair, spanning the gD, gI, and gE genes, was used, a DNA fragment of about 2,900 bp was produced. These results were consistent with the presence of polycistronic transcript including the gD, gI, and gE genes.
Northern blot analysis of mRNA from MDV-infected cells using riboprobes.
To further characterize the low-abundance large transcript, a more sensitive Northern blot analysis was performed with riboprobes and a larger amount of mRNA. MDV-infected CEF cells were used instead of DEF cells in this experiment to determine if the transcription pattern was different between these two cell culture systems.
In the MDV Us region, the SORF3 and US2 genes are leftward oriented while the US3, SORF4, gD, gI, and gE genes are rightward oriented (Fig. 1) (3). DNA fragments including each gene were cloned between the T7 and T3 promoters so that riboprobes could be synthesized. All of the riboprobes were generated as leftward oriented. Thus, they were in antiparallel to the US3, SORF4, gD, gI, and gE genes but parallel to the US2 and SORF3 genes. The US2 probe included nucleotides 3747 to 4837, the US3 probe included nucleotides 4865 to 6269, the SORF4 probe included nucleotides 6334 to 6922, the gD probe included nucleotides 6943 to 8157, the gI probe included nucleotides 8195 to 9354, and the gE probe included nucleotides 9443 to 10973.
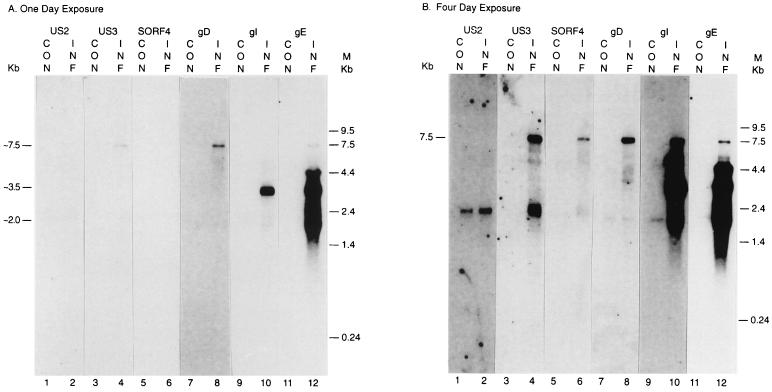
Because riboprobes are much more sensitive than DNA probes and because four times more mRNA was used in these experiments than in the previous Northern blot analysis with DNA probes, the low-abundance 7.5-kb transcript was now more readily detected and analyzed (Fig. 6A, lane 8). Comparing this Northern blot experiment with the previous one, both the gI-gE bicistronic 3.5-kb transcript and the gE-specific 2.0-kb transcript were detected (Fig. 4 and 6A), as expected. A less abundant gE-specific 4.4-kb transcript was also detected by this more sensitive method (Fig. 6B, lane 12). However, after 12 h of exposure the same blot clearly showed only the very abundant 3.5- and 2.0-kb transcripts (data not shown). The low-abundance species would appear to have little significance in the synthesis of gE. Thus, the transcription pattern is very similar between the MDV-infected -CEF cells and DEF cells.
FIG. 6.
Northern blot analysis of mRNA from MDV-infected cells using riboprobes. Lanes: 1, 3, 5, 9, and 11, uninfected CEF cells; 2, 4, 6, 8, 10, and 12, MDV GA-infected CEF cells; 1 and 2, hybridized with a riboprobe which is in the same orientation as the US2 gene; 3 and 4, hybridized with an MDV US3-specific riboprobe; 5 and 6, hybridized with an MDV SORF4-specific riboprobe; 7 and 8, hybridized with an MDV gD-specific riboprobe; 9 and 10, hybridized with an MDV gI-specific riboprobe; 11 and 12, hybridized with an MDV gE-specific riboprobe. (A) One-day exposure. (B) Four-day exposure.
After a fourfold-longer exposure, the 7.5-kb transcript was detected by the US3, SORF4, gD, gI, and gE riboprobes but not by the US2 probe (Fig. 6B). Therefore, the low-abundance large transcript detected by the gD probe does not initiate immediately 5′ of the gD gene but from two ORFs upstream of the gD gene. The 7.5-kb transcript is unlikely to be translated into the gD protein.
RNase protection analysis of the MDV gI and gE transcripts.
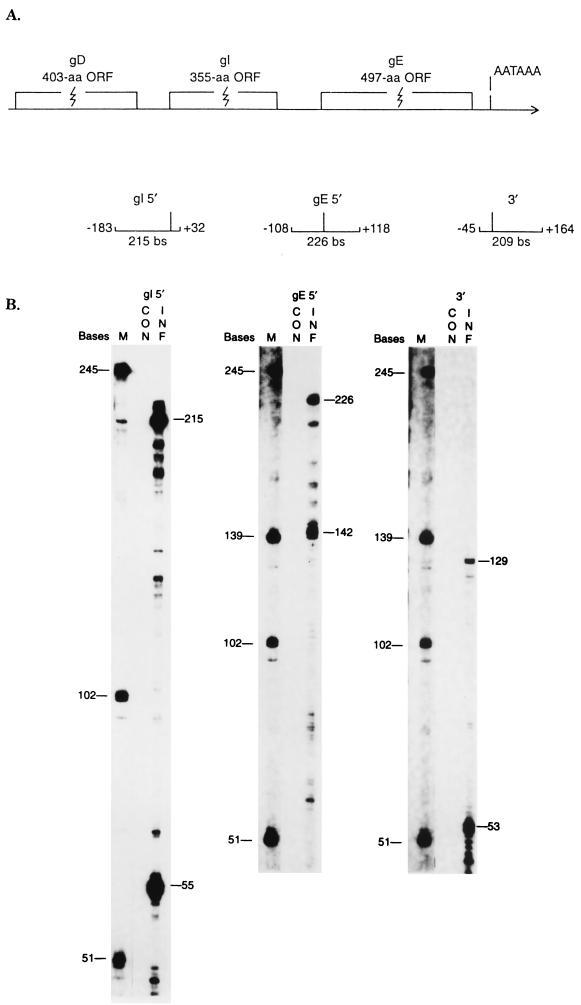
In contrast to the gD gene, the gI and gE genes are transcribed efficiently in MDV-infected cells. To confirm the lack of a gD promoter and to determine the promoter regions and 3′ termini of the gI and gE transcripts, RNase protection analysis was performed. A series of subgenomic fragments inclusive of ATG sites or AATAAA sequences were subcloned into pBCSK(+) plasmids. A series of riboprobes complementary to mRNA were generated (Fig. 7A), hybridized to RNA obtained from mock-infected and MDV-infected cells 72 h postinfection, digested with RNase, and analyzed on sequencing gels. The size of each fragment was calculated based on a comparison with the synthesized RNA marker. No gD gene-specific promoter region could be detected by this method (data not shown).
FIG. 7.
RNase protection assays of RNAs from MDV-infected cells. (A) Locations of probes used for RNase protection. The number adjacent to each probe indicates the distance (in nucleotides) away from the first nucleotide of each ORF. aa, amino acids. (B) CON, RNA from mock-infected DEF cells; INF, RNA from MDV GA-infected DEF cells; M, RNA markers.
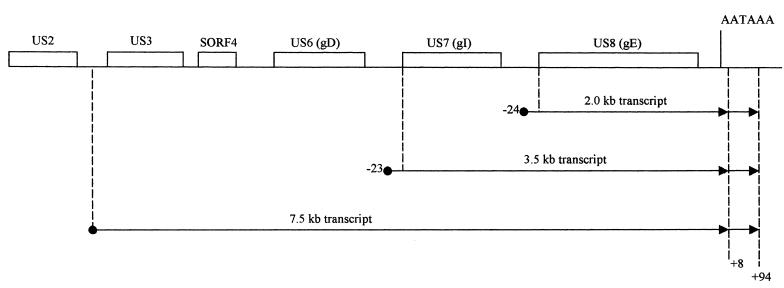
To determine the initiation site proximal to the gI gene, plasmid pBCB1 was constructed. This plasmid contains a 215-bp insert spanning sequences 183 bp upstream to 32 bp downstream of the gI ATG site (Fig. 7A). After digestion with EcoRI, this plasmid could be used to generate a 215-nucleotides antisense probe. RNase protection produced an abundant fragment of 55 nucleotides (Fig. 7B, gI 5′). The size of this product was consistent with an initiation site located 23 nucleotides upstream of the gI ATG site. The result suggests that this transcript is the source of the 3.5-kb gI-gE bicistronic mRNA (Fig. 8). Another, larger fragment (215 bp) was also protected and was probably due to protection from the low-abundance 7.5-kb transcript.
FIG. 8.
MDV transcript termini deduced from Northern blot analysis and RNase protection analysis. Dotted lines indicate the 5′ and 3′ ends of each transcript. There are two possible 3′ termini for each transcript.
To map the initiation site proximal to the gE gene, plasmid pBCKV1 was constructed. The plasmid contains a 226-bp insert spanning the gE ATG site. After digestion with BanI, this plasmid could be used to generate a 226-nucleotide antisense probe initiating 118 nucleotides within the gE gene and extending to 108 nucleotides upstream of the gE ATG codon (Fig. 7A). The full-length fragment derived from RNase protection might result from the 3.5-kb gI-gE bicistronic transcript and/or the low-abundance 7.5-kb polycistronic transcripts. In addition, a smaller fragment (142 nucleotides) was protected (Fig. 7B, gE 5′). The size of this fragment indicates a transcription initiation site 24 nucleotides upstream of the gE ATG site. The result suggests that the gE-specific transcript is the 2.0-kb mRNA (Fig. 8).
Because an AATAAA sequence is located downstream of the gE gene, but not of the gI gene, only one riboprobe was needed to map the 3′ termini of these transcripts. Plasmid pBCSm4, which contains a 209-bp insert spanning sequences 45 bp upstream to 164 bp downstream of the AATAAA sequence, was constructed (Fig. 7A). After digestion with EcoRI, this plasmid could be used to generate a 209-base antisense probe. The probe protected two fragments of 53 and 129 nucleotides (Fig. 7B, 3′). The 129-nucleotide fragment was consistent with transcripts terminating 94 nucleotides downstream of the AATAAA site. The 53-base fragment indicated a transcript terminus 8 nucleotides downstream of the AATAAA site (Fig. 8). It is likely that both the 3.5- and 2.0-kb transcripts can terminate at either site.
DISCUSSION
This study confirmed that MDV gD expression is below the limit of detection in MDV-infected cell culture and showed that this is because of inefficient gD gene-specific transcription. The only transcript clearly detected by the gD gene probe initiates two ORFs upstream of the gD gene. Research on HSV-1 has revealed that each viral gene has its own promoter and that each transcript contains a single functional ORF. Although some HSV-1 mRNAs contain additional protein-coding sequences downstream of their primary ORFs, proteins are not translated from these downstream regions. Instead, abundant proteins are translated from the mRNAs initiating immediately 5′ to their genes (22), consistent with the overwhelmingly monocistronic nature of eukaryotic genes. Thus, a priori, the possibility of significant gD gene translation from the 7.5-kb transcript is very unlikely.
HSV-1 gD gene transcription initiates about 85 nucleotides upstream of its ATG codon. Two TATA motifs are located 108 and 126 nucleotides 5′ to the ATG codon. Furthermore, DNA within the 168 nucleotides upstream is required for regulated expression of the HSV-1 gD gene, and the consensus ICP4 binding motif ATCGTC is found in this region (21). In the upstream region of the MDV gD gene, three TATA motifs are located at 27, 36, and 50 nucleotides 5′ to the ATG codon. No ICP4 binding motif is found there. A consensus poly(A) signal AATAAA sequence is located 60 nucleotides 5′ to the gD start codon, which is very close to the TATA motifs. The proximity of the AATAAA sequence may destabilize the transcription initiation complex formed on the TATA sequences and abort the transcription initiation from the gD gene.
MDV gI gene transcription is initiated 23 nucleotides 5′ to its ATG site, in contrast to its homolog, the HSV-1 gI transcript, which is initiated 91 nucleotides 5′ to its ATG site. Similarly, the MDV gE untranslated region is 24 bases long, only one-third the length of its HSV-1 homolog (73 bases). Considering that the region between MDV gD and gI genes and the region between the gI and gE genes are much shorter than the corresponding regions among the HSV-1 gD, gI, and gE genes, the relatively shorter untranslated regions of MDV gI and gE transcripts are expected.
Either the TATATA sequence or the TATAG sequence upstream of the gI gene CAP site may serve as the TATA box for transcription initiation. Similarly, the TATAT sequence, the TTTAAA sequence, or the TATAA sequence upstream of the gE initiation site may serve as the TATA box for gE transcription initiation. Since the 5′ termini of the gI and gE transcripts have been mapped by the RNase protection assay, their promoter regions have been identified and can be studied. The VZV gpIV (gI homolog) promoter has a very low basal level of transcription, but viral factors or virus-induced factors can significantly activate its promoter activity (19). Whether the MDV gI and gE promoters share similar features remains to be determined.
The 2.0- and 3.5-kb transcripts of gI and gE appear to coterminate at a typical AATAAA motif. It is very common among alphaherpesvirus transcription that one polyadenylation signal is shared by several genes, each initiating from its own promoter (17, 22). Two possible 3′ termini of the gI and gE transcripts were identified in the RNase protection assay. Heterogeneous 3′ ends have been described previously for the VZV gpIV (gI homolog) transcript (19). It is very likely that both the 2.0-kb and 3.5-kb transcripts can terminate at either site.
Because Fig. 6 is a composite made up from the development of 12 different films, those that had been fixed somewhat longer have a darker background (Fig. 6A lanes 7 and 8; Fig. 6B, lanes 1, 2, 9, and 10). Longer exposure of the Northern blot showed several very low abundance fragments smaller than the low-abundance 7.5-kb transcript (Fig. 6B, lanes 4, 6, and 8). These signals may represent nonspecifically hybridized RNA or may represent transcripts present in extremely low abundance. Translation from these uncharacterized transcripts and leaky scanning translation from the 7.5-kb transcript may be able to produce very few gD molecules. Nonetheless, even if these rare translations take place, the few gD molecules could play no significant role in MDV infection. This notion is supported by a study by Morgan's group in which an MDV gD lacZ insertion mutant was made which had similar growth kinetics to the wild-type MDV in both cell culture and chickens (1, 25).
Among the other alphaherpesviruses, HSV-1 has an essential gD gene; VZV, a highly cell-associated virus, lacks a gD homolog altogether; and PRV and BHV-1 are cell-free viruses, but their gD mutants become cell associated, although their infectivity is greatly impaired (12, 18, 28). In keeping with these observations, MDV, a highly cell-associated virus in cell culture, lacks detectable gD expression in cell culture. The FFE of MDV-infected chickens is the only known tissue producing cell-free virus, and whether MDV gD is expressed there is a very interesting question. To address this question, other groups have made monoclonal antibody and detected MDV-gD expression in a few feather follicles (24).
Whether the MDV gD expression phenomenon (perhaps limited only to a few FFE cells) is unique to gD or whether other MDV genes also exhibit this pattern needs to be characterized, and related mechanisms need to be further explored. Learning from research on HSV-1, we can assume that there are specific cellular receptors for MDV. However, since currently known HSV-1 receptors bind to gD, the receptors for MDV probably are of a different type or react with a different viral protein.
ACKNOWLEDGMENTS
This research was supported by the State of Michigan Research Excellence Fund, through Michigan State University's Center for Animal Production Enhancement, and by the Cooperative State Research Service, U.S. Department of Agriculture, under Agreement 90-37266-5589.
We thank Ruth A. Vrable for excellent technical assistance. The critical reading of the manuscript by Bill MacCarthur, Robert Silva, Masahiro Niikura, and Calvin Keeler is appreciated. X.T. also thanks guidance committee members Paul Coussens, Don Salter, Roger Maes, and Richard Schwartz for careful reviews of the manuscript and many helpful suggestions.
Footnotes
Dedicated to the memory of Leland F. Velicer, who passed away on 27 December 2000.
REFERENCES
- 1.Anderson A S, Parcells M S, Morgan R W. The glycoprotein D (US6) homolog is not essential for oncogenicity or horizontal transmission of Marek's disease virus. J Virol. 1998;72:2548–2553. doi: 10.1128/jvi.72.3.2548-2553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunovskis P, Chen X, Velicer L F. Proceedings of the 19th World's Poultry Congress. 1. 4th International Symposium on Marek's Disease. Wageningen, The Netherlands: Part Posen and Looijen; 1992. Analysis of Marek's disease virus glycoproteins D, I and E; pp. 118–122. [Google Scholar]
- 3.Brunovskis P, Velicer L F. The Marek's disease virus (MDV) unique short region: alphaherpesvirus-homologous, fowlpox virus-homologous, and MDV-specific genes. Virology. 1995;206:324–338. doi: 10.1016/s0042-6822(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 4.Burke R L. HSV vaccine development. In: Roizman B, Lopez C, editors. The herpesviruses. New York, N.Y: Raven Press; 1993. pp. 367–380. [Google Scholar]
- 5.Calnek B W, Witter R L. Marek's disease. In: Calnek B W, editor. Diseases of poultry. Ames: Iowa State University Press; 1991. pp. 342–385. [Google Scholar]
- 6.Card J P, Whealy M E, Robbins A K, Enquist L W. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J Virol. 1992;66:3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chase C C L, Carter-Allen K, Lohff C, Letchworth G., III Bovine cells expressing bovine herpesvirus 1 glycoprotein IV resist infection by BHV-1, herpes simplex virus, and pseudorabies virus. J Virol. 1990;64:4866–4872. doi: 10.1128/jvi.64.10.4866-4872.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Velicer L F. Expression of the Marek's disease virus homolog of herpes simplex virus glycoprotein B in Escherichia coli and its identification as B antigen. J Virol. 1992;66:4390–4398. doi: 10.1128/jvi.66.7.4390-4398.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui Z, You Y, Lee L F. Proceedings of the 19th World's Poultry Congress. 1. 4th International Symposium on Marek's Disease. Wageningen, The Netherlands: Part, Pasen and Luoijen; 1992. Identification and localization of the Marek's disease virus group-common antigen p79 gene; pp. 89–92. [Google Scholar]
- 10.Davison, A. J. (ed.). 1993. HSV and other alphaherpesviruses. Semin. Virol. 4.
- 11.Fuller A O, Lee W-C. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J Virol. 1992;66:5002–5012. doi: 10.1128/jvi.66.8.5002-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heffner S, Kovacs F, Klupp B G, Mettenleiter T C. Glycoprotein gp50-negative pseudorabies virus: a novel approach toward a nonspreading live herpesvirus vaccine. J Virol. 1993;67:1529–1537. doi: 10.1128/jvi.67.3.1529-1537.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isfort R J, Sithole I, Kung H, Velicer L F. Molecular characterization of Marek's disease herpesvirus B antigen. J Virol. 1986;59:411–419. doi: 10.1128/jvi.59.2.411-419.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson D C, Frame M C, Ligas M W, Cross A M, Stow N G. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988;62:1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson D C, Ligas M W. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J Virol. 1988;62:4605–4612. doi: 10.1128/jvi.62.12.4605-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson R M, Spear P G. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J Virol. 1989;63:819–827. doi: 10.1128/jvi.63.2.819-827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinchington P R, Vergnes J, Defechereux P, Piette J, Turse S E. Transcriptional mapping of the varicella-zoster virus regulatory genes encoding open reading frames 4 and 63. J Virol. 1994;68:3570–3581. doi: 10.1128/jvi.68.6.3570-3581.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang X, Pyne C, Li Y, Babiuk L A, Kowalski J. Delineation of the essential function of bovine herpesvirus 1 gD: an indication for the modulatory role of gD in virus entry. Virology. 1995;207:429–441. doi: 10.1006/viro.1995.1102. [DOI] [PubMed] [Google Scholar]
- 19.Ling P, Kimchington P R, Sadeghi-zadeh M, Ruyechan W T, Hay J. Transcription from varicella-zoster virus gene 67 (glycoprotein IV) J Virol. 1992;66:3690–3698. doi: 10.1128/jvi.66.6.3690-3698.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litwin V, Jackson W, Grose C. Receptor property of two varicella-zoster virus glycoproteins gpI and gpIV homologous to herpes simplex virus gE and gI. J Virol. 1992;66:3643–3651. doi: 10.1128/jvi.66.6.3643-3651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGeoch D J, Dolan A, Donald S, Rixon F J. Sequence determination and genetic content of the short unique region in the genome of HSV1. J Mol Biol. 1985;181:1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- 22.McGeoch D J. Correlation between HSV-1 DNA sequence and viral transcription maps. In: Wagner E K, editor. Herpesvirus transcription and its regulation. Boca Raton, Fla: CRC Press, Inc.; 1992. [Google Scholar]
- 23.Neidhardt H, Schroder C H, Kaerner H C. Herpes simplex virus type 1 glycoprotein E is not indispensable for viral infection. J Virol. 1987;61:600–603. doi: 10.1128/jvi.61.2.600-603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niikura M, Witter R L, Jang H-K, Ono M, Mikami T, Silva R F. MDV glycoprotein D is expressed in the feather follicle epithelium of infected chickens. Acta Virol. 1999;43:159–163. [PubMed] [Google Scholar]
- 25.Parcells M S, Anderson A S, Morgan R W. Characterization of a Marek's disease virus mutant containing a LacZ insertion in the US6 (gD) homolog gene. Virus Genes. 1994;9:5–13. doi: 10.1007/BF01703430. [DOI] [PubMed] [Google Scholar]
- 26.Peeters B, Pol J, Gielkens A, Moormann R. Envelope glycoprotein gp50 of pseudorabies virus is essential for virus entry but is not required for viral spread in mice. J Virol. 1993;67:170–177. doi: 10.1128/jvi.67.1.170-177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrovskis E A, Meyer A L, Post L E. Reduced yield of infectious pseudorabies virus and herpes simplex virus from cell lines producing viral glycoprotein gp50. J Virol. 1988;62:2196–2199. doi: 10.1128/jvi.62.6.2196-2199.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauh I, Mettenleiter T C. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol. 1991;65:5348–5356. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. New York, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]