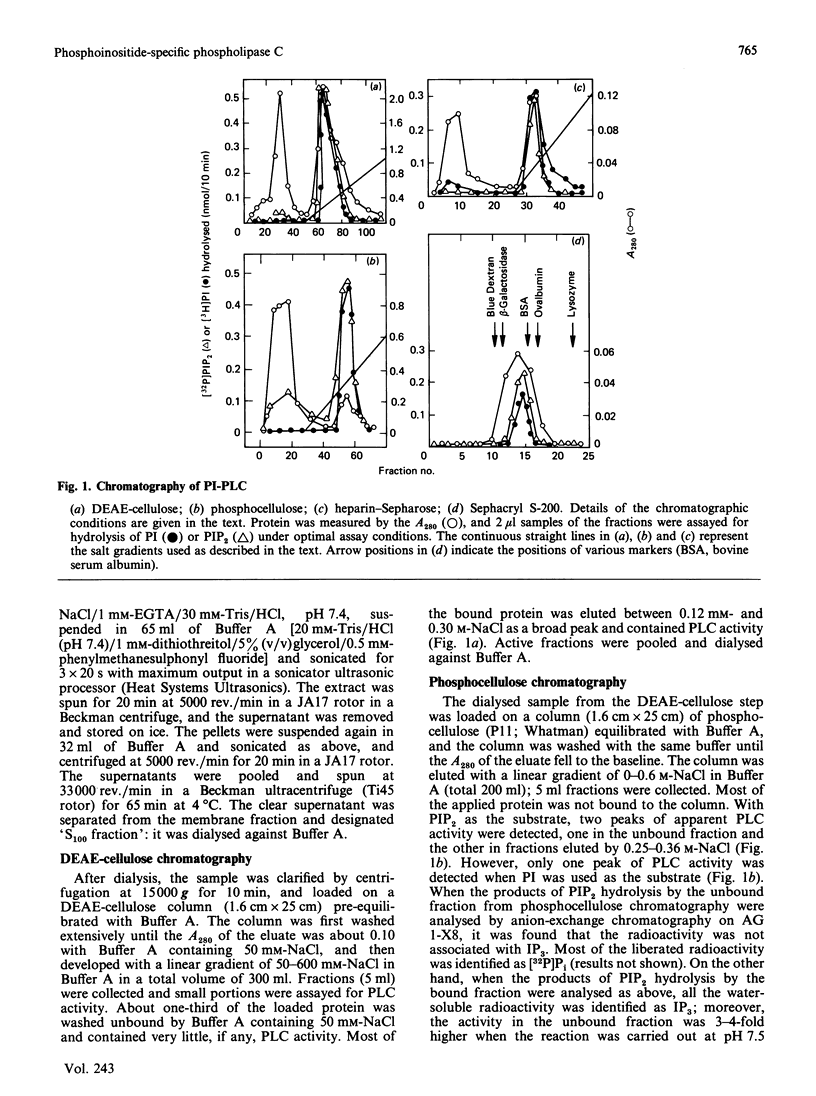
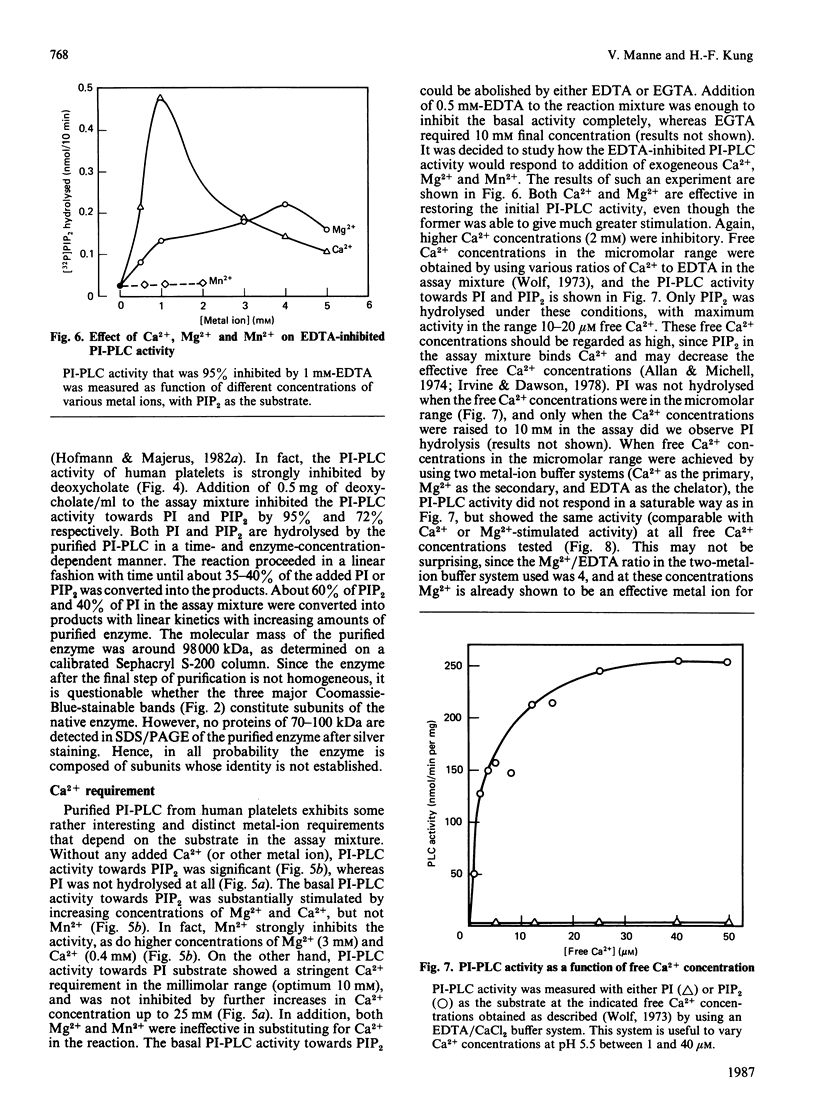
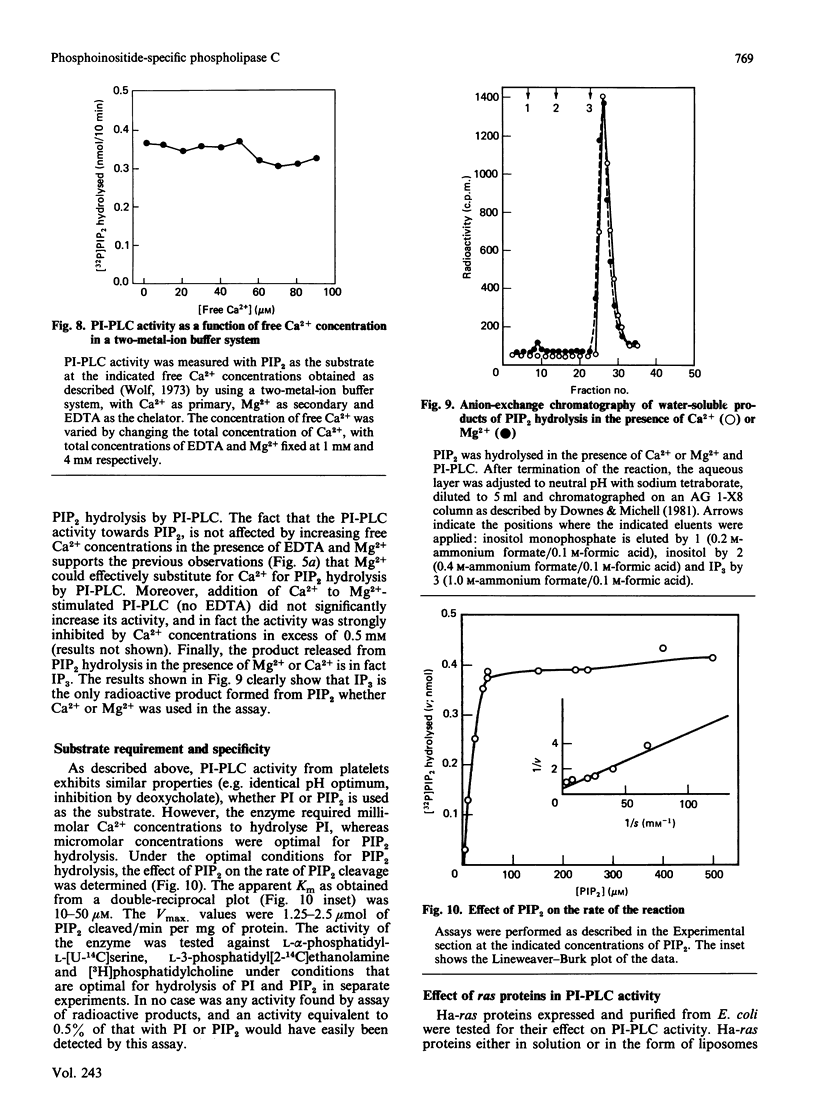
Abstract
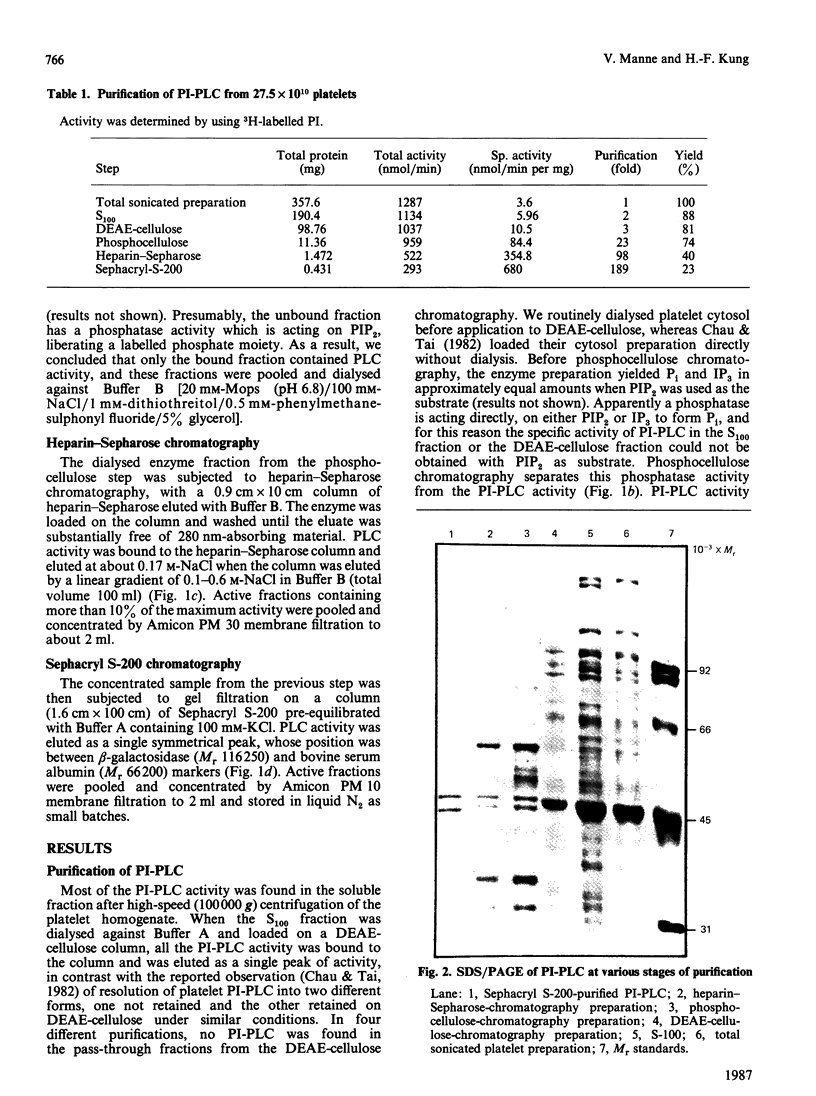
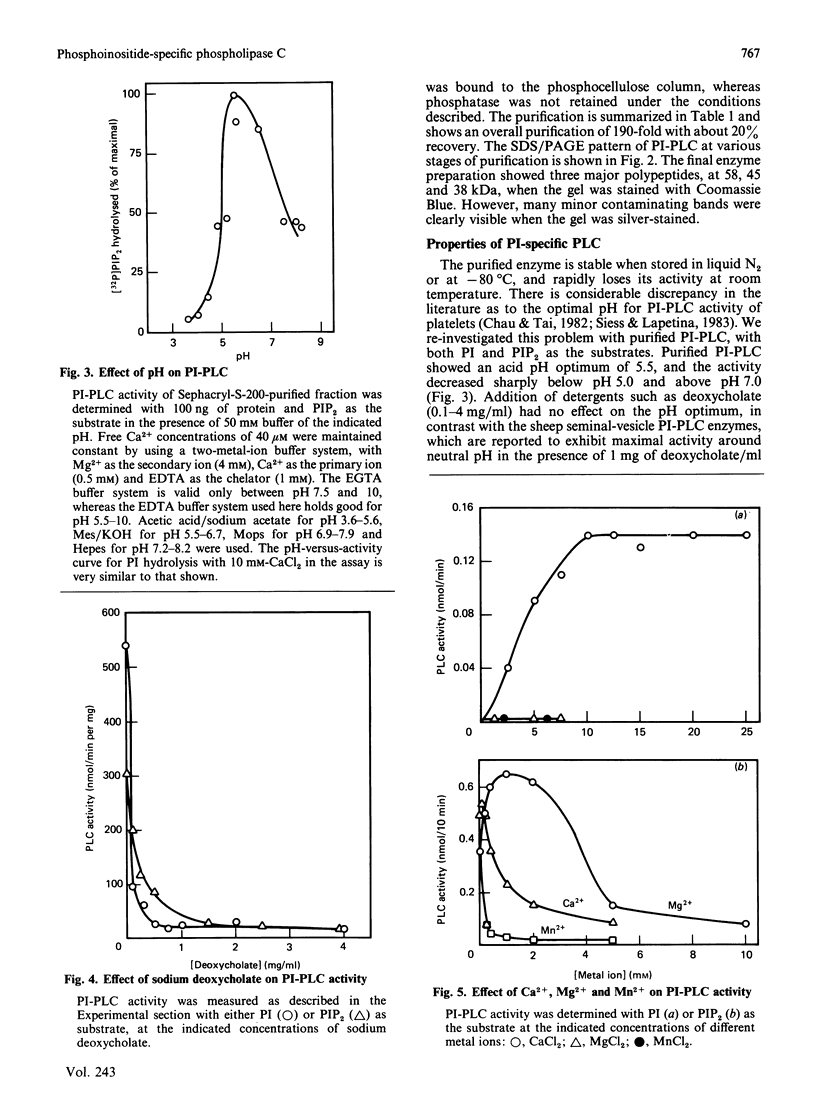
Phosphoinositide-specific phospholipase C (PI-PLC) from human platelet cytosol was purified 190-fold to a specific activity of 0.68 mumol of phosphatidylinositol (PI) cleaved/min per mg of protein. It hydrolyses PI and phosphatidylinositol 4,5-bisphosphate (PIP2), but not phosphatidylcholine, phosphatidylserine or phosphatidylethanolamine. The enzyme exhibits an acid pH optimum of 5.5 and has a molecular mass of 98 kDa as determined by Sephacryl S-200 gel filtration. It required millimolar concentrations of Ca2+ for PI hydrolysis, whereas micromolar concentrations are optimal for PIP2 hydrolysis. Mg2+ could substitute for Ca2+ when PIP2, but not PI, was used as the substrate. EDTA was more effective than EGTA in inhibiting the basal PI-PLC activity towards PIP2. Sodium deoxycholate strongly inhibits the purified PI-PLC activity with either PI or PIP2 as substrate. Ras proteins, either alone or in the form of liposomes, have no effect on PI-PLC activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A., Akhtar R. A., Hawthorne J. N. Acetylcholine increases the breakdown of triphosphoinositide of rabbit iris muscle prelabelled with [32P] phosphate. Biochem J. 1977 Jan 15;162(1):61–73. doi: 10.1042/bj1620061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agranoff B. W., Murthy P., Seguin E. B. Thrombin-induced phosphodiesteratic cleavage of phosphatidylinositol bisphosphate in human platelets. J Biol Chem. 1983 Feb 25;258(4):2076–2078. [PubMed] [Google Scholar]
- Akhtar R. A., Abdel-Latif A. A. Studies on the properties of triphosphoinositide phosphomonoesterase and phosphodiesterase of rabbit iris smooth muscle. Biochim Biophys Acta. 1978 Nov 10;527(1):159–170. doi: 10.1016/0005-2744(78)90265-6. [DOI] [PubMed] [Google Scholar]
- Allan D., Michell R. H. Phosphatidylinositol cleavage in lymphocytes. Requirement for calcium ions at a low concentration and effects of other cations. Biochem J. 1974 Sep;142(3):599–604. doi: 10.1042/bj1420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. L., Baenziger N. L., Majerus P. W. Bradykinin-stimulated release of arachidonate from phosphatidyl inositol in mouse fibrosarcoma cells. Prostaglandins. 1980 Aug;20(2):269–274. doi: 10.1016/s0090-6980(80)80045-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phosphatidylinositol-specific phospholipase-C of platelets: association with 1,2-diacyglycerol-kinase and inhibition by cyclic-AMP. Biochem Biophys Res Commun. 1979 Sep 12;90(1):92–98. doi: 10.1016/0006-291x(79)91594-8. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 and phospholipase C activities of platelets. Differential substrate specificity, Ca2+ requirement, pH dependence, and cellular localization. J Biol Chem. 1980 Nov 10;255(21):10227–10231. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Rapid decrease of phosphatidylinositol 4,5-bisphosphate in thrombin-stimulated platelets. J Biol Chem. 1982 Nov 10;257(21):12705–12708. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Chau L. Y., Tai H. H. Resolution into two different forms and study of the properties of phosphatidylinositol-specific phospholipase C from human platelet cytosol. Biochim Biophys Acta. 1982 Nov 12;713(2):344–351. [PubMed] [Google Scholar]
- Cockcroft S., Baldwin J. M., Allan D. The Ca2+-activated polyphosphoinositide phosphodiesterase of human and rabbit neutrophil membranes. Biochem J. 1984 Jul 15;221(2):477–482. doi: 10.1042/bj2210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M. Studies on the enzymic hydrolysis of monophosphoinositide by phospholipase preparations from P. notatum and ox pancreas. Biochim Biophys Acta. 1959 May;33(1):68–77. doi: 10.1016/0006-3002(59)90499-8. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Freinkel N., Jungalwala F. B., Clarke N. The enzymic formation of myoinositol 1:2-cyclic phosphate from phosphatidylinositol. Biochem J. 1971 May;122(4):605–607. doi: 10.1042/bj1220605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman L. F., Chahwala S. B., Cantley L. ras-transformed cells: altered levels of phosphatidylinositol-4,5-bisphosphate and catabolites. Science. 1986 Jan 24;231(4736):407–410. doi: 10.1126/science.3001936. [DOI] [PubMed] [Google Scholar]
- Friedel R. O., Brown J. D., Durell J. The enzymic hydrolysis of phosphatidyl inositol by guinea pig brain: Sub-cellular distribution and hydrolysis products. J Neurochem. 1969 Mar;16(3):371–378. doi: 10.1111/j.1471-4159.1969.tb10376.x. [DOI] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Hakata H., Kambayashi J., Kosaki G. Purification and characterization of phosphatidylinositol-specific phospholipase C from bovine platelets. J Biochem. 1982 Sep;92(3):929–935. doi: 10.1093/oxfordjournals.jbchem.a134008. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M. Guanine nucleotides decrease the free [Ca2+] required for secretion of serotonin from permeabilized blood platelets. Evidence of a role for a GTP-binding protein in platelet activation. FEBS Lett. 1984 Aug 20;174(1):90–95. doi: 10.1016/0014-5793(84)81084-4. [DOI] [PubMed] [Google Scholar]
- Hofmann S. L., Majerus P. W. Identification and properties of two distinct phosphatidylinositol-specific phospholipase C enzymes from sheep seminal vesicular glands. J Biol Chem. 1982 Jun 10;257(11):6461–6469. [PubMed] [Google Scholar]
- Hofmann S. L., Majerus P. W. Modulation of phosphatidylinositol-specific phospholipase C activity by phospholipid interactions, diglycerides, and calcium ions. J Biol Chem. 1982 Dec 10;257(23):14359–14364. [PubMed] [Google Scholar]
- Igarashi Y., Kondo Y. Demonstration and characterization of partial glyceride specific lipases in pig thyroid plasma membranes. Biochem Biophys Res Commun. 1980 Nov 28;97(2):766–771. doi: 10.1016/0006-291x(80)90330-7. [DOI] [PubMed] [Google Scholar]
- Imai A., Nakashima S., Nozawa Y. The rapid polyphosphoinositide metabolism may be a triggering event for thrombin-mediated stimulation of human platelets. Biochem Biophys Res Commun. 1983 Jan 14;110(1):108–115. doi: 10.1016/0006-291x(83)91267-6. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Dawson R. M. The distribution of calcium-dependent phosphatidylinositol-specific phosphodiesterase in rat brain. J Neurochem. 1978 Dec;31(6):1427–1434. doi: 10.1111/j.1471-4159.1978.tb06568.x. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Hemington N., Dawson R. M. Phosphatidylinositol-degrading enzymes in liver lysosomes. Biochem J. 1977 Apr 15;164(1):277–280. doi: 10.1042/bj1640277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Hemington N., Dawson R. M. The calcium-dependent phosphatidylinositol-phosphodiesterase of rat brain. Mechanisms of suppression and stimulation. Eur J Biochem. 1979 Sep;99(3):525–530. doi: 10.1111/j.1432-1033.1979.tb13284.x. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Hemington N., Dawson R. M. The hydrolysis of phosphatidylinositol by lysosomal enzymes of rat liver and brain. Biochem J. 1978 Nov 15;176(2):475–484. doi: 10.1042/bj1760475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Dawson R. M. Phosphatidylinositol-4,5-bisphosphate phosphodiesterase and phosphomonoesterase activities of rat brain. Some properties and possible control mechanisms. Biochem J. 1984 Feb 15;218(1):177–185. doi: 10.1042/bj2180177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles J., Zwiers H., van Dongen C. J., Schotman P., Wirtz K. W., Gispen W. H. Modulation of brain polyphosphoinositide metabolism by ACTH-sensitive protein phosphorylation. Nature. 1980 Aug 7;286(5773):623–625. doi: 10.1038/286623a0. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Jungalwala F. B., Freinkel N., Dawson R. M. The metabolism of phosphatidylinositol in the thyroid gland of the pig. Biochem J. 1971 Jun;123(1):19–33. doi: 10.1042/bj1230019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerly D. A., Sullivan T. J., Sylwester P., Parker C. W. Diacylglycerol metabolism in mast cells: a potential role in membrane fusion and arachidonic acid release. J Exp Med. 1979 Oct 1;150(4):1039–1044. doi: 10.1084/jem.150.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Billah M. M., Cuatrecasas P. The initial action of thrombin on platelets. Conversion of phosphatidylinositol to phosphatidic acid preceding the production of arachidonic acid. J Biol Chem. 1981 May 25;256(10):5037–5040. [PubMed] [Google Scholar]
- Lapetina E. G., Billah M. M., Cuatrecasas P. The phosphatidylinositol cycle and the regulation of arachidonic acid production. Nature. 1981 Jul 23;292(5821):367–369. doi: 10.1038/292367a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Grosman M., Canessa de Scarnati O. Phosphatidylinositol-cleaving activity in smooth muscle from rat vas deferens. Int J Biochem. 1976;7(9-10):507–513. doi: 10.1016/0020-711x(76)90053-7. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Michell R. H. A membrane-bound activity catalysing phosphatidylinositol breakdown to 1,2-diacylglycerol, D-myoinositol 1:2-cyclic phosphate an D-myoinositol 1-phosphate. Properties and subcellular distribution in rat cerebral cortex. Biochem J. 1973 Mar;131(3):433–442. doi: 10.1042/bj1310433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenstra R., Mauco G., Chap H., Douste-Blazy L. Studies on enzymes related to diacylglycerol production in activated platelets. I. Phosphatidylinositol-specific phospholipase C: further characterization using a simple method for determination of activity. Biochim Biophys Acta. 1984 Feb 9;792(2):199–206. doi: 10.1016/0005-2760(84)90223-6. [DOI] [PubMed] [Google Scholar]
- Litosch I., Lin S. H., Fain J. N. Rapid changes in hepatocyte phosphoinositides induced by vasopressin. J Biol Chem. 1983 Nov 25;258(22):13727–13732. [PubMed] [Google Scholar]
- Low M. G., Carroll R. C., Cox A. C. Characterization of multiple forms of phosphoinositide-specific phospholipase C purified from human platelets. Biochem J. 1986 Jul 1;237(1):139–145. doi: 10.1042/bj2370139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Carroll R. C., Weglicki W. B. Multiple forms of phosphoinositide-specific phospholipase C of different relative molecular masses in animal tissues. Evidence for modification of the platelet enzyme by Ca2+-dependent proteinase. Biochem J. 1984 Aug 1;221(3):813–820. doi: 10.1042/bj2210813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus P. W., Neufeld E. J., Wilson D. B. Production of phosphoinositide-derived messengers. Cell. 1984 Jul;37(3):701–703. doi: 10.1016/0092-8674(84)90405-7. [DOI] [PubMed] [Google Scholar]
- Majumder A. L., Eisenberg F., Jr The formation of cyclic inositol 1,2-monophosphate, inositol 1-phosphate, and glucose 6-phosphate by brain preparations stimulated with deoxycholate and calcium: a gas chromatographic study. Biochem Biophys Res Commun. 1974 Sep 9;60(1):133–139. doi: 10.1016/0006-291x(74)90182-x. [DOI] [PubMed] [Google Scholar]
- Manne V., Bekesi E., Kung H. F. Ha-ras proteins exhibit GTPase activity: point mutations that activate Ha-ras gene products result in decreased GTPase activity. Proc Natl Acad Sci U S A. 1985 Jan;82(2):376–380. doi: 10.1073/pnas.82.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne V., Kung H. F. Effect of divalent metal ions and glycerol on the GTPase activity of H-ras proteins. Biochem Biophys Res Commun. 1985 May 16;128(3):1440–1446. doi: 10.1016/0006-291x(85)91101-5. [DOI] [PubMed] [Google Scholar]
- Manne V., Yamazaki S., Kung H. F. Guanosine nucleotide binding by highly purified Ha-ras-encoded p21 protein produced in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6953–6957. doi: 10.1073/pnas.81.22.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauco G., Chap H., Douste-Blazy L. Characterization and properties of a phosphatidylinositol phosphodiesterase (phospholipase C) from platelet cytosol. FEBS Lett. 1979 Apr 15;100(2):367–370. doi: 10.1016/0014-5793(79)80371-3. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Kambayashi J., Suga K., Hakata H., Mori T. Hydrolysis of polyphosphoinositides in human platelets. Thromb Res. 1985 Jun 1;38(5):513–525. doi: 10.1016/0049-3848(85)90184-7. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Sagawa N., Okita J. R., Bleasdale J. E., MacDonald P. C., Johnston J. M. Diacylglycerol metabolism and arachidonic acid release in human fetal membranes and decidua vera. J Biol Chem. 1981 Jul 25;256(14):7316–7321. [PubMed] [Google Scholar]
- Perret B. P., Plantavid M., Chap H., Douste-Blazy L. Are polyphosphoinositides involved in platelet activation? Biochem Biophys Res Commun. 1983 Jan 27;110(2):660–667. doi: 10.1016/0006-291x(83)91200-7. [DOI] [PubMed] [Google Scholar]
- Richards D. E., Irvine R. F., Dawson R. M. Hydrolysis of membrane phospholipids by phospholipases of rat liver lysosomes. Biochem J. 1979 Aug 15;182(2):599–606. doi: 10.1042/bj1820599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse S. E. Preparation of selectively labeled phosphatidylinositol and assay of phosphatidylinositol-specific phospholipase C. Methods Enzymol. 1982;86:3–11. doi: 10.1016/0076-6879(82)86161-2. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973 Dec;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Papageorge A. G., Shih T. Y. Guanine nucleotide-binding activity as an assay for src protein of rat-derived murine sarcoma viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5355–5359. doi: 10.1073/pnas.76.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Shih T. Y., Maryak J., Ellis R., Chang E., Lowy D. Guanine nucleotide binding activity of the src gene product of rat-derived murine sarcoma viruses. Ann N Y Acad Sci. 1980;354:398–409. doi: 10.1111/j.1749-6632.1980.tb27981.x. [DOI] [PubMed] [Google Scholar]
- Shukla S. D. Minireview. Phosphatidylinositol specific phospholipases C. Life Sci. 1982 Apr 19;30(16):1323–1335. doi: 10.1016/0024-3205(82)90016-9. [DOI] [PubMed] [Google Scholar]
- Siess W., Lapetina E. G. Properties and distribution of phosphatidylinositol-specific phospholipase C in human and horse platelets. Biochim Biophys Acta. 1983 Jul 12;752(2):329–338. doi: 10.1016/0005-2760(83)90131-5. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Nagai Y. Purification of phosphatidylinositol-specific phospholipase C from rat liver. J Biol Chem. 1981 Jul 10;256(13):6769–6775. [PubMed] [Google Scholar]
- Thompson W., Dawson R. M. The triphosphoinositide phosphodiesterase of brain tissue. Biochem J. 1964 May;91(2):237–243. doi: 10.1042/bj0910237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen C. J., Zwiers H., Gispen W. H. Purification and partial characterization of the phosphatidylinositol 4-phosphate kinase from rat brain. Biochem J. 1984 Oct 1;223(1):197–203. doi: 10.1042/bj2230197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooijen L. A., Seguin E. B., Agranoff B. W. Phosphodiesteratic breakdown of endogenous polyphosphoinositides in nerve ending membranes. Biochem Biophys Res Commun. 1983 May 16;112(3):919–926. doi: 10.1016/0006-291x(83)91705-9. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Bross T. E., Hofmann S. L., Majerus P. W. Hydrolysis of polyphosphoinositides by purified sheep seminal vesicle phospholipase C enzymes. J Biol Chem. 1984 Oct 10;259(19):11718–11724. [PubMed] [Google Scholar]
- Wilson D. B., Bross T. E., Sherman W. R., Berger R. A., Majerus P. W. Inositol cyclic phosphates are produced by cleavage of phosphatidylphosphoinositols (polyphosphoinositides) with purified sheep seminal vesicle phospholipase C enzymes. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4013–4017. doi: 10.1073/pnas.82.12.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]