Abstract
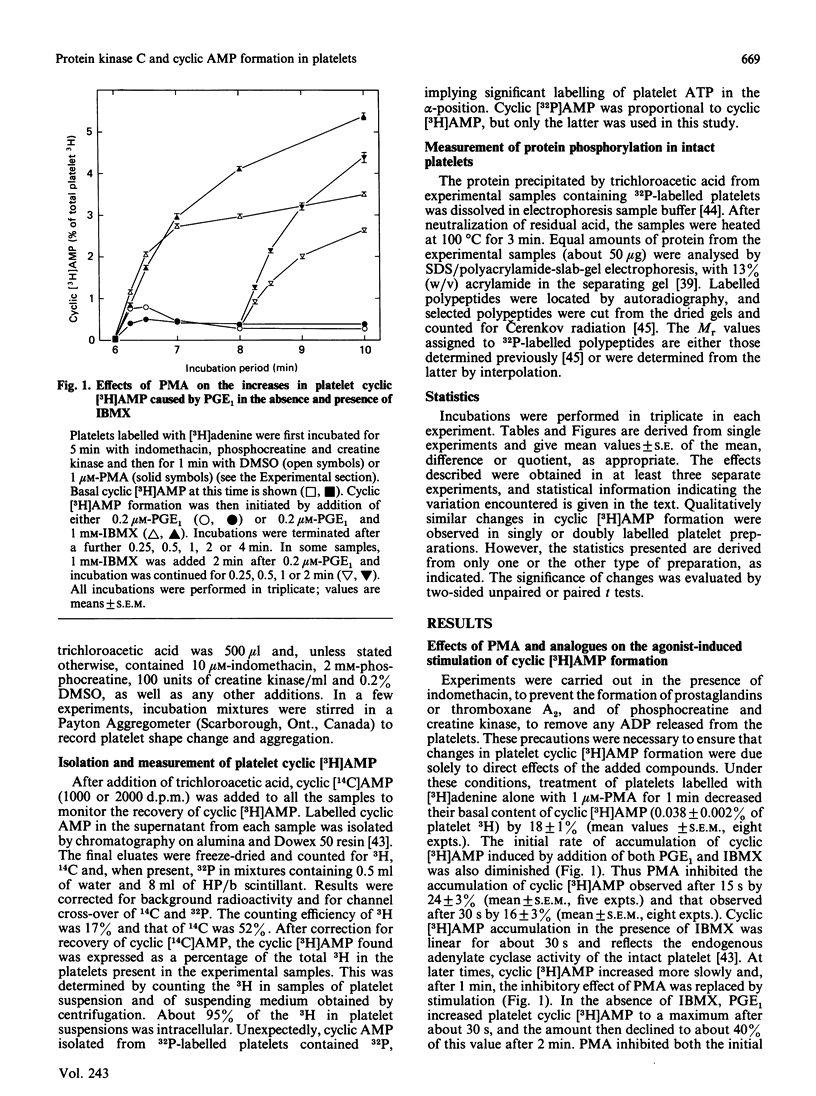
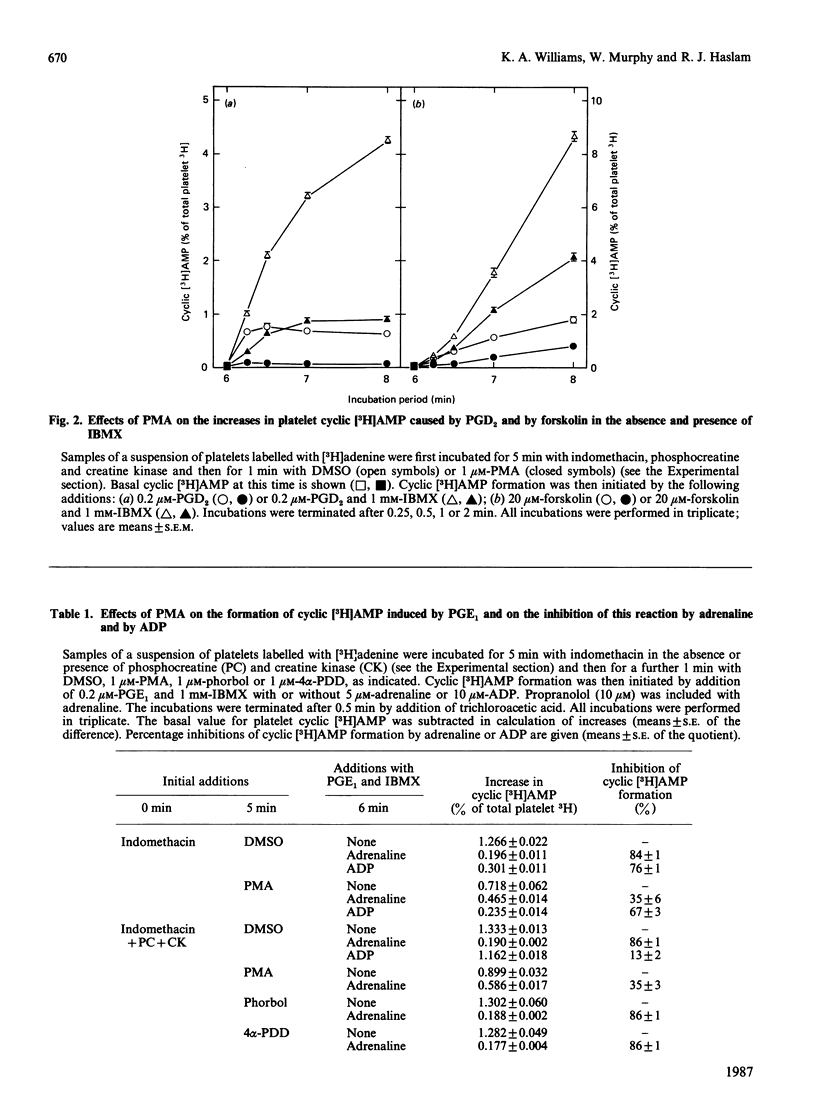
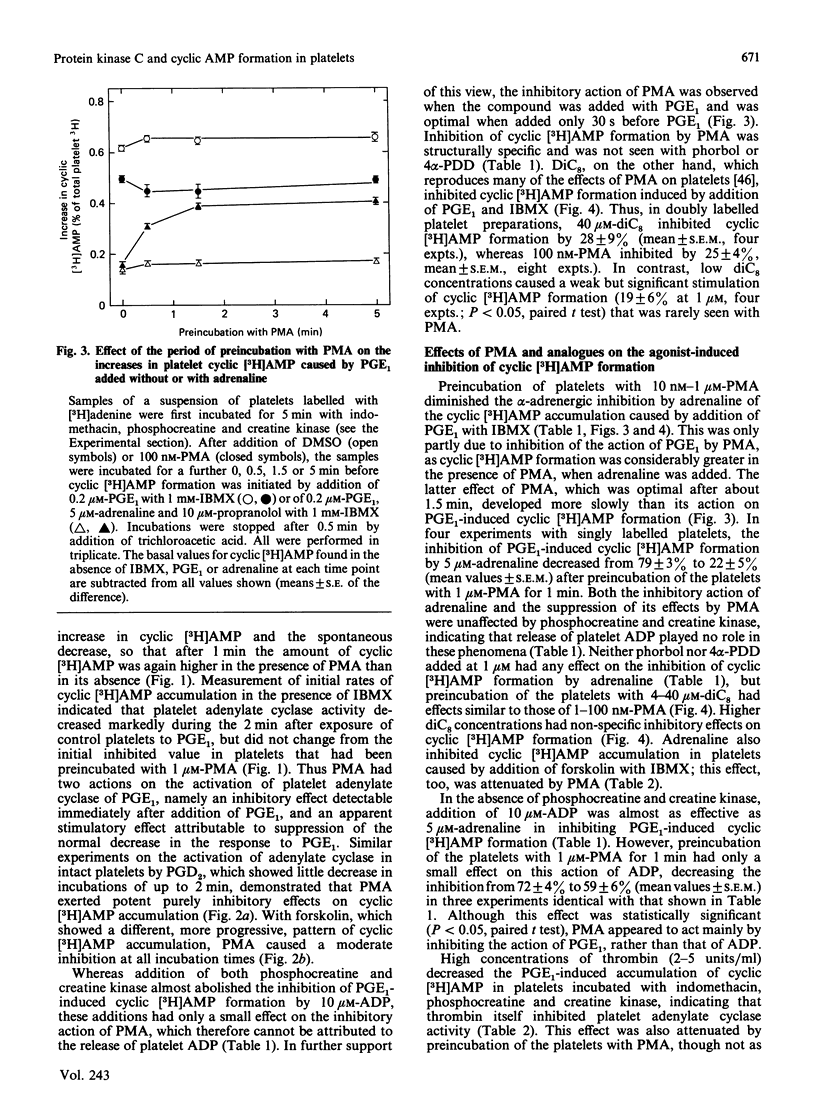
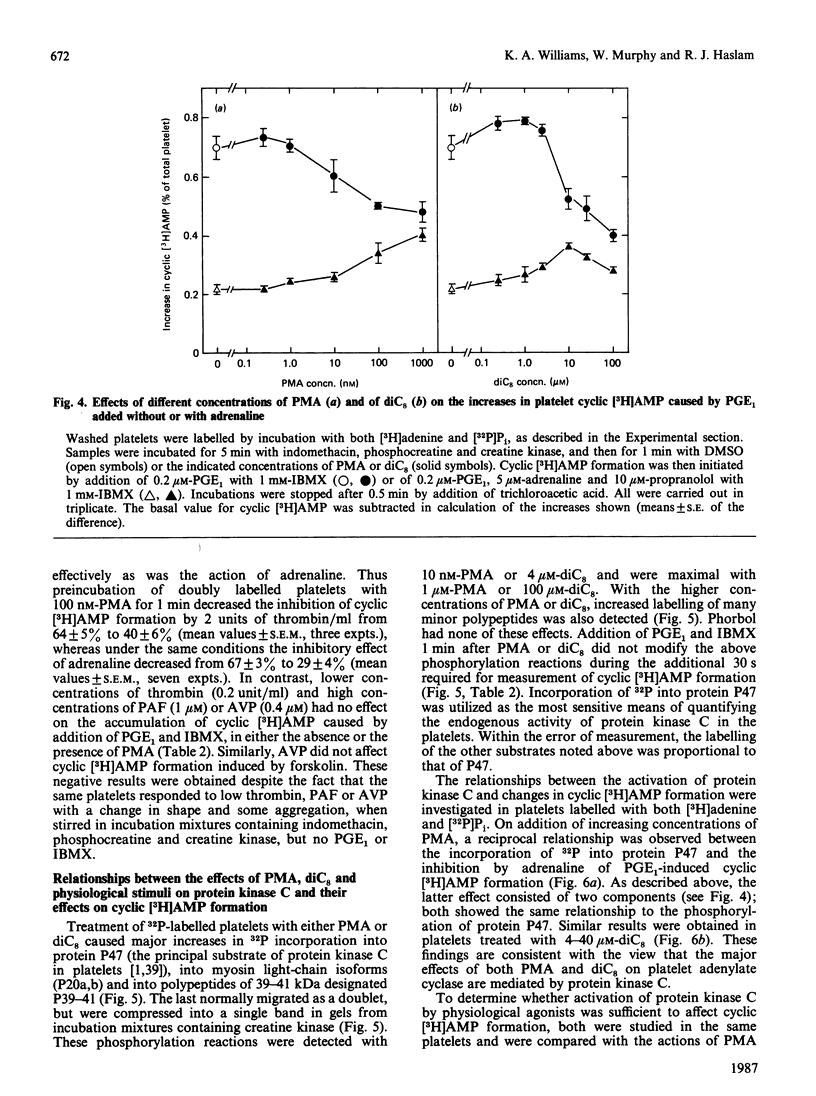
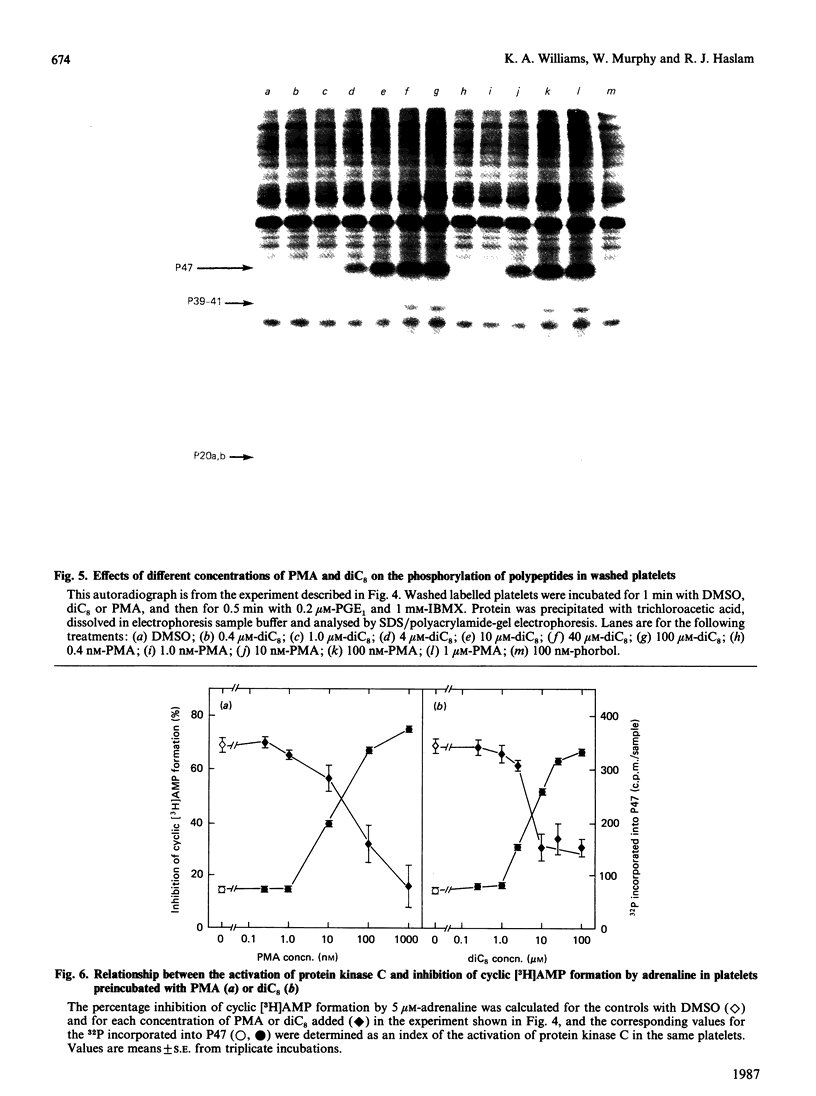
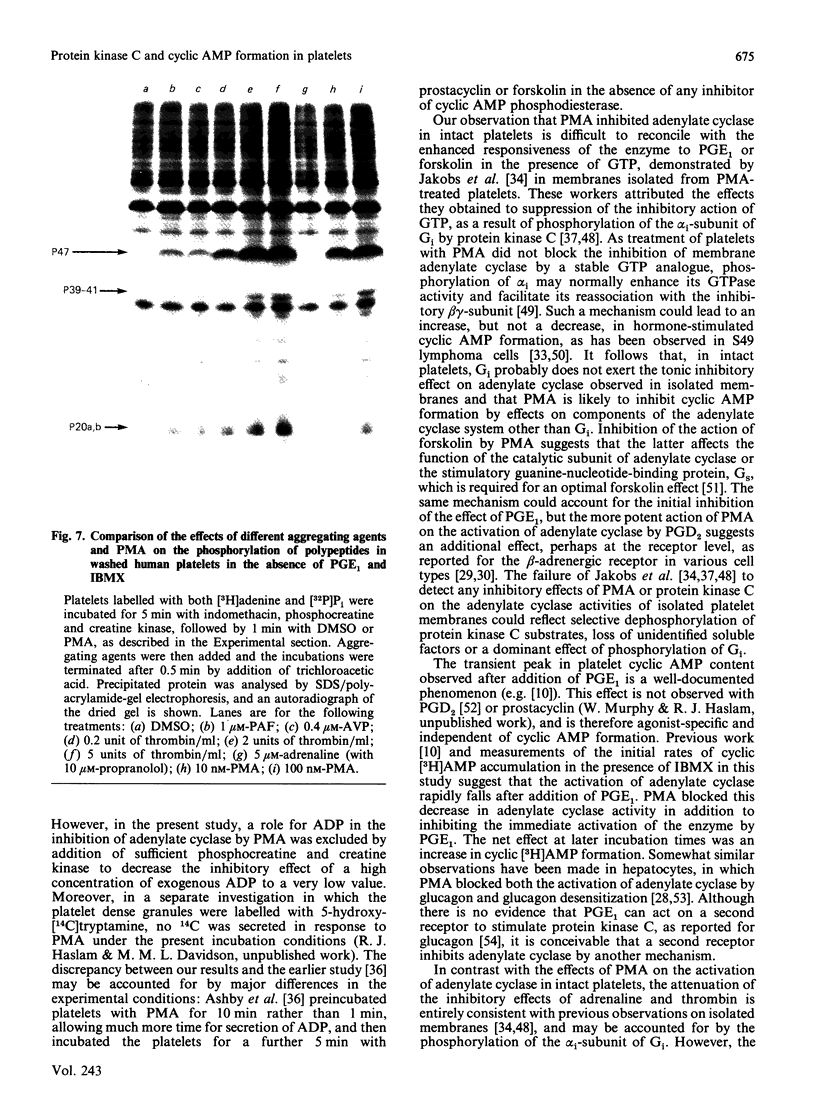
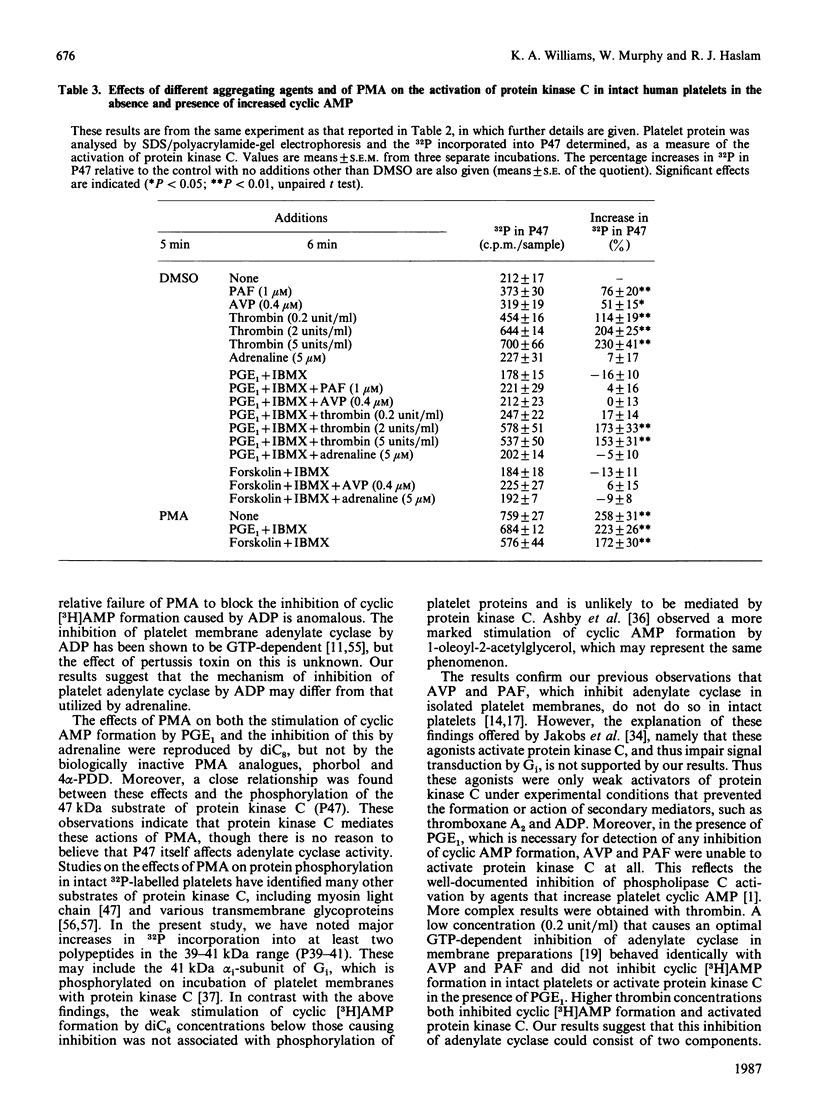
Jakobs, Bauer & Watanabe [(1985) Eur. J. Biochem. 151, 425-430] reported that treatment of platelets with phorbol 12-myristate 13-acetate (PMA) prevented GTP- and agonist-induced inhibition of adenylate cyclase in membranes from the platelets. This was attributed to the phosphorylation of the inhibitory guanine nucleotide-binding protein (Gi) by protein kinase C. In the present study, the effects of PMA on cyclic [3H]AMP formation and protein phosphorylation were studied in intact human platelets labelled with [3H]adenine and [32P]Pi. Incubation mixtures contained indomethacin to block prostaglandin synthesis, phosphocreatine and creatine kinase to remove ADP released from the platelets, and 3-isobutyl-1-methylxanthine to inhibit cyclic AMP phosphodiesterases. Under these conditions, PMA partially inhibited the initial formation of cyclic [3H]AMP induced by prostaglandin E1 (PGE1), but later enhanced cyclic [3H]AMP accumulation by blocking the slow decrease in activation of adenylate cyclase that follows addition of PGE1. PMA had more marked and exclusively inhibitory effects on cyclic [3H]AMP formation induced by prostaglandin D2 and also inhibited the action of forskolin. Adrenaline, high thrombin concentrations and, in the absence of phosphocreatine and creatine kinase, ADP inhibited cyclic [3H]AMP formation induced by PGE1. The actions of adrenaline and thrombin were attenuated by PMA, but that of ADP was little affected, suggesting differences in the mechanisms by which these agonists inhibit adenylate cyclase. sn-1,2-Dioctanoylglycerol (diC8) had effects similar to those of PMA. The actions of increasing concentrations of PMA or diC8 on the modulation of cyclic [3H]AMP formation by PGE1 or adrenaline correlated with intracellular protein kinase C activity, as determined by 32P incorporation into the 47 kDa substrate of the enzyme. Parallel increases in phosphorylation of 20 kDa and 39-41 kDa proteins were also observed. Platelet-activating factor, [Arg8]vasopressin and low thrombin concentrations, all of which inhibit adenylate cyclase in isolated platelet membranes, did not affect cyclic [3H]AMP formation in intact platelets. However, the activation of protein kinase C by these agonists was insufficient to account for their failure to inhibit cyclic [3H]AMP formation. Moreover, high thrombin concentrations simultaneously activated protein kinase C and inhibited cyclic [3H]AMP formation. The results show that, in the intact platelet, the predominant effects of activation of protein kinase C on adenylate cyclase activity are inhibitory, suggesting actions additional to inactivation of Gi.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTER R. H., JANDL J. H. PLATELET SEQUESTRATION IN MAN. I. METHODS. J Clin Invest. 1964 May;43:843–855. doi: 10.1172/JCI104970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktories K., Jakobs K. H. Ni-mediated inhibition of human platelet adenylate cyclase by thrombin. Eur J Biochem. 1984 Dec 3;145(2):333–338. doi: 10.1111/j.1432-1033.1984.tb08558.x. [DOI] [PubMed] [Google Scholar]
- Ashby B., Kowalska M. A., Wernick E., Rigmaiden M., Daniel J. L., Smith J. B. Differences in the mode of action of 1-oleoyl-2-acetyl-glycerol and phorbol ester in platelet activation. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(5):473–483. [PubMed] [Google Scholar]
- Avdonin P. V., Svitina-Ulitina I. V., Leytin V. L., Tkachuk V. A. Interaction of stable prostaglandin endoperoxide analogs U46619 and U44069 with human platelet membranes: coupling of receptors with high-affinity GTPase and adenylate cyclase. Thromb Res. 1985 Oct 1;40(1):101–112. doi: 10.1016/0049-3848(85)90354-8. [DOI] [PubMed] [Google Scholar]
- Bauer S., Jakobs K. H. Phorbol ester treatment impairs hormone- but not stable GTP analog-induced inhibition of adenylate cyclase. FEBS Lett. 1986 Mar 17;198(1):43–46. doi: 10.1016/0014-5793(86)81181-4. [DOI] [PubMed] [Google Scholar]
- Bell J. D., Brunton L. L. Enhancement of adenylate cyclase activity in S49 lymphoma cells by phorbol esters. Withdrawal of GTP-dependent inhibition. J Biol Chem. 1986 Sep 15;261(26):12036–12041. [PubMed] [Google Scholar]
- Bell J. D., Buxton I. L., Brunton L. L. Enhancement of adenylate cyclase activity in S49 lymphoma cells by phorbol esters. Putative effect of C kinase on alpha s-GTP-catalytic subunit interaction. J Biol Chem. 1985 Mar 10;260(5):2625–2628. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Best L. C., McGuire M. B., Martin T. J., Preston F. E., Russell R. G. Effects of epoxymethano analogues of prostaglandin endoperoxides on aggregation, on release of 5-hydroxytryptamine and on the metabolism of 3',5'-cyclic AMP and cyclic GMP in human platelets. Biochim Biophys Acta. 1979 Mar 22;583(3):344–351. doi: 10.1016/0304-4165(79)90458-6. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Walker G., Bourguignon G. J. Phorbol ester-induced phosphorylation of a transmembrane glycoprotein (GP 180) in human blood platelets. J Biol Chem. 1985 Sep 25;260(21):11775–11780. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cooper D. M., Rodbell M. ADP is a potent inhibitor of human platelet plasma membrane adenylate cyclase. Nature. 1979 Nov 29;282(5738):517–518. doi: 10.1038/282517a0. [DOI] [PubMed] [Google Scholar]
- Daly J. W. Forskolin, adenylate cyclase, and cell physiology: an overview. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:81–89. [PubMed] [Google Scholar]
- Feuerstein N., Monos D. S., Cooper H. L. Phorbol ester effect in platelets, lymphocytes, and leukemic cells (HL-60) is associated with enhanced phosphorylation of class I HLA antigens. Coprecipitation of myosin light chain. Biochem Biophys Res Commun. 1985 Jan 16;126(1):206–213. doi: 10.1016/0006-291x(85)90592-3. [DOI] [PubMed] [Google Scholar]
- Fisher G. J., Bakshian S., Baldassare J. J. Activation of human platelets by ADP causes a rapid rise in cytosolic free calcium without hydrolysis of phosphatidylinositol-4,5-bisphosphate. Biochem Biophys Res Commun. 1985 Jun 28;129(3):958–964. doi: 10.1016/0006-291x(85)91984-9. [DOI] [PubMed] [Google Scholar]
- Garte S. J., Belman S. Tumour promoter uncouples beta-adrenergic receptor from adenyl cyclase in mouse epidermis. Nature. 1980 Mar 13;284(5752):171–173. doi: 10.1038/284171a0. [DOI] [PubMed] [Google Scholar]
- Grandt R., Aktories K., Jakobs K. H. Evidence for two GTPases activated by thrombin in membranes of human platelets. Biochem J. 1986 Aug 1;237(3):669–674. doi: 10.1042/bj2370669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Agonists stimulate divalent cation channels in the plasma membrane of human platelets. FEBS Lett. 1985 Jul 8;186(2):175–179. doi: 10.1016/0014-5793(85)80703-1. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M., Desjardins J. V. Inhibition of adenylate cyclase by adenosine analogues in preparations of broken and intact human platelets. Evidence for the unidirectional control of platelet function by cyclic AMP. Biochem J. 1978 Oct 15;176(1):83–95. doi: 10.1042/bj1760083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M. Receptor-induced diacylglycerol formation in permeabilized platelets; possible role for a GTP-binding protein. J Recept Res. 1984;4(1-6):605–629. doi: 10.3109/10799898409042576. [DOI] [PubMed] [Google Scholar]
- Haslam R. J. Interactions of the pharmacological receptors of blood platelets with adenylate cyclase. Ser Haematol. 1973;6(3):333–350. [PubMed] [Google Scholar]
- Haslam R. J., Lynham J. A., Fox J. E. Effects of collagen, ionophore A23187 and prostaglandin E1 on the phosphorylation of specific proteins in blood platelets. Biochem J. 1979 Feb 15;178(2):397–406. doi: 10.1042/bj1780397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam R. J., McClenaghan M. D. Measurement of circulating prostacyclin. Nature. 1981 Jul 23;292(5821):364–366. doi: 10.1038/292364a0. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Vanderwel M. Inhibition of platelet adenylate cyclase by 1-O-alkyl-2-O-acetyl-sn-glyceryl-3-phosphorylcholine (platelet-activating factor). J Biol Chem. 1982 Jun 25;257(12):6879–6885. [PubMed] [Google Scholar]
- Haslam R. J., Williams K. A., Davidson M. M. Receptor-effector coupling in platelets: roles of guanine nucleotides. Adv Exp Med Biol. 1985;192:265–280. doi: 10.1007/978-1-4615-9442-0_19. [DOI] [PubMed] [Google Scholar]
- Heyworth C. M., Whetton A. D., Kinsella A. R., Houslay M. D. The phorbol ester, TPA inhibits glucagon-stimulated adenylate cyclase activity. FEBS Lett. 1984 May 7;170(1):38–42. doi: 10.1016/0014-5793(84)81364-2. [DOI] [PubMed] [Google Scholar]
- Heyworth C. M., Wilson S. P., Gawler D. J., Houslay M. D. The phorbol ester TPA prevents the expression of both glucagon desensitisation and the glucagon-mediated block of insulin stimulation of the peripheral plasma membrane cyclic AMP phosphodiesterase in rat hepatocytes. FEBS Lett. 1985 Aug 5;187(2):196–200. doi: 10.1016/0014-5793(85)81241-2. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Bojanic D., Wilson A. Platelet activating factor and U44069 stimulate a GTPase activity in human platelets which is distinct from the guanine nucleotide regulatory proteins, Ns and Ni. Biochem J. 1986 Mar 15;234(3):737–740. doi: 10.1042/bj2340737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. B., Lee C. S., Cheah M. J., Shen T. Y. Specific receptor sites for 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine (platelet activating factor) on rabbit platelet and guinea pig smooth muscle membranes. Biochemistry. 1983 Sep 27;22(20):4756–4763. doi: 10.1021/bi00289a022. [DOI] [PubMed] [Google Scholar]
- Imaoka T., Lynham J. A., Haslam R. J. Purification and characterization of the 47,000-dalton protein phosphorylated during degranulation of human platelets. J Biol Chem. 1983 Sep 25;258(18):11404–11414. [PubMed] [Google Scholar]
- Jakobs K. H., Bauer S., Watanabe Y. Modulation of adenylate cyclase of human platelets by phorbol ester. Impairment of the hormone-sensitive inhibitory pathway. Eur J Biochem. 1985 Sep 2;151(2):425–430. doi: 10.1111/j.1432-1033.1985.tb09119.x. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Saur W., Schultz G. Inhibition of platelet adenylate cyclase by epinephrine requires GTP. FEBS Lett. 1978 Jan 1;85(1):167–170. doi: 10.1016/0014-5793(78)81272-1. [DOI] [PubMed] [Google Scholar]
- Kassis S., Zaremba T., Patel J., Fishman P. H. Phorbol esters and beta-adrenergic agonists mediate desensitization of adenylate cyclase in rat glioma C6 cells by distinct mechanisms. J Biol Chem. 1985 Jul 25;260(15):8911–8917. [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Kelleher D. J., Pessin J. E., Ruoho A. E., Johnson G. L. Phorbol ester induces desensitization of adenylate cyclase and phosphorylation of the beta-adrenergic receptor in turkey erythrocytes. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4316–4320. doi: 10.1073/pnas.81.14.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G. Metabolism of inositides and the activation of platelets. Life Sci. 1983 May 2;32(18):2069–2082. doi: 10.1016/0024-3205(83)90094-2. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Reep B., Ganong B. R., Bell R. M. Exogenous sn-1,2-diacylglycerols containing saturated fatty acids function as bioregulators of protein kinase C in human platelets. J Biol Chem. 1985 Feb 10;260(3):1358–1361. [PubMed] [Google Scholar]
- MOLNAR J., LORAND L. Studies on apyrases. Arch Biochem Biophys. 1961 May;93:353–363. doi: 10.1016/0003-9861(61)90278-8. [DOI] [PubMed] [Google Scholar]
- MacIntyre D. E., McNicol A., Drummond A. H. Tumour-promoting phorbol esters inhibit agonist-induced phosphatidate formation and Ca2+ flux in human platelets. FEBS Lett. 1985 Jan 28;180(2):160–164. doi: 10.1016/0014-5793(85)81063-2. [DOI] [PubMed] [Google Scholar]
- McGowan E. B., Detwiler T. C. Modified platelet responses to thrombin. Evidence for two types of receptors or coupling mechanisms. J Biol Chem. 1986 Jan 15;261(2):739–746. [PubMed] [Google Scholar]
- Mellwig K. P., Jakobs K. H. Inhibition of platelet adenylate cyclase by ADP. Thromb Res. 1980 Apr 1;18(1-2):7–17. doi: 10.1016/0049-3848(80)90166-8. [DOI] [PubMed] [Google Scholar]
- Mills D. C., Macfarlane D. E. Stimulation of human platelet adenylate cyclase by prostaglandin D2. Thromb Res. 1974 Sep;5(3):401–412. doi: 10.1016/0049-3848(74)90176-5. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Ardlie N. G., Packham M. A. Preparation of suspensions of washed platelets from humans. Br J Haematol. 1972 Feb;22(2):193–204. doi: 10.1111/j.1365-2141.1972.tb08800.x. [DOI] [PubMed] [Google Scholar]
- Naka M., Nishikawa M., Adelstein R. S., Hidaka H. Phorbol ester-induced activation of human platelets is associated with protein kinase C phosphorylation of myosin light chains. Nature. 1983 Dec 1;306(5942):490–492. doi: 10.1038/306490a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Quilliam L. A., Dobson P. R., Brown B. L. Modulation of cyclic AMP accumulation in GH3 cells by a phorbol ester and thyroliberin. Biochem Biophys Res Commun. 1985 Jun 28;129(3):898–903. doi: 10.1016/0006-291x(85)91976-x. [DOI] [PubMed] [Google Scholar]
- Siess W., Weber P. C., Lapetina E. G. Activation of phospholipase C is dissociated from arachidonate metabolism during platelet shape change induced by thrombin or platelet-activating factor. Epinephrine does not induce phospholipase C activation or platelet shape change. J Biol Chem. 1984 Jul 10;259(13):8286–8292. [PubMed] [Google Scholar]
- Sugden D., Vanecek J., Klein D. C., Thomas T. P., Anderson W. B. Activation of protein kinase C potentiates isoprenaline-induced cyclic AMP accumulation in rat pinealocytes. 1985 Mar 28-Apr 3Nature. 314(6009):359–361. doi: 10.1038/314359a0. [DOI] [PubMed] [Google Scholar]
- Sweatt J. D., Blair I. A., Cragoe E. J., Limbird L. E. Inhibitors of Na+/H+ exchange block epinephrine- and ADP-induced stimulation of human platelet phospholipase C by blockade of arachidonic acid release at a prior step. J Biol Chem. 1986 Jul 5;261(19):8660–8666. [PubMed] [Google Scholar]
- Vanderwel M., Lum D. S., Haslam R. J. Vasopressin inhibits the adenylate cyclase activity of human platelet particulate fraction through V1-receptors. FEBS Lett. 1983 Dec 12;164(2):340–344. doi: 10.1016/0014-5793(83)80313-5. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Murphy G. J., Hruby V. J., Houslay M. D. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature. 1986 Sep 4;323(6083):68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Horn F., Bauer S., Jakobs K. H. Protein kinase C interferes with Ni-mediated inhibition of human platelet adenylate cyclase. FEBS Lett. 1985 Nov 11;192(1):23–27. doi: 10.1016/0014-5793(85)80035-1. [DOI] [PubMed] [Google Scholar]
- Watson S. P., Lapetina E. G. 1,2-Diacylglycerol and phorbol ester inhibit agonist-induced formation of inositol phosphates in human platelets: possible implications for negative feedback regulation of inositol phospholipid hydrolysis. Proc Natl Acad Sci U S A. 1985 May;82(9):2623–2626. doi: 10.1073/pnas.82.9.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. A., Haslam R. J. Effects of NaCl and GTP on the inhibition of platelet adenylate cyclase by 1-O-octadecyl-2-O-acetyl-sn-glyceryl-3-phosphorylcholine (synthetic platelet-activating factor). Biochim Biophys Acta. 1984 Mar 14;770(2):216–223. doi: 10.1016/0005-2736(84)90133-0. [DOI] [PubMed] [Google Scholar]
- Zavoico G. B., Halenda S. P., Sha'afi R. I., Feinstein M. B. Phorbol myristate acetate inhibits thrombin-stimulated Ca2+ mobilization and phosphatidylinositol 4,5-bisphosphate hydrolysis in human platelets. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3859–3862. doi: 10.1073/pnas.82.11.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. B., Troll W., Belman S. The tumor-promoter phorbol ester (12-O-tetradecanoyl-phorbol-13-acetate), a potent aggregating agent for blood platelets. J Cell Biol. 1974 Feb;60(2):325–336. doi: 10.1083/jcb.60.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]