Abstract
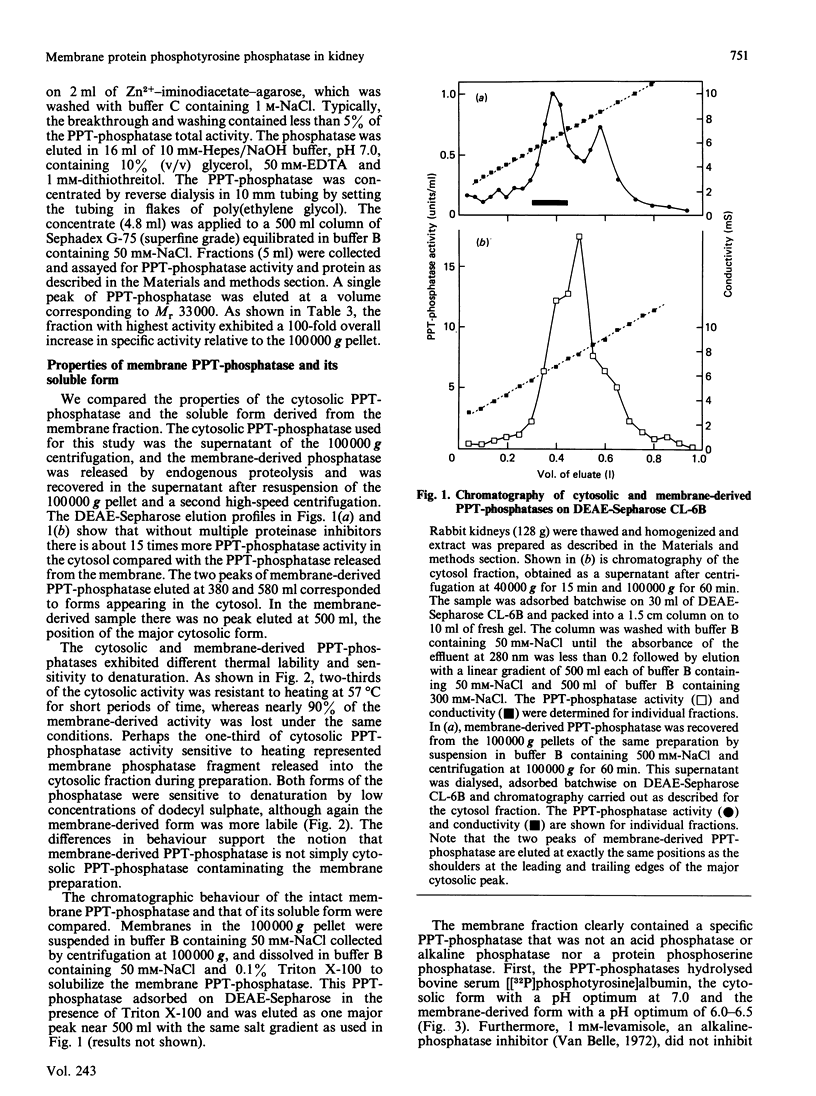
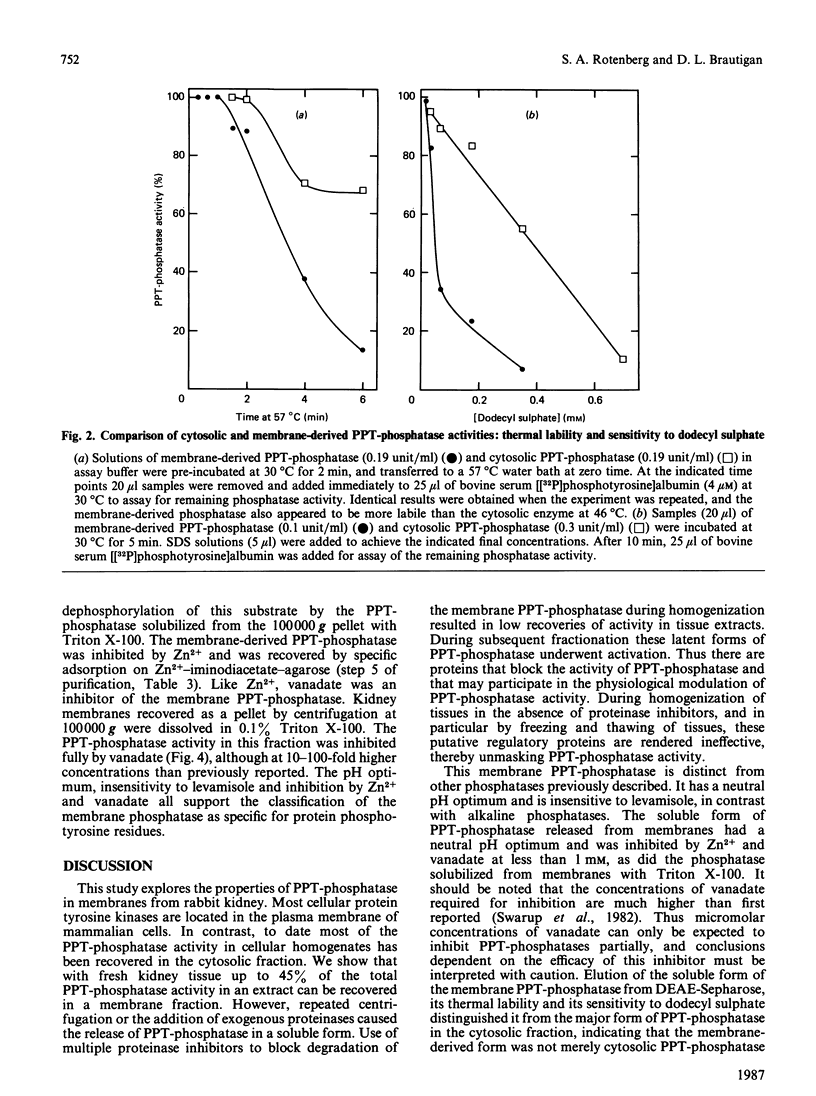
Most protein phosphotyrosine phosphatases (PPT-phosphatases) have been recovered from the cytosol of various cell types and tissues. The present study explores the properties of PPT-phosphatases in rabbit kidney membranes prepared by centrifugation at 100,000 g. More of the total activity was recovered in membranes from fresh (45%) compared with frozen-and-thawed (36%) tissue. However, extracts of fresh tissue had only 15-30% as much total PPT-phosphatase activity. Up to 3-fold activation of cytosolic and membrane PPT-phosphatases occurred during preparation, an effect most evident when fresh tissue was homogenized in buffers containing multiple proteinase inhibitors. These inhibitors apparently block some, but not all, digestion of proteins that mask PPT-phosphatase activity. Incubation of membranes prepared from fresh tissue with added trypsin, papain or thermolysin in each case caused activation of PPT-phosphatase as well as generation of a soluble catalytic fragment. The fragment also was generated by the action of endogenous proteinases during repeated centrifugation and was isolated from these supernatants by DEAE-Sepharose, Zn2+-affinity and gel-filtration chromatography. The fragment had Mr approx. 33,000, had a neutral pH optimum, was inhibited by 50% by 100 microM-vanadate, and was insensitive to the alkaline-phosphatase inhibitors EDTA and levamisole. Although the chromatographic behaviour and lability of the fragment were distinct from those of the predominant cytosolic PPT-phosphatase, some cytosolic PPT-phosphatases exhibited properties consistent with the suggestion that they are fragments derived by proteolysis of PPT-phosphatases in membranes. Localization of PPT-phosphatases in plasma membranes would facilitate reaction with receptor/kinases in vivo.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Bornstein P., Gallis B. Phosphotyrosyl-protein phosphatase. Specific inhibition by Zn. J Biol Chem. 1981 Jul 10;256(13):6519–6522. [PubMed] [Google Scholar]
- Brunati A. M., Pinna L. A. Isolation and partial characterization of distinct species of phosphotyrosyl protein phosphatases from rat spleen. Biochem Biophys Res Commun. 1985 Dec 31;133(3):929–936. doi: 10.1016/0006-291x(85)91225-2. [DOI] [PubMed] [Google Scholar]
- Chan J. R., Stinson R. A. Dephosphorylation of phosphoproteins of human liver plasma membranes by endogenous and purified liver alkaline phosphatases. J Biol Chem. 1986 Jun 15;261(17):7635–7639. [PubMed] [Google Scholar]
- Chernoff J., Li H. C. A major phosphotyrosyl-protein phosphatase from bovine heart is associated with a low-molecular-weight acid phosphatase. Arch Biochem Biophys. 1985 Jul;240(1):135–145. doi: 10.1016/0003-9861(85)90016-5. [DOI] [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Foulkes J. G., Erikson E., Erikson R. L. Separation of multiple phosphotyrosyl-and phosphoseryl-protein phosphatases from chicken brain. J Biol Chem. 1983 Jan 10;258(1):431–438. [PubMed] [Google Scholar]
- Gruppuso P. A., Johnson G. L., Constantinides M., Brautigan D. L. Phosphorylase phosphatase regulatory subunit. "Western" blotting with immunoglobulins against inhibitor-2 reveals a protein of Mr = 60,000. J Biol Chem. 1985 Apr 10;260(7):4288–4294. [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Huang F. L., Glinsmann W. H. Separation and characterization of two phosphorylase phosphatase inhibitors from rabbit skeletal muscle. Eur J Biochem. 1976 Nov 15;70(2):419–426. doi: 10.1111/j.1432-1033.1976.tb11032.x. [DOI] [PubMed] [Google Scholar]
- Hörlein D., Gallis B., Brautigan D. L., Bornstein P. Partial purification and characterization of phosphotyrosyl-protein phosphatase from Ehrlich ascites tumor cells. Biochemistry. 1982 Oct 26;21(22):5577–5584. doi: 10.1021/bi00265a030. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Kull F. C., Jr, Earp H. S., Svoboda M. E., Van Wyk J. J., Cuatrecasas P. Somatomedin-C stimulates the phosphorylation of the beta-subunit of its own receptor. J Biol Chem. 1983 Aug 25;258(16):9581–9584. [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- Lau K. H., Farley J. R., Baylink D. J. Phosphotyrosyl-specific protein phosphatase activity of a bovine skeletal acid phosphatase isoenzyme. Comparison with the phosphotyrosyl protein phosphatase activity of skeletal alkaline phosphatase. J Biol Chem. 1985 Apr 25;260(8):4653–4660. [PubMed] [Google Scholar]
- Leis J. F., Kaplan N. O. An acid phosphatase in the plasma membranes of human astrocytoma showing marked specificity toward phosphotyrosine protein. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6507–6511. doi: 10.1073/pnas.79.21.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. C., Chernoff J., Chen L. B., Kirschonbaum A. A phosphotyrosyl-protein phosphatase activity associated with acid phosphatase from human prostate gland. Eur J Biochem. 1984 Jan 2;138(1):45–51. doi: 10.1111/j.1432-1033.1984.tb07879.x. [DOI] [PubMed] [Google Scholar]
- Lin M. F., Clinton G. M. Human prostatic acid phosphatase has phosphotyrosyl protein phosphatase activity. Biochem J. 1986 Apr 15;235(2):351–357. doi: 10.1042/bj2350351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. L., Branton P. E. Identification, purification, and characterization of phosphotyrosine-specific protein phosphatases from cultured chicken embryo fibroblasts. Mol Cell Biol. 1984 Jun;4(6):1003–1012. doi: 10.1128/mcb.4.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. B., Shia M. A., Pilch P. F. Stimulation of tyrosine-specific phosphorylation in vitro by insulin-like growth factor I. 1983 Sep 29-Oct 5Nature. 305(5933):438–440. doi: 10.1038/305438a0. [DOI] [PubMed] [Google Scholar]
- Shriner C. L., Brautigan D. L. Cytosolic protein phosphotyrosine phosphatases from rabbit kidney. Purification of two distinct enzymes that bind to Zn2+-iminodiacetate agarose. J Biol Chem. 1984 Sep 25;259(18):11383–11390. [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun. 1982 Aug;107(3):1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Selective dephosphorylation of proteins containing phosphotyrosine by alkaline phosphatases. J Biol Chem. 1981 Aug 10;256(15):8197–8201. [PubMed] [Google Scholar]
- Tal M., Silberstein A., Nusser E. Why does Coomassie Brilliant Blue R interact differently with different proteins? A partial answer. J Biol Chem. 1985 Aug 25;260(18):9976–9980. [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Van Belle H. Kinetics and inhibition of alkaline phosphatases from canine tissues. Biochim Biophys Acta. 1972 Nov 10;289(1):158–168. doi: 10.1016/0005-2744(72)90118-0. [DOI] [PubMed] [Google Scholar]


