Abstract
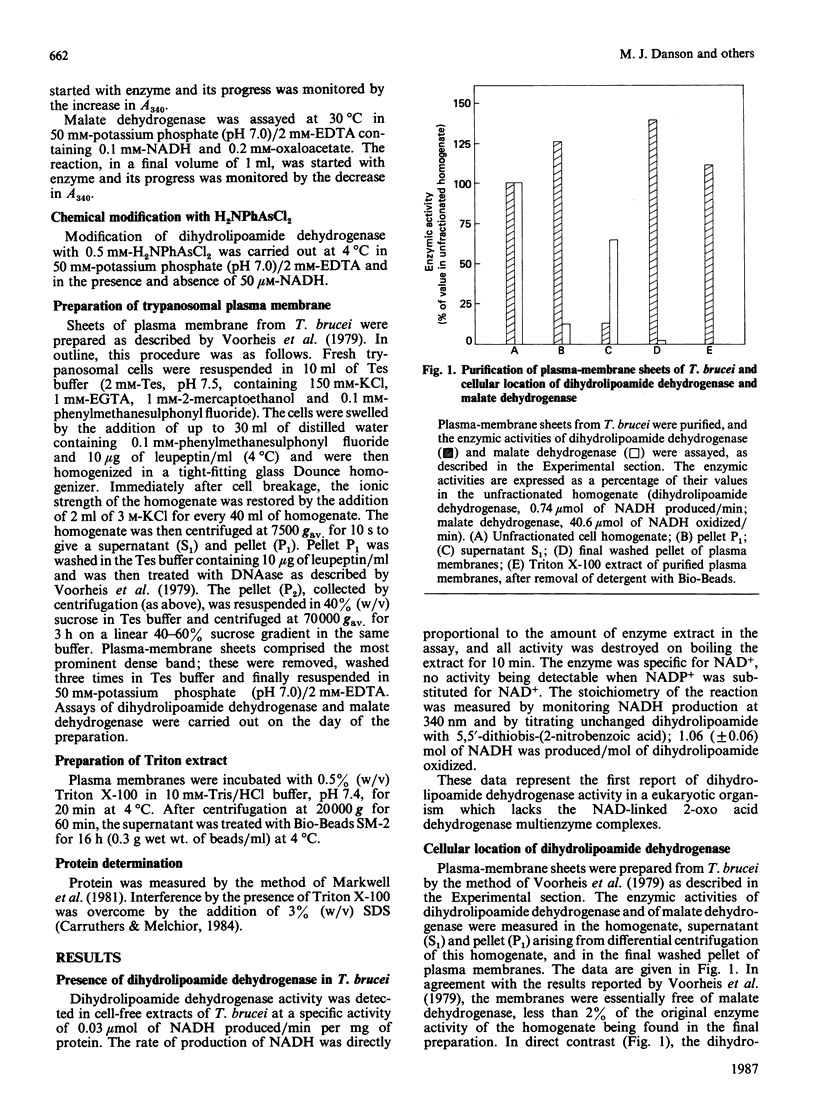
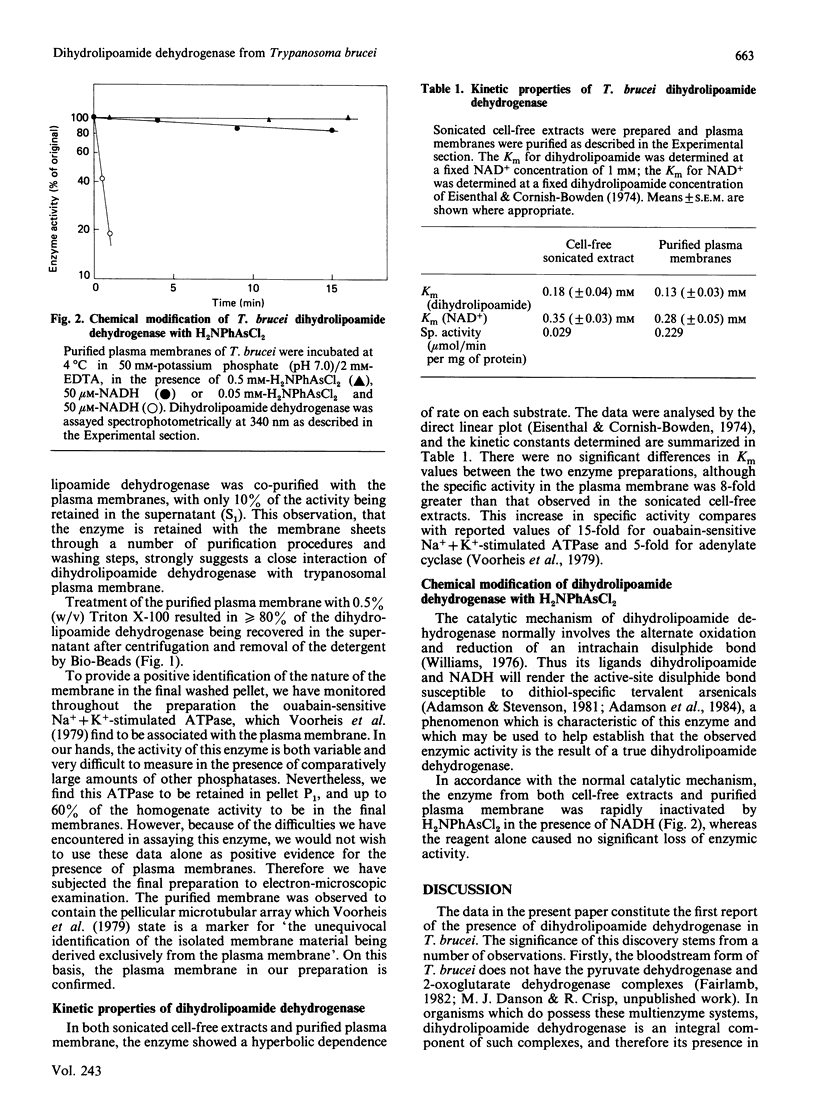
Dihydrolipoamide dehydrogenase has been discovered in the bloodstream form of the eukaryotic African parasite, Trypanosoma brucei. The enzyme catalysed the stoichiometric oxidation of dihydrolipoamide by NAD+ and exhibited a hyperbolic dependence of catalytic activity on the concentrations of both dihydrolipoamide and NAD+. Chemical modification with the tervalent arsenical reagent p-aminophenyldichloroarsine indicates the involvement in catalysis of a reversibly reducible disulphide bond. Plasma-membrane sheets were purified from T. brucei, and it was shown that virtually all the dihydrolipoamide dehydrogenase remained closely associated with this membrane preparation. T. brucei apparently lacks the 2-oxoacid dehydrogenase multienzyme complexes of which dihydrolipoamide dehydrogenase is usually an integral component. In the context of this absence, the possible function of trypanosomal dihydrolipoamide dehydrogenase is discussed, with particular reference to its cellular location in the plasma membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. R., Robinson J. A., Stevenson K. J. Inhibition of pyruvate dehydrogenase multienzyme complex from Escherichia coli with a radiolabeled bifunctional arsenoxide: evidence for an essential histidine residue at the active site of lipoamide dehydrogenase. Biochemistry. 1984 Mar 13;23(6):1269–1274. doi: 10.1021/bi00301a039. [DOI] [PubMed] [Google Scholar]
- Adamson S. R., Stevenson K. J. Inhibition of pyruvate dehydrogenase multienzyme complex from Escherichia coli with a bifunctional arsenoxide: selective inactivation of lipoamide dehydrogenase. Biochemistry. 1981 Jun 9;20(12):3418–3424. doi: 10.1021/bi00515a018. [DOI] [PubMed] [Google Scholar]
- Carruthers A., Melchior D. L. A rapid method of reconstituting human erythrocyte sugar transport proteins. Biochemistry. 1984 Jun 5;23(12):2712–2718. doi: 10.1021/bi00307a027. [DOI] [PubMed] [Google Scholar]
- Danson M. J., Eisenthal R., Hall S., Kessell S. R., Williams D. L. Dihydrolipoamide dehydrogenase from halophilic archaebacteria. Biochem J. 1984 Mar 15;218(3):811–818. doi: 10.1042/bj2180811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Panes A. The aerobic/anaerobic transition of glucose metabolism in Trypanosoma brucei. FEBS Lett. 1985 Feb 11;181(1):23–27. doi: 10.1016/0014-5793(85)81106-6. [DOI] [PubMed] [Google Scholar]
- Frost S. C., Lane M. D. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3-L1 adipocytes. J Biol Chem. 1985 Mar 10;260(5):2646–2652. [PubMed] [Google Scholar]
- Game S., Holman G., Eisenthal R. Sugar transport in Trypanosoma brucei: a suitable kinetic probe. FEBS Lett. 1986 Jan 1;194(1):126–130. doi: 10.1016/0014-5793(86)80063-1. [DOI] [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Tolbert N. E., Bieber L. L. Protein determination in membrane and lipoprotein samples: manual and automated procedures. Methods Enzymol. 1981;72:296–303. doi: 10.1016/s0076-6879(81)72018-4. [DOI] [PubMed] [Google Scholar]
- REED L. J., KOIKE M., LEVITCH M. E., LEACH F. R. Studies on the nature and reactions of protein-bound lipoic acid. J Biol Chem. 1958 May;232(1):143–158. [PubMed] [Google Scholar]
- RYLEY J. F. Studies on the metabolism of the protozoa. 9. Comparative metabolism of blood-stream and culture forms of Trypanosoma rhodesiense. Biochem J. 1962 Oct;85:211–223. doi: 10.1042/bj0850211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richarme G., Heine H. G. Galactose- and maltose-stimulated lipoamide dehydrogenase activities related to the binding-protein-dependent transport of galactose and maltose in toluenized cells of Escherichia coli. Eur J Biochem. 1986 Apr 15;156(2):399–405. doi: 10.1111/j.1432-1033.1986.tb09596.x. [DOI] [PubMed] [Google Scholar]
- Richarme G. Possible involvement of lipoic acid in binding protein-dependent transport systems in Escherichia coli. J Bacteriol. 1985 Apr;162(1):286–293. doi: 10.1128/jb.162.1.286-293.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson K. J., Hale G., Perham R. N. Inhibition of pyruvate dehydrogenase multienzyme complex from Escherichia coli with mono- and bifunctional arsenoxides. Biochemistry. 1978 May 30;17(11):2189–2192. doi: 10.1021/bi00604a026. [DOI] [PubMed] [Google Scholar]
- Voorheis H. P., Gale J. S., Owen M. J., Edwards W. The isolation and partial characterization of the plasma membrane from Trypanosoma brucei. Biochem J. 1979 Apr 15;180(1):11–24. doi: 10.1042/bj1800011. [DOI] [PMC free article] [PubMed] [Google Scholar]


