Abstract
The floating freshwater fern Azolla is the only plant that retains an endocyanobiont, Nostoc azollae (aka Anabaena azollae), during its sexual and asexual reproduction. The increased interest in Azolla as a potential source of food and its unique evolutionary history have raised questions about its cyanotoxin content and genome. Cyanotoxins are potent toxins synthesized by cyanobacteria which have an anti-herbivore effect but have also been linked to neurodegenerative disorders including Alzheimer’s and Parkinson’s diseases, liver and kidney failure, muscle paralysis, and other severe health issues. In this study, we investigated 48 accessions of Azolla–Nostoc symbiosis for the presence of genes coding microcystin, nodularin, cylindrospermopsin and saxitoxin, and BLAST analysis for anatoxin-a. We also investigated the presence of the neurotoxin β-N-methylamino-L-alanine (BMAA) in Azolla and N. azollae through LC-MS/MS. The PCR amplification of saxitoxin, cylindrospermospin, microcystin, and nodularin genes showed that Azolla and its cyanobiont N. azollae do not have the genes to synthesize these cyanotoxins. Additionally, the matching of the anatoxin-a gene to the sequenced N. azollae genome does not indicate the presence of the anatoxin-a gene. The LC-MS/MS analysis showed that BMAA and its isomers AEG and DAB are absent from Azolla and Nostoc azollae. Azolla therefore has the potential to safely feed millions of people due to its rapid growth while free-floating on shallow fresh water without the need for nitrogen fertilizers.
Keywords: Azolla, Nostoc, Anabaena, symbiosis, BMAA, microcystins, nodularin, anatoxin-a, cylindrospermopsin, saxitoxin
1. Introduction
Azolla Lam. is the only plant with a permanent nitrogen-fixing cyanobacterial symbiont (cyanobiont) that has chains of cells (filaments) comprising photosynthetic vegetative cells and thicker-walled heterocysts that contain the nitrogen-fixing enzyme nitrogenase [1]. The cyanobiont has been assigned to both Anabaena azollae and Nostoc azollae because its morphology resembles free-living species of both genera, including their change into motile hormogonia and akinetes (resting cells) that ensure survival during stressed conditions [2].
Genetic and paleontological data indicate that the Azolla–N. azollae symbiosis originated 80 million years ago in North America following whole-genome duplication (WGD) that increased the genome of Azolla’s immediate ancestor [3]. Nostoc azollae’s subsequent coevolution with Azolla caused extensive changes in the cyanobiont’s genome compared to free-living species of Anabaena and Nostoc [3,4,5,6,7,8]. Some changes involved the upregulation of genes that enhanced N. azollae’s sequestration of atmospheric nitrogen and provision of nitrogen-based compounds to Azolla, increasing the plant’s speed of growth while free-floating on fresh water. The downregulation, loss, or conversion to pseudogenes of other genes changed N. azollae’s ancestors from independent free-living organisms into obligate endosymbionts, reflecting N. azollae’s permanent location inside the leaves and female megasporocarps of Azolla. These included genes that previously expressed proteins involved in the synthesis of carotenoid and chlorophyll pigments, so that A. azollae is reliant on Azolla’s cellular pigments for protection against photooxidative damage [6].
Colonies of N. azollae live in specialized cavities in Azolla’s dorsal floating leaves, providing nitrogen-based nutrients to the plant that enable it to double its biomass in less than two days while free-floating on fresh water [9,10]. As a result, Azolla has been used for hundreds of years in India and the Far East as a nitrogen biofertilizer for paddy rice, reducing mosquito breeding populations by 95% [11,12,13] and emissions of the potent greenhouse gas methane from paddies by 25–50% [14,15,16]. Azolla also provides livestock feed, biofuel and biofertilizer for other plants, alleviating shortages of the ‘three Fs’ that increasingly threaten food supplies globally: feed, fuel, and fertilizer. It absorbs and removes phosphates and nitrates from water contaminated by chemical fertilizers, industrial pollutants, and animal and human waste that trigger toxic cyanobacterial (aka blue-green algal) blooms in rivers and lakes. The symbionts’ combined CO2 sequestration also increases Azolla’s carbon capture so that it can sequester large amounts of atmospheric CO2, with the plants being compressed and stored to reduce anthropogenic climate change through carbon capture and storage (CCS). Azolla can, therefore, mitigate many of the threats arising from the ‘perfect storm’, as our population increases by more than a million every three days. Its remarkable properties are increasingly recognised, and one study has designated it a unique superorganism [1].
Azolla has the potential to help feed millions of people because of its rapid growth, ease of outdoor cultivation in tropical and temperate regions, and global production using the indoor Azolla biosystem described by Bujak & Bujak (2020) in ‘The Azolla Story’ [2]. The use of Azolla for human consumption was thought to be limited by its high total polyphenolic content (TPC), but Winstead et al. (2024) [17] showed that the TPC of raw Azolla caroliniana, which is native in the eastern United States, has only 4.26 g gallic acid equivalent (GAE) kg−1 DW and that simple cooking methods can decrease TPC in all Azolla species. They also demonstrated that its protein content is 19% DW and its apparent protein digestibility is 78.45%, with a yield of 173 g FW m−2 day−1 and 5.53 g DW m−2 day−1, confirming Azolla’s potential for cultivation and domestication as a nutritious food. This raises the question as to whether Azolla is safe to eat because of the presence of harmful cyanotoxins in many cyanobacteria.
Cyanotoxins are produced by cyanobacteria of the genera Anabaena and Nostoc among others and include some of the most powerful natural poisons that target the nervous system (neurotoxins such as BMAA, saxitoxin, and anatoxin-a), the liver (hepatotoxins such as microcystins and nodularins), protein synthesis and DNA modification (cylindrospermopsin), and the skin (dermatoxins, such as nodularins). Cyanotoxins are alkaloids (anatoxin-a, saxitoxin, cylindrospermopsin) or peptides (pentapeptide nodularin or the heptapeptide microcystin) [18,19,20,21]. Upon their release in water, they are ingested by zooplankton and animals or absorbed by phytoplankton and plants, which can have acute or chronic effects when eaten by humans. This is a global health issue, owing to bioconcentration and bioaccumulation in the food chain and poisoning through ingestion of contaminated food, so cyanotoxins are now widely analyzed and studied to determine their effects on plants and animals [22]. For example, the World Health Organization (WHO) recommends a value of 1 μg/L for microcystin-LR in drinking water [23].
BMAA (β-N-methylamino-L-alanine) is a non-proteinogenic amino acid produced by free-living cyanobacteria in marine, freshwater, and terrestrial environments [24,25]. It has been detected in plants with endosymbiotic cyanobacteria including lichens, hornworts, the leaf petioles of the tropical flowering plant Gunnera, and the cycad Cycas circinalis [24,25,26] and linked to the amyotrophic lateral sclerosis/Parkinson–dementia complex (ALS/PDC) detected among Chamorro people living on the Pacific island of Guam [24,25]. BMAA, like other cyanotoxins, can be biomagnified in seafood eaten by people, including fish [27,28], shrimps [29], mussels, oysters, and crabs [30]. BMAA can also be synthetized by eukaryotes such as diatoms [31] and dinoflagellates [32], which are food sources for crustaceans, fish and shellfish [33]. However, the genes related to the BMAA biosynthetic pathway are not known.
These observations raise the question as to whether eating Azolla may be harmful to humans due to the possible production of BMAA and other cyanotoxins by N. azollae. Unlike free-living Anabaena and Nostoc, the loss or conversion to pseudogenes of genes involved in cyanotoxin and/or BMAA production may have occurred in N. azollae because they were no longer needed by the permanently enclosed cyanobiont. The following analyses were therefore undertaken on all seven extant Azolla species and their cyanobionts to determine if they can be safely eaten by people.
The presence of genes coding for microcystin, nodularin, cylindrospermopsin, and saxitoxin.
The presence of the anatoxin-a/homoanatoxin-a gene cluster by bioinformatic tools.
The presence of BMAA.
The seven examined species of Azolla are A. caroliniana, A. filiculoides, A. mexicana, A. microphylla, A. nilotica, A. rubra and A. pinnata, including its two subspecies A. pinnata subsp. pinnata and A. pinnata subsp. imbricata. Table 1 lists the 48 accessions that provided the Azolla species and subspecies used in this study.
Table 1.
List of Azolla accessions from countries worldwide.
| Accession a | Species Name | Origin and Harvest Year | Source b/ Collector |
|---|---|---|---|
| PI1 *,$ | A. pinnata subsp. imbricata | Philippines, Sto Domingo, Albay, 1975 | IRRI |
| PI2 | Malaysia, Bumbong Lima, Butterworth, 1977 | IRRI | |
| PI23 | India, Cuttack, Orissa, 1978 | CRRI | |
| PI68 | Sri Lanka, Tissa, 1984 | S. Kulasooriya | |
| PI102 | Japan, Okinawa, 1987 | O. Mochida | |
| PI503 | Australia, Murdoch, 1978 | M. Dilworth | |
| PI531 | Indonesia, Bali, 1983 | - | |
| PI540 | China, Putian, 1989 | C. van Hove | |
| FI1001 * | A. filiculoides | East Germany (ex-GDR), 1979 | IB China |
| FI1008 | USA, Cranmore Road, Sutter Co., California, 1981 | D. Rains | |
| FI1010 | Peru, PUFFI, Lima, 1982 | CIAT | |
| FI1042 | Brazil, Parana, 1987 | I. Watanabe | |
| FI1052 | South of France, North of Lyon, 1989 | P. Roger | |
| FI1090 | Japan, Tanabe-cho, 1992 | S. Kitoh | |
| FI1501 | Belgian, Harchies, 1987 | A. Lawalree | |
| FI1505 | South Africa, Verwoerd dam, 1987 | D. Toerien | |
| FI1507 $ | Colombia, Zipaquira, 1987 | Y. Lopez | |
| FI1522 | Switzerland, Zurich Botanical Garden, 1987 | - | |
| FI-BGLU | Botanical Garden of Lisbon University, 2009 | A.L. Pereira | |
| FI-BGM | Botanical Garden of Madeira, Funchal, 2010 | C. Lobo | |
| ME2001 * | A. mexicana | USA, Graylodge, California, 1978 | D. Rains |
| ME2008 | Colombia, CIAT, Cali, 1982 | CIAT | |
| ME2011 | Japan, Osaka, 1984 | T. Lumpkin | |
| ME2026 $ | Brazil, Solimoes river, Pacencia Island, Iranduba, Amazonas (BLCC 18), 1984 | T. Lumpkin | |
| CA3001 *,$ | A. caroliniana | USA, Ohio, 1978 | D. Rains |
| CA3017 | Brazil, Rio Grande do Sul, 1987 | I. Watanabe | |
| CA3502 | Egypt, Moshtohr University, 1987 | C. Myttenaere | |
| CA3507 | Suriname, Boxel, 1987 | H. Lardinois | |
| CA3513 | Zimbabwe, Causeway Botanical Garden, 1987 | T. Muller | |
| CA3524 | Holland, 1987 | E. Ohoto | |
| CA3525 | Ruanda, Cyili Rice Research Center, 1987 | C. van Hove | |
| MI4018 * | A. microphylla | Paraguay, 1981 | D. Rains |
| MI4021 $ | Equator, Santa Cruz Island, Galapagos, 1982 | T. Lumpkin | |
| MI4028 | Philippines, hybrid (MI4018xFI1001), 1985 | Do Van Cat | |
| MI4054 | Brazil, Baía, 1987 | I. Watanabe | |
| MI4510 | Philippines, Los Baños, IRRI, 1987 | C. van Hove | |
| NI5001 *,$ | A. nilotica | Sudan, Kosti, 1982 | T. Lumpkin |
| NI5002 # | Sudan, Kosti, 1989 | T. Lumpkin | |
| NI5501 | Burundi, Bujumbura, 1987 | J. Bouharmont | |
| RU6010 * | A. rubra | New Zealand, Nouville, 1986 | C. van Hove |
| RU6502 | Australia, Victoria (37.40 S–144.40 E), 1985 | - | |
| RU6503 | New Zealand, between Lumdsen and Kingston, 1986 | C. van Hove | |
| PP7001 *,$ | A. pinnata subsp. pinnata | Australia, Kakadu Northern Park Northern Territory, 1982 | Yatazawa |
| PP7506 | Sierra Leone, 1982 | C Dixon | |
| PP7509 | Nigeria, Moor plantation, 987 | C. van Hove | |
| PP7511 | Guinea-Bissau, Contuboel, 1987 | H. Diara | |
| PP7512 | Zaire, Kisantu, 1987 | B. Bruyneel | |
| PP7546 | Madagascar, Antsahavory, East zone, 1991 | C. van Hove |
a Accession numbers are listed according to IRRI code number except for Portuguese accessions (FI-BGLU and FI-BGM) from an unknown collector or germplasm source. b CIAT: International Centre for Tropical Agriculture, Colombia; CRRI: Cyili Rice Research Center; IB China: Institute of Botany, Academia Sinica, Beijing, China; IRRI: International Rice Research Institute. # N. azollae was not isolated from this Azolla accession. * BMAA extracted from Azolla and Nostoc azollae (isolated from Azolla, see Section 4.2) with method 1 (see Section 4.3.1). $ BMAA extracted from Azolla accessions with method 2 (see Section 4.3.2).
2. Results
2.1. The Cyanotoxins Microcystin, Nodularin, Saxitoxin, Cylindrospermopsin and Anatoxin-a
The PCR amplification of 12 genes that encode for 4 cyanotoxins (cylindrospermopsin, nodularin, saxitoxin and microcystin) was determined for all 7 Azolla species and 2 A. pinnata subspecies from 48 Azolla accessions listed in Table 1. The global distribution of the accessions’ countries of origin is shown in Supplementary Material Figure S25. The results indicate that cyanotoxin genes amplified on Azolla accessions were negative when matched with positive and negative controls for 12 genes: cyl, mcy A, mcy B, mcy B domain A, mcy C, mcy C domain A, mcy D domain ACP, mcy D domain KS, mcyE/ndaF, mcy E domain GSA-AMT, mcy G domain CM, and sxt.
The results also showed that same 12 genes in N. azollae isolated from the 47 Azolla accessions were not amplified compared with positive and negative controls. This indicates that both Azolla and N. azollae do not have genes that biosynthesize those cyanotoxins. Photographs of all gels from the PCR amplifications are shown in Supplementary Material Figures S1–S24.
The BLAST search for the anatoxin-a and homoanatoxin-a gene cluster showed a query cover of only 3% and percentage identity of 73.04%. Most of these alignments were partial segments comprising less than 250 bp of the anaH transposase gene with identities around 70%. The aligned genes in N. azollae are only associated with pseudogenes and not with protein coding genes. All other alignments were partial, and none included any of the whole genes associated with the anatoxin-a biosynthesis gene cluster.
2.2. Detection of BMAA (β-N-Methylamino-L-Alanine)
The detection of the non-proteinogenic amino acid BMAA by LC-MS/MS with method 1 on all seven Azolla species (A. caroliniana, A. filiculoides, A. microphylla, A. mexicana, A. nilotica, A. rubra and the two subspecies of A. pinnata) showed that BMAA was absent from both Azolla and its cyanobiont N. azollae, since the retention time for BMAA and the isomer 2,4-DAB (2,4-diaminobutyric acid) could not be found when compared with their standards.
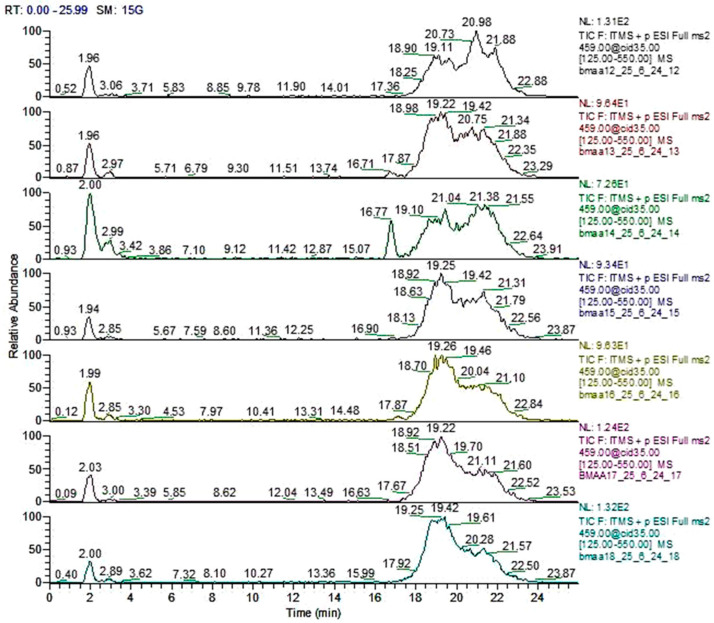
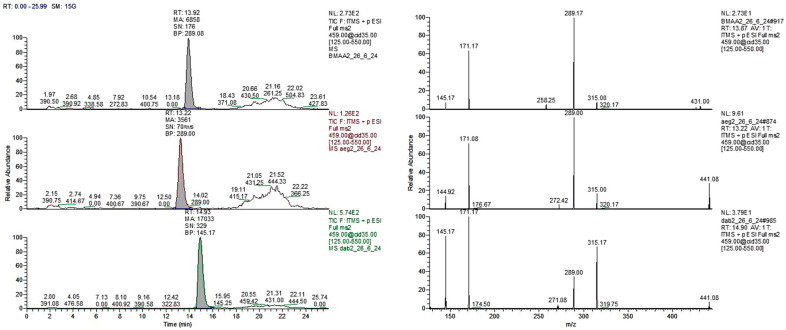
Re-analysis of six of the Azolla species (excluding A. rubra, which was not re-analyzed) using method 2, in which the samples were derivatized, corroborated the results obtained with method 1. This indicates that the Azolla species shown in Figure 1 did not show the retention times for BMAA (RT = 13.92 min) and the two isomers 2,4-DAB (RT = 14.93 min) and AEG (N-(2-aminoethyl)-glycine) (RT = 13.22 min) when compared with their standards (Figure 2).
Figure 1.
Total ion chromatogram of six Azolla species for the detection of BMAA with method 2. From top to bottom: A. caroliniana (CA 3001), A. filiculoides (FI 1507), A. pinnata subsp. pinnata (PP 7001), A. nilotica (NI 5001), A. mexicana (ME 2026), A. microphylla (MI 4021), A. pinnata subsp. imbricata (PI 1).
Figure 2.
Total ion chromatogram (left) and CID spectra (right) of derivatized standards at 1 ppm. BMAA (top, RT = 13.92 min), AEG (middle, RT = 13.22 min), and 2,4-DAB (bottom, RT = 14.93 min).
3. Discussion
Most genera of free-living cyanobacteria synthesize cyanotoxins, but their expression depends on environmental factors such as nutrients, light and temperature [22]. Some cyanobacteria also have temporary symbioses with plants, so that the host plant has the potential to assimilate and bioaccumulate the cyanotoxins, discussed above. There are few published studies documenting genetic and chromatographic detection of cyanotoxins in cyanobionts. Cyanobacteria from lichens have been analysed and contain genes that encode nodularin and microcystin and can translate the peptides nodularin and microcystins [34,35,36]. The synthesis of those two cyanotoxins may be linked to the temperature and humidity in which the lichens grow and may be important for the maintenance of lichens in diverse ecological habitats [37].
Unlike lichens, the fern Azolla has a permanent symbiosis with the cyanobacteria N. azollae, giving this symbiosis a unique evolution pattern and, ultimately, the loss of genes by the cyanobiont [4,5,6,7]. Our genetic analyses show, for or the first time, that all seven Azolla species and their cyanobiont, N. azollae, do not possess genes associated with the synthesis of microcystin, nodularin, saxitoxin, cylindrospermopsin, anatoxin-a, and homoanatoxin-a. The biosynthetic pathways of microcystins [38] and anatoxin-a/homoanatoxin-a [39] are a multi-step process that requires several genes to synthesize both cyanotoxins. All genes associated with microcystin synthesis were, therefore, amplified by specific primers with Azolla and N. azollae DNA, and the complete anatoxin-a gene cluster was BLAST-searched against the N. azollae genome. There were no matches, thereby supporting the hypothesis that N. azollae lost the ability to synthesise microcystin, nodularin, saxitoxin, cylindrospermopsin, anatoxin-a, and homoanatoxin-a due to downregulation of cyanotoxin biosynthesis genes or loss of the genes during the co-evolution of Azolla and N. azollae.
BMAA was isolated in 1967 from seeds of Cycas circinalis (cycad) [24,25,26] and identified as the primary cause of amyotrophic lateral sclerosis/Parkinson–dementia complex (ALS/PDC) in the Chamorro people on the Pacific island of Guam [40,41], with the high levels of the neurotoxin resulting from biomagnification through the food chain [27,28,33,42,43]. BMAA is a cyanotoxin that can cross the blood–brain barrier where it forms a reservoir [44]) and can be inserted into proteins instead of the amino acid L-serine, causing protein misfolding and aggregation [45,46,47]. BMAA can induce changes in the expression of genes in brain cells, thus resulting in a wide range of other neurodegenerative disorders [19]. Alzheimer’s, Parkinson’s and other neurological diseases including amyotrophic lateral sclerosis (ALS), progressive supranuclear palsy (PSP), and dementia with Lewy bodies (DLB) [48] may therefore be partially caused or facilitated by BMAA. However, the gene/genes for the codification of BMAA are not known in any cyanobacteria and plant, so that their presence can only be detected by analytical methods, including those used in this study.
BMAA was detected in examples of plant–cyanobacteria symbiosis such as hornworts, liverwort, lichens, cycads, and Gunnera [24], and also in A. filiculoides with 2 μg/g [42]. Some analytical methods of detecting this non-proteinogenic molecule can result in erroneous interpretations due to structural isomers DAB (2,4-diaminobutyric) and AEG (N-(2-aminoethyl)-glycine, which can co-elute and be mis-identified as BMAA [49,50]. For the present study, two methods were therefore used to detect BMAA, DAB, and AEG; we did not detect BMAA, AEG, or DAB in any of the analyzed Azolla and N. azollae. These data indicate that the previous reported detection of 2 μg/g BMAA in Azolla [42] is incorrect. It is possible that environmental factors affect the syntheses of cyanotoxins, including BMAA. Future studies could therefore analyze plant–cyanobacteria symbiosis collected from a variety of locations to determine if environments conditions influence the synthesis of cyanotoxins, including BMAA.
Harmful algal blooms (HABs) of other cyanobacteria species also release cyanotoxins upon cell necrosis. The uptake and bioaccumulation of cyanotoxins from irrigated water for crop and non-crop plants therefore also need to be evaluated to determine if Azolla species may bioaccumulate cyanotoxins. Previous studies have shown that A. filiculoides does now uptake or bioaccumulate microcystin [51] or cylindrospermospin [52], confirming that Azolla can be safely eaten.
4. Materials and Methods
4.1. Azolla Accessions and Culturing
Seven Azolla species, including two A. pinnata subspecies from the germplasm collection at IRRI (International Rice Research Institute, Laguna, Philippines) and two A. filiculoides accessions from Portugal (FI-BGLU and FI-BGM), were used to detect the cyanotoxin genes of microcystin, nodularin, cylindrospermospin, and saxitoxin (by PCR) and BMAA (by LC-MS/MS) (Table 1). The 48 Azolla accessions have a global distribution throughout 33 countries (Supplementary Material, Figure S25). The Azolla species were cultured in Hoagland medium (H-40), pH 6.1–6.2, with a controlled temperature (23–24 °C), photoperiod (16 h light/8 h dark), and light intensity (6.0 Wm−2) [53]. After 28 days, the biomass of fully developed Azolla was collected, washed in distilled water, frozen at −80 °C, lyophilized, and weighed.
4.2. Isolation of Nostoc azollae from Azolla Accessions
Nostoc azollae cyanobionts were isolated from the dorsal foliar cavities of 48 Azolla accessions (Table 1) using the gentle roller method [54,55] with the following modifications. Roots were cut off, and sporophytes were disinfected in aqueous sodium hypochlorite (1 mL NaClO:10 mL distilled water, v:v) for 20 min, followed by three washes in ultrapure water (Millipore, Madrid, Spain). Sporophytes were sectioned and squeezed with a roller to separate the cyanobiont from Azolla cavities. The extract (Azolla + water + N. azollae) was centrifuged twice at 3000× g for 3 min to settle fern debris. The recovered supernatant with N. azollae filaments was centrifuged twice at 1000× g for 1 min to free cyanobionts from the cellular debris. The recovered dark green pellet was centrifuged at 11,000× g for 10 min, stored at −20 °C, frozen at −80°C, lyophilized, and weighted.
4.3. Detection and Analysis of BMAA (β-N-Methylamino-L-Alanine)
4.3.1. Method 1
The methodology, including reagents and materials, described by Baptista et al., (2015) [56] was used., with extraction of BMAA and quantification by LC-MS/MS using validated analytical methods [50,56]. Lyophilized Azolla biomass and N. azollae isolated from Azolla (10 mg each sample) were acid-digested in 6 M HCl at 90 °C for 20 min, using a high-pressure microwave system (Milestone-Ethos 1, Sorisole, Italy). After evaporation with nitrogen, 20 mM of HCl was added to samples and filtered (0.22 μm Millipore, Burlington, MA, USA).
Analyses of BMAA by LC-MS/MS were performed in a Thermo LCQ Fleet Ion Trap LC/MSn system (Thermo Scientific, Waltham, MA, USA) using a 2.1 × 100 mm, 5 μm diameter ZIC-HILIC column (SeQuant, Geneva, Switzerland) and a 14 × 1 mm, 5 μm guard column (SeQuant). The mobile phase was acetonitrile (0.1% formic acid) and deionized water (0.1% formic acid). A linear gradient of 90% acetonitrile for 20 min was followed by 60% acetonitrile for 15 min and 90% acetonitrile for 5 min. The flow rate was 0.5 mL min−1, and the injection volume was 10 μL; the column temperature was 40 °C, and we used the positive mode in electrospray ionization (ESI). Nitrogen was the sheath gas at a rate of 45 (unitless) and the auxiliary gas at a rate of 20 (unitless). The capillary temperature was held at 250 °C. A mass-to-charge ratio (m/z) scan was performed from 50 to 150, and the ion m/z 119 was monitored to assess 2,4-DAB (2,4-diaminobutyric acid). The occurrence of the product ions m/z 102, 88 and 76 was verified at a collision energy of 14 V for the presence of BMAA.
4.3.2. Method 2
Method 2 followed that described by Pravadali-Cekic S. et al., (2023) [49] with some modifications for the amount of starting material, chromatographic column, eluents and mode of mass detection. Lyophilized Azolla biomass (100 mg) was dissolved in 3 mL of trichloacetic acid (TCA) 10% (v/v) and sonicated on ice (5 min, 70% amplitude, 20 Hz), followed by overnight precipitation at 4 °C. The mixture was then centrifuged (5000× g, 15 min, 4 °C), the supernatant reserved, and the pellet submitted to a second extraction cycle. The third extraction step used 10% TCA/acetone. The pellet as the bound fraction was transferred to a glass vial with acetone (100%), centrifuged, and the supernatant added to the free fraction. The pooled free fraction was then evaporated to dry in a SpeedVac (Büchi, Maia, Portugal) and kept at −80°C. Pellets were also dried using the SpeedVac, and acid hydrolysis was carried out by adding 3 mL of 6 M HCl overnight at 110 °C. The hydrolyzed pellet was re-suspended in 1 mL ultrapure water and added to the free fraction. Samples were then filtered. This was carried out with a 20 µL standard mix solution or sample extract, 20 µL of derivatizing reagent, and 60 µL of borate buffer. Using an AccQ-Tag Ultra Derivatisation Kit in accordance with the manufacturer’s guidelines, the mixture was vortexed for several seconds and placed in a thermocycler at 55 °C for 10 min. The final extract was then transferred to a 1.5 mL vial for LC/MS/MS analysis.
Samples were injected into a liquid chromatograph Thermo Finnigan Surveyor HPLC System (Thermo Scientific, Waltham, MA, USA), coupled with a mass spectrometry LCQ Fleet™ Ion Trap Mass Spectrometer (Thermo Scientific, Waltham, MA, USA). We optimized the parameters of the XcaliburTM version 2 mass spectrometer tune method for data acquisition and processing using direct injection of BMMA and co-occurring isomers in a solution of 1 ppm in LCMS-grade water (Table 2). The mass spectrometer operated in electrospray positive polarity mode using collision ionisation dissociation (CID) corresponding to the [M + H]+ BMAA, AEG (N-(2-aminoethyl)-glycine) and 2,4-DAB molecules ion precursors and respective diagnostic fragments. The spray voltage was maintained at 3.5 kV, capillary temperature at 350 °C, and capillary voltage at 20 kV and tube lens at 120 kV. Nitrogen was used as the sheath and auxiliary gas, with collision energy at 20 eV in collision-induced dissociation mode. Separation was achieved on an ACE Excel C18 (50 × 2.1 mm I.D., 1.7 μm, Batch: V17-1253, Avantor®, ACE®, VWR, PT) at 18 °C, with a flow rate of 0.3 mL/min injected at a volume of 10 μL in no-waste mode. The eluents used were methanol (A) and water (B), both acidified with formic acid at 0.1% (v/v). The gradient program started at 13% A, increasing to 90% A in 20 min before turning back to initial conditions in 5 min and equilibrating for an additional 10 min with 20% A. See Table 2 for the chromatographic and mass parameters.
Table 2.
Chromatographic and mass parameters for the BMAA and the detection of isomers AEG and DAB.
| Target | Retention Time (min) | Derivatized Ion Precursor (m/z) | CID Fragments (m/z) | CID Colission Energy (V) | Calibration: Curve and Linear Interval (µg/L) |
LOD (µg/L) |
LOQ (µg/L) |
|---|---|---|---|---|---|---|---|
| BMAA | 13.92 ± 0.08 | 459.00 | 320, 315, 289, 258, 171, 145 | 35 | y = 1377.4x − 2154.1 r2 = 0.9947 3-250 |
7.99 | 24.2 |
| AEG | 13.22 ± 0.11 | 459.00 | 320, 315, 289, 272, 171, 145 | 35 | y = 889.91x + 4972.2 r2 = 0.9993 3-150 |
57.4 | 174 |
| 2,4-DAB | 14.93 ± 0.15 | 459.00 | 320, 315, 289, 271, 171, 145 | 35 | y = 2785x + 5538.1 r2 = 0.9934 7-250 |
70 | 212 |
LOD—limit of detection; LOQ—limit of quantification; CID—collision-induced dissociation.
4.4. Cyanotoxin Genes in Azolla Accessions and Nostoc Azollae
DNA Extraction
DNA from N. azollae isolated from Azolla accessions was extracted with a PureLink® Genomic DNA MiniKit (Invitrogen, Carlsbad, CA, USA), and DNA from Azolla accessions was extracted with Genomic DNA from Plant NucleoSpin® Plant II (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions. DNA was stored at −20 °C. The DNA was quantified in a Qubit fluorometer (Invitrogen) using the Quant-iT® dsDNA HS assay following the manufacturer’s instructions. A working DNA concentration of 0.1 μg/μL was made with sterile ultrapure water, and saxitoxin, nodularin, and microcystin were assessed by specific primers (Table 3). A Biometra TProfessional (Goettingen, Germany) thermocycler was used for PCR amplification using the conditions listed in Table 4 for each gene, with a hold at 4 °C for all the programs. Each 20 μL reaction contained 1 μL of 0.5 µM of each primer (Invitrogen, Waltham, MA, USA), 2 μL of 0.1 μg/μL DNA, 9 μL Supreme NZYTaq 2x Green Master Mix (NZYTech, Lisbon, Portugal), and 7 μL of ultrapure sterile water. Negative (with sterile ultrapure water) and positive (Microcystis aeruginosa LEGE91094 for microcystin and microcystin/nodularin genes, Aphanizomenon ovalisporum for the cylindrospermopsin gene, and Aphanizomenon gracillaris LMECYA 40 from INSA for the saxitoxin gene) controls were included. The amplification products were separated via 1.5% agarose gel electrophoresis running in TAE 1× at 150 V for 25–30 min and stained with 0.2 μg/mL ethidium bromide (BioRad, Hercules, CA, USA). The 1 Kb Plus DNA ladder (Invitrogen) was used as a molecular size marker.
Table 3.
Primers used to amplify cyanotoxic genes in Azolla and N. azollae DNA.
| Gene | Primer | Sequence Primer (5′ → 3′) | Size (bp) | Reference |
|---|---|---|---|---|
| Saxitoxin (sxt) | SXT683F | GGATCTCAAACATGATCCCA | 195 | [57] |
| SXT877R | GCCAAACGCAGTACCACTT | |||
| Cylindrospermopsin (cyl) (poliketide synthase) | K18F | CCTCGCACATAGCCATTTGC | 422 | [58] |
| M4R | GAAGCTCTGGAATCCGGTAA | |||
| Cylindrospermopsin (cyl) (peptide synthase) | M13 | GGCAAATTGTGATAGCCACGAGC | 597 | [59] |
| M14 | GATGGAACATCGCTCACTGGTG | [58] | ||
| Microcystin/Nodularin synthetase (mcyE/ndaF) | HepF | TTTGGGGTTAACTTTTTTGGCCATAGTC | 472 | [60] |
| HepR | AATTCTTGAGGCTGTAAATCGGGTTT | |||
| Microcystin synthetase (mcy A) | mcyA-Cd1F | AAAATTAAAAGCCGTATCAAA | 297 | [61] |
| mcyA-Cd1R | AAAAGTGTTTTATTAGCGGCTCAT | |||
| Microcystin synthetase (mcy B) | 2959F | TGGGAAGATGTTCTTCAGGTATCCAA | 350 | [62] |
| 3278R | AGAGTGGAAACAATATGATAAGCTAC | |||
| Microcystin (mcy C) | FAA | CTATGTTATTTATACATCAGG | 758 | [63] |
| RAA | CTCAGCTTAACTTGATTATC | |||
| Microcystin (mcy B, domain A) | 2156F | ATCACTTCAATCTAACGACT | 955 | [64] |
| 3111R | GTTGCTGCTGTAAGAAA | |||
| Microcystin (mcy C, domain A) | PSCF1 | GCAACATCCCAAGAGCAAAG | 674 | [65] |
| PSCR1 | CCGACAACATCACAAAGGC | |||
| Microcystin (mcy D, domain ACP) | PKDF1 | GACGCTCAAATGATGAAACT | 647 | [65] |
| PKDR1 | GCAACCGATAAAAACTCCC | |||
| Microcystin (mcy D, domain KS) | PKDF2 | AGTTATTCTCCTCAAGCC | 859 | [65] |
| PKDR2 | CATTCGTTCCACTAAATCC | |||
| Microcystin (mcy E, domain GSA-AMT) | PKEF1 | CGCAAACCCGATTTACAG | 755 | [65] |
| PKER1 | CCCCTACCATCTTCATCTTC | |||
| Microcystin (mcy G, domain CM) | PKGF1 | ACTCTCAAGTTATCCTCCCTC | 425 | [65] |
| PKGR1 | AATCGCTAAAACGCCACC |
Table 4.
Amplification conditions for the cyanotoxic genes in Azolla and N. azollae DNA.
| Gene | Initial Denaturation | Denaturation | Annealing | Extension | Final Extension | Reference |
|---|---|---|---|---|---|---|
| sxt | 94 °C; 3 min | 35 cycles | 72 °C; 7 min | [57] | ||
| 94 °C; 10 s | 52 °C; 20 s | 72 °C; 1 min | ||||
| cyl | 94 °C; 10 min | 30 cycles | 72 °C; 7 min | [59] | ||
| 94 °C; 30 s | 55 °C; 30 s | 72 °C; 7 min | ||||
| mcyE/ndaF | 92 °C; 2 min | 35 cycles | 72 °C; 5 min | [60] | ||
| 92 °C; 20 s | 56 °C; 30 s | 72 °C; 1 min | ||||
| mcy A | 95 °C; 2 min | 35 cycles | 72 °C; 7 min | [61] | ||
| 95 °C; 90 s | 56 °C; 30 s | 72 °C; 50 s | ||||
| mcy B | 94 °C; 2 min | 35 cycles | 72 °C; 5 min | [62] | ||
| 94 °C; 30 s | 59 °C; 45 s | 72 °C; 1 min | ||||
| mcy C | 94 °C; 2 min | 35 cycles | 72 °C; 7 min | [63] | ||
| 94 °C; 10 s | 50 °C; 20 s | 72 °C; 1 min | ||||
| mcy B, domain A | 94 °C; 4 min | 30 cycles | 72 °C; 7 min | [64] | ||
| 95 °C; 30 s | 52 °C; 30 s | 72 °C; 1 min | ||||
| mcy C, domain A | 94 °C; 5 min | 35 cycles | 72 °C; 7 min | [65] | ||
| 95 °C; 1 min | 52 °C; 30 s | 72 °C; 1 min | ||||
| mcy D, domain ACP | 94 °C; 5 min | 35 cycles | 72 °C; 7 min | [65] | ||
| 95 °C; 1 min | 52 °C; 30 s | 72 °C; 1 min | ||||
| mcy D, domain KS | 94 °C; 5 min | 35 cycles | 72 °C; 7 min | [65] | ||
| 95 °C; 1 min | 52 °C; 30 s | 72 °C; 1 min | ||||
| mcy E, domain GST-AMT | 94 °C; 5 min | 35 cycles | 72 °C; 7 min | [65] | ||
| 95 °C; 1 min | 52 °C; 30 s | 72 °C; 1 min | ||||
| mcy G, domain CM | 94 °C; 5 min | 35 cycles | 72 °C; 7 min | [65] | ||
| 95 °C; 1 min | 52 °C; 30 s | 72 °C; 1 min | ||||
4.5. BLAST of Anatoxin-a Genes against Nostoc azollae
To determine if N. azollae produces anatoxin-a, a nucleotide BLAST (BLASTN) search was performed for anatoxin-a coding genes. Since the anatoxin-a gene cluster was discovered after the PCR and gel analysis of the other cyanotoxins performed in this study, analysis of its presence was carried out separately through BLAST rather than as the query sequence [66]. This was a 34,682 bp sequence encoding for proteins associated with the biosynthesis of these toxins. The nucleotide query was applied to the full genome of Nostoc azollae 0708 (taxid: 551115). Matches with E-values from the BLASTN of less than 0.01 were investigated and analyzed.
5. Conclusions
Our LC-MS/MS results show the Azolla–Nostoc azollae superorganism does not contain BMAA or their isomers DAB and AEG and that Azolla and N. azollae do not synthesize other common cyanotoxins, indicating that Azolla is a nutritious food that can be safely eaten.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants13192707/s1, Supplementary Materials 1, Figures S1–S24: Agarose gel from the PCR amplification of genes in Azolla and N. azollae. Figure S25: Map showing the countries of origin of Azolla accessions used in the present study. Figure S1: Agarose gel from the PCR amplification of the gene microcystin/nodularin synthetase for nodularin in 48 Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control. The first line is the ladder. Figure S2: Agarose gel from the PCR amplification of the gene saxitoxin in 48 Azolla accessions (see Table 1 from the manuscript). C+: positive control (A. gracillaris LMECYA 40), C−: negative control; the first line is the ladder. Figure S3: Agarose gel from the multiplex PCR amplification of the genes poliketide synthase and peptide synthase for cylindrospermopsin in 48 Azolla accessions (see Table 1 from the manuscript). C+: positive control (A. ovalisporum), C−: negative control; the first line is the ladder. Figure S4: Agarose gel from the PCR amplification of the gene microcystin synthetase (mcy A) for microcystin in 48 Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S5: Agarose gel from the PCR amplification of the gene microcystin synthetase (mcy B) for microcystin in 48 Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S6: Agarose gel from the PCR amplification of the gene microcystin C for microcystin in 48 Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S7: Agarose gel from the PCR amplification of the gene microcystin B A-domain for microcystin in 48 Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S8: Agarose gel from the PCR amplification of the gene microcystin C A-domain for microcystin in 48 Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S9: Agarose gel from the PCR amplification of the gene microcystin D ACP-domain for microcystin in 48 Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S10: Agarose gel from the PCR amplification of the gene microcystin D KS-domain for microcystin in 48 Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S11: Agarose gel from the PCR amplification of the gene microcystin E GSA-AMT-domain for microcystin in 48 Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S12: Agarose gel from the PCR amplification of the gene microcystin G CM-domain for microcystin in 48 Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S13: Agarose gel from the PCR amplification of the gene microcystin/nodularin synthetase for nodularin in 47 Nostoc azollae isolated from Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S14: Agarose gel from the PCR amplification of the gene saxitoxin in 47 Nostoc azollae isolated from Azolla accessions (see Table 1 from the manuscript). C+: positive control (A. gracillaris LMECYA 40), C−: negative control; the first line is the ladder. Figure S15: Agarose gel from the multiplex PCR amplification of the genes poliketide synthase and peptide synthase for cylindrospermopsin in 47 Nostoc azollae isolated from Azolla accessions (see Table 1 from the manuscript). C+: positive control (A. ovalisporum), C−: negative control; the first line is the ladder. Figure S16: Agarose gel from the PCR amplification of the gene microcystin synthetase (mcy A) for microcystin in 47 Nostoc azollae isolated from Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S17: Agarose gel from the PCR amplification of the gene microcystin synthetase (mcy B) for microcystin in 47 Nostoc azollae isolated from Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S18: Agarose gel from the PCR amplification of the gene microcystin C for microcystin in 47 Nostoc azollae isolated from Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S19: Agarose gel from the PCR amplification of the gene microcystin B A-domain for microcystin in 47 Nostoc azollae isolated from Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S20: Agarose gel from the PCR amplification of the gene microcystin C A-domain for microcystin in 47 Nostoc azollae isolated from Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S21: Agarose gel from the PCR amplification of the gene microcystin D ACP-domain for microcystin in 47 Nostoc azollae isolated from Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S22: Agarose gel from the PCR amplification of the gene microcystin D KS-domain for microcystin in 47 Nostoc azollae isolated from Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S23: Agarose gel from the PCR amplification of the gene microcystin E GSA-AMT-domain for microcystin in 47 Nostoc azollae isolated from Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S24: Agarose gel from the PCR amplification of the gene microcystin G CM-domain for microcystin in 47 Nostoc azollae isolated from Azolla accessions (see Table 1 from the manuscript). C+: positive control (M. aeruginosa LEGE 91094), C−: negative control; the first line is the ladder. Figure S25: Map showing the countries of origin of Azolla accessions used in the present study. Legend: a—A. pinnata subsp. imbricata; b—A. filiculoides; c—A. mexicana; d—A. caroliniana; e—A. microphylla; f—A. nilotica; g—A. rubra; h—A. pinnata subsp. pinnata. Supplementary Materials 2: Sequence alignment from the BLASTN query between the Anatoxin-a gene cluster and the Nostoc azollae genome.
Author Contributions
Conceptualization, J.P.B., A.L.P., A.A.B., M.P.G., V.L., T.S. and D.J.W.; Formal analysis, A.L.P., J.A., V.V. and D.J.W.; Investigation, A.L.P., V.V. and D.J.W.; Methodology, J.P.B., A.L.P., J.A., T.S. and D.J.W.; Project administration, J.P.B.; Resources, J.A.; Supervision, J.P.B.; Validation, A.L.P.; Visualization, J.P.B., A.L.P., A.A.B., V.L. and D.J.W.; Writing—original draft, J.P.B., A.L.P., A.A.B. and D.J.W.; Writing—review and editing, A.L.P., A.A.B. and D.J.W. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Contributions made by D. Winstead were supported financially by Open Philanthropy for the Penn State University—Research on Emergency Food Resilience project.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Carrapiço F. Azolla as a Superorganism. Its Implication in Symbiotic Studies. In: Seckbach J., Grube M., editors. Symbioses and Stress: Joint Ventures in Biology. Springer; Dordrecht, The Netherlands: 2010. pp. 225–241. [Google Scholar]
- 2.Singh P., Khan A., Srivastava A. Chapter 16—Heterocyst and Akinete Differentiation in Cyanobacteria: A View toward Cyanobacterial Symbiosis. In: Singh P.K., Kumar A., Singh V.K., Shrivastava A.K., editors. Advances in Cyanobacterial Biology. Academic Press; Cambridge, MA, USA: 2020. pp. 235–248. [Google Scholar]
- 3.Bujak J.P., Bujak A.A. Origin and Evolution of the Azolla Superorganism. Plants. 2024;13:2106. doi: 10.3390/plants13152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekman M., Tollbäck P., Klint J., Bergman B. Protein Expression Profiles in an Endosymbiotic Cyanobacterium Revealed by a Proteomic Approach. Mol. Plant-Microbe Interact. 2006;19:1251–1261. doi: 10.1094/MPMI-19-1251. [DOI] [PubMed] [Google Scholar]
- 5.Ekman M., Tollbäck P., Bergman B. Proteomic Analysis of the Cyanobacterium of the Azolla Symbiosis: Identity, Adaptation, and NifH Modification. J. Exp. Bot. 2008;59:1023–1034. doi: 10.1093/jxb/erm282. [DOI] [PubMed] [Google Scholar]
- 6.Larsson J., Nylander J.A., Bergman B. Genome Fluctuations in Cyanobacteria Reflect Evolutionary, Developmental and Adaptive Traits. BMC Evol. Biol. 2011;11:187. doi: 10.1186/1471-2148-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ran L., Larsson J., Vigil-Stenman T., Nylander J.A.A., Ininbergs K., Zheng W.-W., Lapidus A., Lowry S., Haselkorn R., Bergman B. Genome Erosion in a Nitrogen-Fixing Vertically Transmitted Endosymbiotic Multicellular Cyanobacterium. PLoS ONE. 2010;5:e11486. doi: 10.1371/annotation/835c5766-5128-41c4-b636-adfe0c503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bujak J., Bujak A. The Azolla Story: A Message from the Future. The Azolla Foundation; Blackpool, UK: 2020. [Google Scholar]
- 9.Bujak A., Bujak J. Azolla’s Use as a Biofertilizer and Livestock Feed. In: Marimuthu J., Fernández H., Kumar A., Thangaiah S., editors. Ferns. Springer; Singapore: 2022. pp. 671–695. [Google Scholar]
- 10.Watanabe I., Berja N.S. The Growth of Four Species of Azolla as Affected by Temperature. Aquat. Bot. 1983;15:175–185. doi: 10.1016/0304-3770(83)90027-X. [DOI] [Google Scholar]
- 11.Ansari M.A., Sharma V.P. Role of Azolla in Controlling Mosquito Breeding in Ghaziabad District Villages (U.P.) Indian J. Malariol. 1991;28:51–54. [PubMed] [Google Scholar]
- 12.Mwingira V., Mayala B., Senkoro K., Rumisha S., Shayo H., Elizabeth Mlozi P., Mboera L. Mosquito Larval Productivity in Rice-Fields Infested with Azolla in Mvomero District, Tanzania. Tanzan. J. Health Res. 2009;11:17–22. doi: 10.4314/thrb.v11i1.43246. [DOI] [PubMed] [Google Scholar]
- 13.Rajendran R., Reuben R. Evaluation of the Water Fern Azolla microphylla for Mosquito Population Management in the Rice-Land Agro-Ecosystem of South India. Med. Vet. Entomol. 1991;5:299–310. doi: 10.1111/j.1365-2915.1991.tb00556.x. [DOI] [PubMed] [Google Scholar]
- 14.Bharati K. Influence of Incorporation or Dual Cropping of Azolla on Methane Emission from a Flooded Alluvial Soil Planted to Rice in Eastern India. Agric. Ecosyst. Environ. 2000;79:73–83. doi: 10.1016/S0167-8809(99)00148-6. [DOI] [Google Scholar]
- 15.Mujiyo, Sunarminto B., Hanudin E., Widada J., Syamsiyah J. Methane Emission on Organic Rice Experiment Using Azolla. Int. J. Appl. Environ. Sci. 2016;11:295–308. [Google Scholar]
- 16.Xu H., Zhu B., Liu J., Li D., Yang Y., Zhang K., Jiang Y., Hu Y., Zeng Z. Azolla Planting Reduces Methane Emission and Nitrogen Fertilizer Application in Double Rice Cropping System in Southern China. Agron. Sustain. Dev. 2017;37:29. doi: 10.1007/s13593-017-0440-z. [DOI] [Google Scholar]
- 17.Winstead D., Di Gioia F., Jauregui M., Jacobson M. Nutritional Properties of Raw and Cooked Azolla caroliniana Willd., an Aquatic Wild Edible Plant. Food Sci. Nutr. 2024;12:2050–2060. doi: 10.1002/fsn3.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bláha L., Babica P., Maršálek B. Toxins Produced in Cyanobacterial Water Blooms—Toxicity and Risks. Interdiscip. Toxicol. 2009;2:36–41. doi: 10.2478/v10102-009-0006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burton B., Collins K., Brooks J., Marx K., Renner A., Wilcox K., Moore E., Osowski K., Riley J., Rowe J., et al. The Biotoxin BMAA Promotes Dysfunction via Distinct Mechanisms in Neuroblastoma and Glioblastoma Cells. PLoS ONE. 2023;18:e0278793. doi: 10.1371/journal.pone.0278793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chorus I., Bartram J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management. CRC Press; Boca Raton, FL, USA: 1999. [Google Scholar]
- 21.Chorus I., Welker M. Toxic Cyanobacteria in Water. 2nd ed. CRC Press; Boca Raton, FL, USA: 2021. [Google Scholar]
- 22.Merel S., Walker D., Chicana R., Snyder S., Baurès E., Thomas O. State of Knowledge and Concerns on Cyanobacterial Blooms and Cyanotoxins. Environ. Int. 2013;59:303–327. doi: 10.1016/j.envint.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Funari E., Testai E. Human Health Risk Assessment Related to Cyanotoxins Exposure. Crit. Rev. Toxicol. 2008;38:97–125. doi: 10.1080/10408440701749454. [DOI] [PubMed] [Google Scholar]
- 24.Cox P.A., Banack S.A., Murch S.J., Rasmussen U., Tien G., Bidigare R.R., Metcalf J.S., Morrison L.F., Codd G.A., Bergman B. Diverse Taxa of Cyanobacteria Produce β-N-Methylamino-l-Alanine, a Neurotoxic Amino Acid. Proc. Natl. Acad. Sci. USA. 2005;102:5074–5078. doi: 10.1073/pnas.0501526102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esterhuizen M., Downing T.G. Beta-N-Methylamino-L-Alanine (BMAA) in Novel South African Cyanobacterial Isolates. Ecotoxicol. Environ. Saf. 2008;71:309–313. doi: 10.1016/j.ecoenv.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Vega A., Bell E.A. α-Amino-β-Methylaminopropionic Acid, a New Amino Acid from Seeds of Cycas circinalis. Phytochemistry. 1967;6:759–762. doi: 10.1016/S0031-9422(00)86018-5. [DOI] [Google Scholar]
- 27.Al-Sammak M.A., Hoagland K.D., Cassada D., Snow D.D. Co-Occurrence of the Cyanotoxins BMAA, DABA and Anatoxin-a in Nebraska Reservoirs, Fish, and Aquatic Plants. Toxins. 2014;6:488–508. doi: 10.3390/toxins6020488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammerschlag N., Davis D.A., Mondo K., Seely M.S., Murch S.J., Glover W.B., Divoll T., Evers D.C., Mash D.C. Cyanobacterial Neurotoxin BMAA and Mercury in Sharks. Toxins. 2016;8:238. doi: 10.3390/toxins8080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holtcamp W. The Emerging Science of BMAA: Do Cyanobacteria Contribute to Neurodegenerative Disease? Environ. Health Perspect. 2012;120:a110–a116. doi: 10.1289/ehp.120-a110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masseret E., Banack S., Boumédiène F., Abadie E., Brient L., Pernet F., Juntas-Morales R., Pageot N., Metcalf J., Cox P., et al. Dietary BMAA Exposure in an Amyotrophic Lateral Sclerosis Cluster from Southern France. PLoS ONE. 2013;8:e83406. doi: 10.1371/journal.pone.0083406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang L., Eriksson J., Lage S., Jonasson S., Shams S., Mehine M., Ilag L.L., Rasmussen U. Diatoms: A Novel Source for the Neurotoxin BMAA in Aquatic Environments. PLoS ONE. 2014;9:e84578. doi: 10.1371/journal.pone.0084578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang L., Ilag L. Detection of Endogenous BMAA in Dinoflagellate (Heterocapsa triquetra) Hints at Evolutionary Conservation and Environmental Concern. PubRaw Sci. 2014;1:1–8. [Google Scholar]
- 33.Lage S., Costa P.R., Moita T., Eriksson J., Rasmussen U., Rydberg S.J. BMAA in Shellfish from Two Portuguese Transitional Water Bodies Suggests the Marine Dinoflagellate Gymnodinium catenatum as a Potential BMAA Source. Aquat. Toxicol. Amst. Neth. 2014;152:131–138. doi: 10.1016/j.aquatox.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 34.Kaasalainen U., Fewer D.P., Jokela J., Wahlsten M., Sivonen K., Rikkinen J. Cyanobacteria Produce a High Variety of Hepatotoxic Peptides in Lichen Symbiosis. Proc. Natl. Acad. Sci. USA. 2012;109:5886–5891. doi: 10.1073/pnas.1200279109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaasalainen U., Fewer D.P., Jokela J., Wahlsten M., Sivonen K., Rikkinen J. Lichen Species Identity and Diversity of Cyanobacterial Toxins in Symbiosis. New Phytol. 2013;198:647–651. doi: 10.1111/nph.12215. [DOI] [PubMed] [Google Scholar]
- 36.Gehringer M.M., Adler L., Roberts A.A., Moffitt M.C., Mihali T.K., Mills T.J., Fieker C., Neilan B.A. Nodularin, a Cyanobacterial Toxin, Is Synthesized in Planta by Symbiotic Nostoc Sp. ISME J. 2012;6:1834–1847. doi: 10.1038/ismej.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koksharova O.A., Safronova N.A. Non-Proteinogenic Amino Acid β-N-Methylamino-L-Alanine (BMAA): Bioactivity and Ecological Significance. Toxins. 2022;14:539. doi: 10.3390/toxins14080539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rouhiainen L., Vakkilainen T., Siemer B.L., Buikema W., Haselkorn R., Sivonen K. Genes Coding for Hepatotoxic Heptapeptides (Microcystins) in the Cyanobacterium anabaena Strain 90. Appl. Environ. Microbiol. 2004;70:686–692. doi: 10.1128/AEM.70.2.686-692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Méjean A., Paci G., Gautier V., Ploux O. Biosynthesis of Anatoxin-a and Analogues (Anatoxins) in Cyanobacteria. Toxicon. 2014;91:15–22. doi: 10.1016/j.toxicon.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Kurland L.T., Mulder D.W. Epidemiologic Investigations of Amyotrophic Lateral Sclerosis. 2. Familial Aggregations Indicative of Dominant Inheritance Part I. Neurology. 1955;5:182–196. doi: 10.1212/WNL.5.3.182. [DOI] [PubMed] [Google Scholar]
- 41.Kurland L.T., Mulder D.W. Epidemiologic Investigations of Amyotrophic Lateral Sclerosis. 2. Familial Aggregations Indicative of Dominant Inheritance Part II. Neurology. 1955;5:249–268. doi: 10.1212/WNL.5.4.249. [DOI] [PubMed] [Google Scholar]
- 42.Cox P.A., Banack S.A., Murch S.J. Biomagnification of Cyanobacterial Neurotoxins and Neurodegenerative Disease among the Chamorro People of Guam. Proc. Natl. Acad. Sci. USA. 2003;100:13380–13383. doi: 10.1073/pnas.2235808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murch S.J., Cox P.A., Banack S.A. A Mechanism for Slow Release of Biomagnified Cyanobacterial Neurotoxins and Neurodegenerative Disease in Guam. Proc. Natl. Acad. Sci. USA. 2004;101:12228–12231. doi: 10.1073/pnas.0404926101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie X., Basile M., Mash D.C. Cerebral Uptake and Protein Incorporation of Cyanobacterial Toxin β-N-Methylamino-L-Alanine. NeuroReport. 2013;24:779. doi: 10.1097/WNR.0b013e328363fd89. [DOI] [PubMed] [Google Scholar]
- 45.Lobner D., Piana P.M.T., Salous A.K., Peoples R.W. Beta-N-Methylamino-L-Alanine Enhances Neurotoxicity through Multiple Mechanisms. Neurobiol. Dis. 2007;25:360–366. doi: 10.1016/j.nbd.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rush T., Liu X., Lobner D. Synergistic Toxicity of the Environmental Neurotoxins Methylmercury and β-N-Methylamino-L-Alanine. Neuroreport. 2012;23:216–219. doi: 10.1097/WNR.0b013e32834fe6d6. [DOI] [PubMed] [Google Scholar]
- 47.Weiss J.H., Koh J.Y., Choi D.W. Neurotoxicity of Beta-N-Methylamino-L-Alanine (BMAA) and Beta-N-Oxalylamino-L-Alanine (BOAA) on Cultured Cortical Neurons. Brain Res. 1989;497:64–71. doi: 10.1016/0006-8993(89)90970-0. [DOI] [PubMed] [Google Scholar]
- 48.Dunlop R.A., Cox P.A., Banack S.A., Rodgers K.J. The Non-Protein Amino Acid BMAA Is Misincorporated into Human Proteins in Place of L-Serine Causing Protein Misfolding and Aggregation. PLoS ONE. 2013;8:e75376. doi: 10.1371/journal.pone.0075376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pravadali-Cekic S., Vojvodic A., Violi J.P., Mitrovic S.M., Rodgers K.J., Bishop D.P. Simultaneous Analysis of Cyanotoxins β-N-Methylamino-L-Alanine (BMAA) and Microcystins-RR, -LR, and -YR Using Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) Molecules. 2023;28:6733. doi: 10.3390/molecules28186733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faassen E.J., Gillissen F., Lürling M. A Comparative Study on Three Analytical Methods for the Determination of the Neurotoxin BMAA in Cyanobacteria. PLoS ONE. 2012;7:e36667. doi: 10.1371/journal.pone.0036667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pereira A.L., Monteiro B., Azevedo J., Campos A., Osório H., Vasconcelos V. Effects of the Naturally-Occurring Contaminant Microcystins on the Azolla filiculoides–Anabaena azollae Symbiosis. Ecotoxicol. Environ. Saf. 2015;118:11–20. doi: 10.1016/j.ecoenv.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Santos C., Azevedo J., Campos A., Vasconcelos V., Pereira A.L. Biochemical and Growth Performance of the Aquatic Macrophyte Azolla filiculoides to Sub-Chronic Exposure to Cylindrospermopsin. Ecotoxicology. 2015;24:1848–1857. doi: 10.1007/s10646-015-1521-x. [DOI] [PubMed] [Google Scholar]
- 53.Pereira A., Carrapico F. Culture of Azolla filiculoides in Artificial Conditions. Plant Biosyst. 2009;2009:431–434. doi: 10.1080/11263500903172110. [DOI] [Google Scholar]
- 54.Peters G.A., Mayne B.C. The Azolla, Anabaena azollae Relationship: I. Initial Characterization of the Association. Plant Physiol. 1974;53:813–819. doi: 10.1104/pp.53.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rai A.K., Rai V. Effect of NaCl on Growth, Nitrate Uptake and Reduction and Nitrogenase Activity of Azolla pinnata–Anabaena azollae. Plant Sci. 2003;164:61–69. doi: 10.1016/S0168-9452(02)00335-7. [DOI] [Google Scholar]
- 56.Baptista M.S., Vasconcelos R.G.W., Ferreira P.C., Almeida C.M.R., Vasconcelos V.M. Assessment of the Non-Protein Amino Acid BMAA in Mediterranean Mussel Mytilus galloprovincialis after Feeding with Estuarine Cyanobacteria. Environ. Sci. Pollut. Res. 2015;22:12501–12510. doi: 10.1007/s11356-015-4516-5. [DOI] [PubMed] [Google Scholar]
- 57.Lopes V.R., Ramos V., Martins A., Sousa M., Welker M., Antunes A., Vasconcelos V.M. Phylogenetic, Chemical and Morphological Diversity of Cyanobacteria from Portuguese Temperate Estuaries. Mar. Environ. Res. 2012;73:7–16. doi: 10.1016/j.marenvres.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Schembri M.A., Neilan B.A., Saint C.P. Identification of Genes Implicated in Toxin Production in the Cyanobacterium Cylindrospermopsis raciborskii. Env. Toxicol. 2001;16:413–421. doi: 10.1002/tox.1051. [DOI] [PubMed] [Google Scholar]
- 59.Fergusson K.M., Saint P.C. Multiplex PCR Assay for Cylindrospermopsis raciborskii and Cylindrospermopsin-Producing Cyanobacteria. Env. Toxicol. 2003;18:120–125. doi: 10.1002/tox.10108. [DOI] [PubMed] [Google Scholar]
- 60.Jungblut A.-D., Neilan B.A. Molecular Identification and Evolution of the Cyclic Peptide Hepatotoxins, Mycrocystin and Nodularin Synthetases Genes in the Three Orders of Cyanobacteria. Arch. Microbiol. 2006;185:107–114. doi: 10.1007/s00203-005-0073-5. [DOI] [PubMed] [Google Scholar]
- 61.Hisbergues M., Christiansen G., Rouhiainen L., Sivonen K., Börner T. PCR-Based Identification of Microcystin-Producing Genotypes of Different Cyanobacterial Genera. Arch. Microbiol. 2003;180:402–410. doi: 10.1007/s00203-003-0605-9. [DOI] [PubMed] [Google Scholar]
- 62.Nonneman D., Zimba P.V. A PCR-Based Test to Assess the Potential for Microcystin Occurrence in Channel Catfish Production Ponds. J. Phycol. 2002;38:230–233. doi: 10.1046/j.1529-8817.2002.01138.x. [DOI] [Google Scholar]
- 63.Neilan B.A., Dittmann E., Rouhiainen L., Bass R.A., Schaub V., Sivonen K., Börner T. Nonribosomal Peptide Synthesis and Toxigenicity of Cyanobacteria. J. Bacteriol. 1999;181:4089–4097. doi: 10.1128/JB.181.13.4089-4097.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mikalsen B., Boison G., Skulberg O.M., Fastner J., Davies W., Gabrielsen T.M., Rudi K., Jakobsen K.S. Natural Variation in the Microcystin Synthetase Operon mcyABC and Impact on Microcystin Production in Microcystis Strains. J. Bacteriol. 2003;185:2774–2785. doi: 10.1128/JB.185.9.2774-2785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ouahid Y., Pérez-Silva G., del Campo F.F. Identification of Potentially Toxic Environmental Microcystis by Individual and Multiple PCR Amplification of Specific Microcystin Synthetase Gene Regions. Env. Toxicol. 2005;20:235–242. doi: 10.1002/tox.20103. [DOI] [PubMed] [Google Scholar]
- 66.Méjean A., Mann S., Maldiney T., Vassiliadis G., Lequin O., Ploux O. Evidence That Biosynthesis of the Neurotoxic Alkaloids Anatoxin-a and Homoanatoxin-a in the Cyanobacterium Oscillatoria PCC 6506 Occurs on a Modular Polyketide Synthase Initiated by L -Proline. J. Am. Chem. Soc. 2009;131:7512–7513. doi: 10.1021/ja9024353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material.