Abstract
Pulmonary embolism is a rare but serious complication in Mycoplasma pneumoniae pneumonia patients, leading to serious sequelae and even death. We aim to retrospectively analyze the clinical features of Mycoplasma pneumoniae pneumonia with pulmonary consolidation in children and to explore the independent risk factors for progression to pulmonary embolism. Clinical data of 207 children with Mycoplasma pneumoniae pneumonia complicated with pulmonary consolidation were collected, and the patients were divided into the pulmonary embolism group (69 patients) and the control group (138 patients). Multivariate logistic regression was used to analyze the risk factors and the predictive efficacy was evaluated by receiver operating characteristic curve. Multivariate logistic regression analysis showed that fever days, D-dimer, immunoglobulin A, chest pain, extra-respiratory symptoms, plastic bronchitis and cutaneous mucosal system complications were the independent risk factors. Fever days ≥ 7.5, D-dimer ≥ 0.895 mg/L, immunoglobulin A ≥ 1.015 g/L, chest pain, extra-respiratory symptoms, plastic bronchitis and cutaneous mucous system complications significantly increased the risk of pulmonary embolism in children with Mycoplasma pneumoniae pneumonia complicated with pulmonary consolidation.
Subject terms: Risk factors, Infectious diseases, Respiratory tract diseases
Introduction
Mycoplasma pneumoniae (MP) infection is responsible for 20 ~ 40% of community-acquired pneumonia cases and is one of the most common pathogens of community-acquired pneumonia in children 5 years of age and older1. Mycoplasma pneumoniae pneumonia (MPP) is considered a mild and self-limiting illness, and is usually characterized by persistent dry cough and fever2. However, the global trend of MPP has increased in recent years, and an increasing number of refractory, severe, fulminant and even fatal MPP cases have been reported3,4. Severe cases of MPP are associated with severe pulmonary and extrapulmonary complications involving the respiratory, circulatory, digestive, blood, urinary, nervous, cutaneous mucosal and other systems1,5.
It has been reported that children with MPP, especially children with severe MPP, often have abnormal coagulation function, and the risk of thrombosis is high6,7. Thromboembolism is a rare but serious extra-respiratory complication that is prone to serious sequelae and even death. Pulmonary embolism (PE) is the most common thromboembolism in MPP patients and is an important cause of pulmonary necrosis, remaining atelectasis and organizing pneumonia8. However, there are few articles on risk factor analysis for PE associated with MPP. The diagnosis of PE in children is difficult, and the clinical manifestations are not typical. The diagnosis of PE depends on computed tomographic pulmonary angiography (CTPA). However, CTPA also has several limitations, such as high radiation, a high risk of contrast media allergy, high medical costs, a low level of child cooperation, increased use of sedatives, and an increased risk of sedation. As a result, the utilization rate of CTPA in the diagnosis of PE associated with MPP in children is less than expected, which easily leads to missed diagnosis and delayed diagnosis and treatment of children with PE. In addition, studies have shown that pulmonary consolidation may be the most closely related risk factor for thrombosis in MPP patients3. Therefore, we retrospectively analyzed the clinical features of MPP with pulmonary consolidation in children and explored the independent risk factors for PE in MPP children with pulmonary consolidation.
Methods
Study population
This retrospective observational study was conducted on 207 MPP children with pulmonary consolidation who were admitted to Henan Children’s Hospital from January 2018 to December 2023. The patients were divided into the PE group (69 patients) and the control group (138 patients) based on CTPA. PE was confirmed by CTPA in patients with high suspicion of PE who had dyspnea, chest pain, pre-syncope or syncope, haemoptysis, or a significant increase in D-dimer (D-D). The patient met the diagnostic criteria for embolism: computed CTPA showing filling defects in the pulmonary artery, and the diagnosis can be made by the reduction or disappearance of distal vascular branches and a wedge-shaped lesion of the lung. This study was approved by the Ethics Committee of the Children’s Hospital of Henan Province (2023-K-128). The need for informed consent was waived given the retrospective nature of the study.
Inclusion criteria: (1) age < 18 years; (2) met the diagnostic criteria for MPP in the Guidelines for Diagnosis and Treatment of Mycoplasma pneumoniae Pneumonia in Children (2023 Edition)8; (3) met the diagnostic criteria for pulmonary consolidation: a diagnosis of lobar/pulmonary segmental consolidation confirmed by chest imaging examination (chest X-ray or chest CT), with a large hyperdense shadow or infiltrative changes in the lung parenchyma.
Exclusion criteria: (1) patients who were admitted to the hospital during the MPP recovery period (those with a disease course of more than four weeks, a stable temperature for more than one week, and imaging absorption improvement); (2) had a previous PE history; (3) other causes of PE, such as air embolism, PE due to congenital heart disease, central vein intubation, nephrotic syndrome, surgery, and tumors; (4) patients with insufficient clinical data; (5) informed consent for CTPA inspection was not obtained.
Data extraction
The following clinical data of all the children were collected retrospectively: (1) general data; (2) clinical manifestations; (3) co-pathogen infection status; (4) laboratory data; (5) treatment of the PE group.
The laboratory data was collected within 24 h after admission to our hospital. Imaging tests were performed within 48 h after admission, and CTPA was performed immediately in patients with suspected PE.
CTPA protocol and imaging analysis
CTPA examinations were obtained with a Philips 256-row spiral CT scanner after intravenous injection of nonionic contrast agent (Omnipaque, iodine concentration 300 mg/mL) at a dose of 2.0 ~ 2.5 mL/kg body weight. The injection flow rate was 0.5 ~ 2.5 mL/s. The CT scan parameters were as follows: 100 kV, automatic tube current, a slice thickness of 0.625 mm. Patients who could not cooperate during the examination underwent CT under sedation. CTPA images were analyzed by two experienced cardiovascular radiologists using a double-blind method.
Statistical analysis
SPSS 27.0 statistical software was used for data analysis. Quantitative data of normal distribution are expressed as the mean ± standard deviation (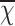 ± S), while non-normally distributed data are expressed as the median (P25, P75). Two-sample t-tests or Mann-Whitney U tests were used for comparisons between groups. The chi-square test was used for categorical data. Logistic regression analysis was used to explore the risk factors for PE in children with MPP pulmonary consolidation. First, univariate logistic regression analysis was performed to screen potential risk factors. Second, multicollinearity analysis was carried out. Finally, multivariate logistic regression analysis was performed to screen out independent risk factors. Receiver operating characteristic (ROC) curves were used to evaluate the efficacy of the study indicators. P < 0.05 was considered statistically significant.
± S), while non-normally distributed data are expressed as the median (P25, P75). Two-sample t-tests or Mann-Whitney U tests were used for comparisons between groups. The chi-square test was used for categorical data. Logistic regression analysis was used to explore the risk factors for PE in children with MPP pulmonary consolidation. First, univariate logistic regression analysis was performed to screen potential risk factors. Second, multicollinearity analysis was carried out. Finally, multivariate logistic regression analysis was performed to screen out independent risk factors. Receiver operating characteristic (ROC) curves were used to evaluate the efficacy of the study indicators. P < 0.05 was considered statistically significant.
Results
General data
There were significant differences in age of onset and hospitalization days between the two groups (P < 0.05, Table 1). There was no significant difference in sex between the two groups (P > 0.05, Table 1). Among all study subjects, the male to female ratio was (1.18:1).
Table 1.
Comparison of general data, clinical manifestations and co-pathogen infection distribution.
| Variables | Control group (n = 138) | PE group (n = 69) | P |
|---|---|---|---|
| Male | 72, 52.20% | 40, 58.00% | 0.430 |
| Age (months) | 67.00 (44.00–84.00) | 84.00 (70.00-96.50) | 0.000 |
| Hospitalization days | 11.00 (8.00–15.00) | 16.00 (13.25–22.25) | 0.000 |
| Symptoms | |||
| Fever days | 8.00 (5.00–12.00) | 12.00 (10.00-16.50) | 0.000 |
| Cough | 135, 97.80% | 69, 100.00% | 0.537 |
| Dyspnea | 42, 30.40% | 22, 31.90% | 0.832 |
| Chest pain | 4, 2.90% | 14, 20.30% | <0.001 |
| Hemoptysis | 0, 0.00% | 9, 13.00% | <0.001 |
| Extra-respiratory symptoms | 15, 10.90% | 21, 30.40% | <0.001 |
| Complication | |||
| Respiratory system complications | 109, 79.00% | 69, 100.00% | <0.001 |
| Endobronchitis | 70, 50.70% | 60, 87.00% | <0.001 |
| Sputum bolt | 11, 8.00% | 29, 42.00% | <0.001 |
| Plastic bronchitis | 2, 1.40% | 11, 15.90% | <0.001 |
| Bronchial stenosis | 15, 10.90% | 12, 17.40% | 0.189 |
| Bronchiectasis | 3, 2.20% | 5, 7.20% | 0.161 |
| Pulmonary atelectasis | 8, 5.80% | 11, 15.90% | 0.017 |
| Pleural effusion | 73, 52.90% | 56, 81.20% | <0.001 |
| External respiratory complications | 49, 35.50% | 47, 68.10% | <0.001 |
| Digestive system | 22, 15.90% | 34, 49.30% | <0.001 |
| Nervous system | 3, 2.20% | 1, 1.40% | 1.000 |
| Circulatory system | 24, 17.40% | 27, 39.10% | <0.001 |
| Blood system | 10, 7.20% | 2, 2.90% | 0.344 |
| Cutaneous mucosal system | 4, 2.90% | 9, 13.00% | 0.011 |
| Urinary system | 2, 1.40% | 1, 1.40% | 1.000 |
| Co-pathogen infection | 58, 42.00% | 25, 36.20% | 0.422 |
| Bacteria (%) | 28, 20.14% | 7, 10.14% | / |
| Fungi (%) | 13, 9, 42% | 8, 11.59% | / |
| Virus (%) | 35, 25.36% | 11, 15.94% | / |
| Number of FBAL | 1.00 (0.00–1.00) | 2.00 (1.00–2.00) | 0.000 |
Clinical manifestations
All the patients had fever. There was no significant difference in the incidence of cough or dyspnea between the two groups (P > 0.05, Table 1).
There were significant differences in fever days, chest pain, hemoptysis, extra-respiratory symptoms (dizziness, headache, abdominal pain, limb pain) and fiberoptic bronchoscopic alveolar lavage (FBAL) times between the two groups, and they were higher in the PE group than in the control group (P < 0.05, Table 1). All the patients underwent fiberoptic bronchoscopy, and 78.26% received FBAL.
The incidence of respiratory complications and external respiratory complications in the PE group was also significantly higher than that in the control group (P < 0.05, Table 1). Respiratory complications were mainly described in Table 1, which also included 11 cases of subcutaneous emphysema, 6 cases of pneumomediastinum or pulmonary interstitial pneumonia, and 1 case of pulmonary hemorrhage. Extra-respiratory complications with statistically significant differences between groups included digestive, circulatory and cutaneous mucosal complications. The most common digestive complications were liver damage (30 patients), ascites (27 patients) and gastrointestinal bleeding (1 patient). Circulatory complications can include myocardial damage, infective endocarditis, arrhythmia, cardiac insufficiency, pericardial effusion, and atrial thrombosis, and others. All patients underwent echocardiography within 48 h of admission. Echocardiography showed infective endocarditis with neoplasm in 7 cases, pericardial effusion in 28 cases, atrial thrombosis in 2 cases, and no right heart failure or pulmonary hypertension. The main complication of the cutaneous mucous system was mild skin rash. There was no statistically significant difference in the proportion of nervous or urinary complications between the two groups (P > 0.05, Table 1). Nervous complications include toxic encephalopathy, encephalitis, intracranial hemorrhage and cerebral hernia. Urinary complications include renal damage, acute renal failure, and renal infarction. The complications of each system may have one or more manifestations.
In addition, sepsis occurred in 2 patients (2.90%) in the PE group and 10 patients (7.20%) in the control group. Five patients (7.25%) in the PE group were complicated with thrombosis in other parts, including splenic embolism, atrial thrombosis, hepatic venous thrombosis, lower extremity venous thrombosis, and renal embolism.
Co-pathogen infection
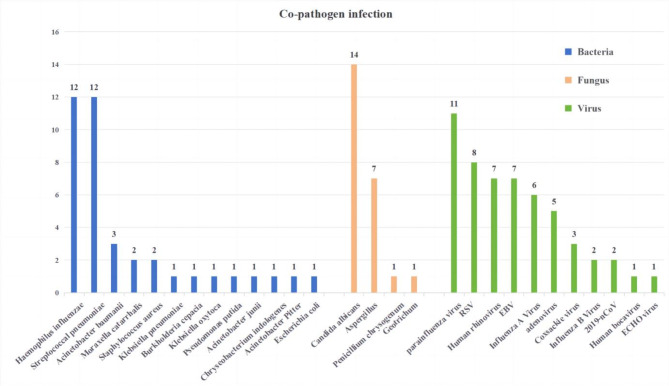
Etiological tests were performed for all patients, and there was no statistically significant difference in co-pathogen infection between the two groups (P > 0.05, Table 1). Of 207 subjects, 83 patients (40.10%) were coinfected with other pathogens, of which viruses (22.22%) were the most common, followed by bacteria (16.91%) and fungi (10.14%). Coinfection with multiple pathogens is not uncommon. The details of the MPP children coinfected with pathogens are shown in Fig. 1.
Fig. 1.
Co-pathogen infection.
Laboratory data
The white blood cell (WBC) count, neutrophil percentage (N%), neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP), prothrombin time (PT), D-D, lactate dehydrogenase (LDH), immunoglobulin M (IgM), immunoglobulin A (IgA) and serum ferritin (SF) were significantly higher than those in the control group (P < 0.05, Table 2). The lymphocyte percentage (L%), activated partial thromboplastin time (APTT), albumin-to-globulin ratio (AGR) and complement C4 levels in the PE group were significantly lower than those in the control group (P < 0.05, Table 2).
Table 2.
Comparison of laboratory variables between the two groups.
| Variables | Control group (n = 138) | PE group (n = 69) | P |
|---|---|---|---|
| WBC (10^9/L) | 8.410 (6.830–12.238) | 15.210 (11.180–17.855) | 0.000 |
| N%, % | (68.986 ± 14.511) | (78.407 ± 10.684) | <0.001 |
| L%, % | 24.750 (15.175–33.025) | 14.700 (9.450–19.600) | 0.000 |
| NLR | 2.781 (1.815–5.396) | 5.439 (3.717–9.209) | <0.001 |
| CRP (mg/L) | 11.310 (2.505–34.640) | 31.750 (17.440–57.315) | 0.000 |
| ESR (mm/h) | 40.000 (24.000–54.000) | 40.000 (26.000–65.500) | 0.287 |
| PT (S) | 12.150 (11.375–12.900) | 12.450 (11.825–13.400) | 0.019 |
| APTT (S) | 29.450 (25.400–34.025) | 26.550 (22.300–30.500) | 0.001 |
| D-D (mg/L) | 0.510 (0.370–1.775) | 3.315 (1.765–5.285) | 0.000 |
| AGR | 1.614 (1.359–1.989) | 1.224 (1.120–1.363) | <0.001 |
| LDH (U/L) | 359.000 (299.850–462.250) | 544.000 (403.000–749.500) | 0.000 |
| IgG (g/L) | 8.850 (7.058–10.560) | 8.870 (8.070–11.025) | 0.161 |
| IgM (g/L) | 1.135 (0.913–1.588) | 2.260 (1.248–3.550) | 0.000 |
| IgA (g/L) | 1.140 (0.623–1.680) | 1.510 (1.180–2.098) | 0.000 |
| Complement C3 (g/L) | (1.359 ± 0.313) | (1.271 ± 0.301) | 0.088 |
| Complement C4 (g/L) | (0.334 ± 0.121) | (0.267 ± 0.125) | 0.001 |
| PCT (ng/ml) | 0.139 (0.078–0.397) | 0.190 (0.089–0.470) | 0.277 |
| SF (ng/mL) | 121.050 (72.925–258.325) | 243.800 (177.500–409.100) | 0.000 |
| IL-6 (pg/ml) | 26.330 (13.110–101.600) | 30.050 (11.030–60.260) | 0.473 |
WBC, White blood cell count; N%, neutrophil percentage; L%, lymphocyte percentage; NLR, neutrophil-to-lymphocyte ratio; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PT, prothrombin time; APTT , activated partial thromboplastin time; D-D, D-dimer; AGR, albumin-to-globulin ratio; LDH, lactate dehydrogenase; IgG, immunoglobulin G; IgM, immunoglobulin M; IgA, immunoglobulin A; PCT, procalcitonin; SF, serum ferritin; IL-6, interleukin-6.
There were no significant differences in erythrocyte sedimentation rate (ESR), immunoglobulin G (IgG), complement C3, procalcitonin (PCT) and interleukin-6 (IL-6) between the two groups (P > 0.05, Table 2).
Treatment of the PE group
All the children were treated with conventional antibiotics. In the PE group, the average number of days from symptom onset to diagnosis was 19.12 days. Low-molecular-weight heparin (100 IU/kg, every 12 h, subcutaneous injection) was applied to all children in the PE group as an anticoagulant; 4 of them received low-molecular-weight heparin combined with rivaroxaban or warfarin anticoagulant therapy, and 1 child was given aspirin antiplatelet therapy. Thrombolytic therapy was performed in 3 patients with urokinase, and thrombectomy was performed in 4 patients. Ten children were treated with assisted mechanical ventilation or Extracorporeal Membrane Oxygenation (ECMO), with fever days ranging from 7 ~ 25 and PE appearing on days (8 ~ 25) of the disease course. All six children who received mechanical ventilation had dyspnea, and their mean duration of mechanical ventilation was 6 days. One of them developed hemodynamic instability and was treated with emergency ECMO-assisted pulmonary artery thrombectomy after 1 day of mechanical ventilation. Unfortunately, the child eventually died of complications of severe intracranial hemorrhage and brain herniation. Furthermore, four children underwent ECMO-assisted removal of heart valve neoplasms.
Logistic regression analysis
Multivariate logistic regression analysis revealed that fever days, D-D, IgA, chest pain, extra-respiratory symptoms, plastic bronchitis, and cutaneous mucosal system complications were the independent risk factors (P < 0.05, Table 3).
Table 3.
Logistic regression analysis of PE associated with MPP in children.
| Variables | Univariate logistic regression | Multivariate logistic regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age (months) | 1.020 | 1.009 ~ 1.030 | < 0.001 | |||
| Hospitalization days | 1.148 | 1.089 ~ 1.211 | < 0.001 | |||
| Symptoms | ||||||
| Fever days | 1.152 | 1.082 ~ 1.226 | < 0.001 | 1.152 | 1.062 ~ 1.249 | <0.001 |
| Chest pain | 8.527 | 2.687 ~ 27.057 | < 0.001 | 11.034 | 1.990 ~ 61.168 | 0.006 |
| Extra-respiratory symptoms | 3.587 | 1.709 ~ 7.533 | 0.001 | 2.905 | 1.009 ~ 8.362 | 0.048 |
| Complication | ||||||
| Respiratory system | ||||||
| Endobronchitis | 6.476 | 2.981 ~ 14.072 | < 0.001 | |||
| Sputum bolt | 8.370 | 3.838 ~ 18.255 | < 0.001 | |||
| Plastic bronchitis | 12.897 | 2.771 ~ 60.017 | 0.001 | 32.821 | 3.460 ~ 311.297 | 0.002 |
| Pulmonary atelectasis | 3.082 | 1.178 ~ 8.064 | 0.022 | |||
| Pleural effusion | 3.836 | 1.924 ~ 7.646 | < 0.001 | |||
| External respiratory complications | ||||||
| Digestive system | 5.122 | 2.658 ~ 9.872 | < 0.001 | |||
| Circulatory system | 3.054 | 1.588 ~ 5.872 | 0.001 | |||
| Cutaneous mucosal system | 5.025 | 1.489 ~ 16.961 | 0.009 | 8.033 | 1.258 ~ 51.310 | 0.028 |
| Number of FBAL | 2.474 | 1.711 ~ 3.578 | < 0.001 | |||
| WBC (10^9/L) | 1.151 | 1.084 ~ 1.222 | < 0.001 | |||
| N%, % | 1.062 | 1.033 ~ 1.090 | < 0.001 | |||
| L%, % | 0.926 | 0.897 ~ 0.957 | < 0.001 | |||
| NLR | 1.121 | 1.049 ~ 1.198 | 0.001 | |||
| CRP (mg/L) | 1.010 | 1.003 ~ 1.017 | 0.005 | |||
| PT (S) | 0.997 | 0.958 ~ 1.039 | 0.903 | |||
| APTT (S) | 1.009 | 0.994 ~ 1.024 | 0.265 | |||
| D-D (mg/L) | 1.307 | 1.153 ~ 1.483 | < 0.001 | 1.198 | 1.032 ~ 1.391 | 0.018 |
| AGR | 0.050 | 0.018 ~ 0.144 | < 0.001 | |||
| LDH (U/L) | 1.002 | 1.001 ~ 1.003 | < 0.001 | |||
| IgM (g/L) | 1.972 | 1.458 ~ 2.669 | < 0.001 | |||
| IgA (g/L) | 2.060 | 1.319 ~ 3.216 | 0.001 | 2.253 | 1.300 ~ 3.904 | 0.004 |
| Complement C4 (g/L) | 0.011 | 0.001 ~ 0.184 | 0.002 | |||
| SF (ng/mL) | 1.001 | 1.000 ~ 1.003 | 0.079 | |||
ROC curve
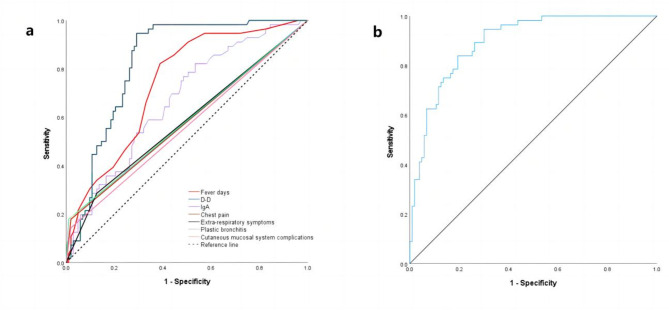
ROC curve analysis showed that fever days ≥ 7.5, D-D ≥ 0.895 mg/L, IgA ≥ 1.015 g/L increased the risk of PE in children with MPP pulmonary consolidation. In addition, the combination of the above indexes with chest pain, extra-respiratory symptoms, plastic bronchitis, and cutaneous mucosal system complications further improved the efficiency [AUC (95% CI) = 0.895 (0.847 ~ 0.943)], and the sensitivity and specificity were 0.839 and 0.806, respectively (Table 4; Fig. 2).
Table 4.
The results of ROC curve.
| Variables | AUC | Cut-off value | Youden index | Sensitivity | Specificity | 95% CI |
|---|---|---|---|---|---|---|
| Fever days | 0.740 | 7.5 | 0.421 | 0.928 | 0.493 | (0.672 ~ 0.808) |
| D-D (mg/L) | 0.826 | 0.895 | 0.625 | 0.956 | 0.669 | (0.770 ~ 0.882) |
| IgA (g/L) | 0.669 | 1.015 | 0.284 | 0.821 | 0.463 | (0.584 ~ 0.753) |
| Joint variable of seven factors | 0.895 | / | 0.645 | 0.839 | 0.806 | (0.847 ~ 0.943) |
Fig. 2.
ROC curve. (a) ROC curves of fever days, D-D, IgA, chest pain, extra-respiratory symptoms, plastic bronchitis, cutaneous mucosal system complications. (b) ROC Curve of joint variable.
Discussion
Most MPP cases are mild, but approximately 12% of MPP children progress to severe cases with serious complications9. PE associated with MPP is a rare but serious extra-respiratory complication in MPP children, with atypical clinical manifestations, high mortality and poor prognosis7. In recent years, embolism caused by MPP has received increasing amounts of attention from paediatricians. The objective of this study was to retrospectively analyze the clinical characteristics of MPP pulmonary consolidation in children and explore the independent risk factors for progression to PE.
The specific mechanism of thrombosis caused by MP infection is still unclear, but it may be related to endothelial cell injury, autoimmune response, coagulation and anticoagulation imbalance. (1) MP may directly damage vascular endothelial cells, leading to local vasculitis and thrombotic vascular occlusion7. (2) MP can activate immune cells to produce cytokines, and produce cross-reactive antibodies by molecular simulation to trigger autoimmune response10. The transient hypercoagulable state caused by antiphospholipid antibodies produced by the autoimmune response induced by MP infection has been considered as one of the possible mechanisms3,11,12. (3) MPP can cause an imbalance of coagulation and anticoagulation in vivo13, which may lead to an abnormal fibrinolytic coagulation system through increased platelet activation14 and activation of the complement system15, increasing the risk of thrombotic complications16.
In our study, fever days, chest pain, extra-respiratory symptoms, D-D, IgA, plastic bronchitis, and cutaneous mucosal system complications were found to be the independent risk factors for progression to PE in children with MPP pulmonary consolidation.
The longer the number of fever days in MPP children, the greater the risk of progression to severe or critical illness8. The median number of fever days in the PE group was 12.00 (10.00 ~ 16.50), which was significantly greater than that in the control group. The extension of fever days is considered to be associated with prolonged disease caused by an excessive inflammatory response and severe lung injury. In addition, we found that fever days ≥ 7.5 was an independent risk factor for PE in children with MPP pulmonary consolidation.
Chest pain, hemoptysis and dyspnea are typical symptoms of PE, but the manifestations in children are atypical, and syncope, abdominal pain and other atypical manifestations may occur7. The diagnosis of early PE requires timely identification of a range of nonspecific signs and symptoms. PE should be suspected when one of the above conditions is present17. In our study, the proportion of patients with chest pain and extra-respiratory symptoms in the PE group was significantly greater than that in the control group, which consistent with the results of other domestic studies8,18. In addition, some studies have indicated that 30% of PE patients associated with MPP also experience pain in different parts of the body, and children with abdominal organ embolism also experience gastrointestinal symptoms such as abdominal pain and vomiting19. In our PE group, 21 (30.40%) children had extra-respiratory symptoms such as dizziness, headache, abdominal pain, and limb pain. The accuracy of the data on chest pain and extra-respiratory symptoms was biased considering that younger children were unable to express or correctly express their subjective feelings.
D-D is an extremely important screening and diagnostic index in the early diagnosis of PE20,21. D-D is a fibrin-degrading protein fragment that represents the activation of coagulation and fibrinolysis systems17. During acute thrombosis, the level of D-D in the blood increases. Previous studies have shown that D-D is highly sensitive in the diagnosis of thromboembolism in children22, and it is also highly sensitive in predicting thromboembolism in MPP patients7,23. Some studies have reported significantly elevated plasma D-D levels in children with MPP, especially in severe cases such as PE and necrotizing pneumonia3,13,19. Furthermore, D-D > 5.0 mg/L, especially D-D > 11.1 mg/L, is helpful for the early diagnosis of thrombosis in MPP patients3. In our study, D-D was found to be an independent risk factor for PE in children with MPP pulmonary consolidation, and the risk significantly increased when D-D was ≥ 0.895 mg/L. The difference from other studies may be related to the fact that we did not detect the peak value. In addition, it is worth mentioning that the higher the level of D-D is, the greater the risk of thrombosis being more widespread and severe3.
The deterioration of the MPP is related to the immune response24. IgA is an important part of the human immune system, and an increasing number of studies have recently focused on IgA10. In addition to its well-known passive function, IgA has recently been found to actively control immune responses. The active immunity of IgA occurs through the regulation of cytokine and chemokine production by human myeloid immune cells25. Studies have shown that the serum IgA concentration is significantly increased in patients with MPP, and this increase is even more significant in patients with severe pneumonia26,27. Our findings are consistent with the above. We also found that IgA was an independent risk factor for PE in children with MPP pulmonary consolidation, and that children with MPP pulmonary consolidation with IgA ≥ 1.015 g/L had a significantly increased risk of PE. In addition, IgA not only has diagnostic value for MPP but also has great therapeutic potential by regulating IgA in vivo to treat many diseases, including severe MPP.
Plastic bronchitis is an acute and critical pulmonary disease in pediatric patients, which has been studied and reported more and more. Plastic bronchitis is one of the important causes of severe MPP and fulminant MPP8. In recent years, the number of plastic bronchitis cases caused by MP infection has steadily increased, and a domestic study has shown that MPP is a common pathogen causing plastic bronchitis28. Plastic bronchitis was present in 13 (6.30%) of our 207 subjects. The incidence of plastic bronchitis in the PE group was 15.90% greater than that in the control group (2.40%). The pathogenesis of plastic bronchitis has not been fully elucidated and may be related to coagulation function, especially D-D29. In addition, the plastic bronchitis is often associated with the presence of neutrophils and eosinophils on pathologic examination. Although the disease is different, Etosis caused by neutrophils and eosinophils has been pointed out as a cause of plastic bronchitis due to A(H1N1)pdm0930. In this study, the WBC of 13 children with plastic bronchitis was neutrophil predominant, and the N% was (62.8% ~ 89.8%). The results of multivariate logistic regression analysis showed that plastic bronchitis was an independent risk factor for PE in children with MPP pulmonary consolidation, with a high specificity of 0.986.
MP can cause a variety of external respiratory complications, and the cutaneous mucosal system is most often involved31. Cutaneous mucosal system complications caused by MP are associated with systemic inflammation and increase the risk of long-term sequelae32. Cutaneous and mucous system complications are more common in severe MPP patients, and most of these complications manifest as rash and mucous inflammation (MIRM), including urticaria, maculopapular rash, and anaphylactoid purpura27. The pathogenesis of MIRM is unclear, and the general hypothesis is that MIRM is caused by the cloning of B cells and the subsequent production of immunoglobulins, resulting in the deposition of skin immune complexes33. MIRM occurred in 13 (6.30%) of our 207 patients and was more common in the PE group. Most of the children in this study presented with mild rash. We also found that cutaneous mucosal system complications were an independent risk factor for PE in MPP children with pulmonary consolidation.
To date, the sample size of our study is the largest among the relevant studies on PE associated with MPP. However, there are some limitations to this study. First, this was a single-center retrospective study, with possible selection bias. Further prospective studies with large sample sizes are needed. Second, without systematic long-term follow-up of children with pulmonary embolism, our study was unable to assess long-term outcomes. In addition, the mechanism of embolism in MPP patients remains unclear, and relevant animal studies need to be established.
Conclusions
In conclusion, fever days ≥ 7, D-D ≥ 0.895 mg/L, IgA ≥ 1.015 g/L, chest pain, extra-respiratory symptoms, plastic bronchitis, and cutaneous mucosal system complications were found to be independent risk factors for PE in children with MPP pulmonary consolidation. For MPP children with pulmonary consolidation suspected of having PE, pediatricians should start early dynamic monitoring and examination of the above indicators, striving to effectively control disease progression and improve prognosis.
Acknowledgements
The authors sincerely thank the participants of the study.
Author contributions
Xue Zhang participated in the research design, data collection, data analysis and writing. Ruiyang Sun, Jiapu Hou, Wanyu Jia and Peng Li participated in the research design and data analysis. Chunlan Song and Yibing Cheng participated in the study design and critically revised the important knowledge content.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due the hospital’s policies or confidentiality agreements but are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Children’s Hospital of Henan Province (2023-K-128). The need for informed consent was waived given the retrospective nature of the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Waites, K. B., Xiao, L., Liu, Y., Balish, M. F. & Atkinson, T. P. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin. Microbiol. Rev.30(3), 747–809. 10.1128/CMR.00114-16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li, Z. J. et al. Etiological and epidemiological features of acute respiratory infections in China. Nat. Commun.12(1), 5026. 10.1038/s41467-021-25120-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu, J. et al. Mycoplasma pneumoniae pneumonia associated thrombosis at Beijing Children’s hospital. BMC Infect. Dis.20(1), 51. 10.1186/s12879-020-4774-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim, K. et al. Global trends in the proportion of macrolide-resistant mycoplasma pneumoniae infections: A systematic review and meta-analysis. JAMA Netw. Open5(7), e2220949. 10.1001/jamanetworkopen.2022.20949 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moynihan, K. M. et al. Severe mycoplasma pneumoniae infection in children admitted to pediatric intensive care. Pediatr. Infect. Dis. J.37(12), e336–e338. 10.1097/inf.0000000000002029 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Jin, X. et al. Assessment of levels of D-dimer and interferon-γ in pediatric patients with Mycoplasma pneumoniae pneumonia and its clinical implication. Exp. Therap. Med.16(6), 5025–5030. 10.3892/etm.2018.6873 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, L. et al. Thromboembolic complications of Mycoplasma pneumoniae pneumonia in children. Clin. Respir. J.17(3), 187–196. 10.1111/crj.13584 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.China, N. H. C o t P s R o, Guidelines for diagnosis and treatment of mycoplasma pneumoniae pneumonia in children. China Licensed Pharmacist20(3), 16–24 (2023).
- 9.Kutty, P. K. et al. Mycoplasma pneumoniae among children hospitalized with community-acquired pneumonia. Clin. Infect. Dis.68(1), 5–12. 10.1093/cid/ciy419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waites, K. B. & Talkington, D. F. Mycoplasma pneumoniae and its role as a human pathogen. Clin. Microbiol. Rev.17(4), 697–728. 10.1128/cmr.17.4.697-728.2004 (2004). table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witmer, C. M., Steenhoff, A. P., Shah, S. S. & Raffini, L. J. Mycoplasma pneumoniae, splenic infarct, and transient antiphospholipid antibodies: A new association? Pediatrics119(1), e292-5. 10.1542/peds.2006-1340 (2007). [DOI] [PubMed]
- 12.Snowden, N., Wilson, P. B., Longson, M. & Pumphrey, R. S. Antiphospholipid antibodies and Mycoplasma pneumoniae infection. Postgrad. Med. J.66(775), 356–362. 10.1136/pgmj.66.775.356 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, T. et al. Evaluation of variation in coagulation among children with Mycoplasma pneumoniae pneumonia: A case-control study. J. Int. Med. Res.45 (6), 2110–2118. 10.1177/0300060517709613 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichota, A., Gwozdzinski, K. & Szewczyk, E. M. Microbial Modulation of Coagulation disorders in venous thromboembolism. J. Inflamm. Res.13, 387–400. 10.2147/jir.S258839 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narita, M. Pathogenesis of neurologic manifestations of Mycoplasma pneumoniae infection. Pediatr. Neurol.41(3). 10.1016/j.pediatrneurol.2009.04.012 (2009). 159 – 66. [DOI] [PubMed]
- 16.Song, S. & Xu, Y. A retrospective study of the clinical characteristics of 9 children with pulmonary embolism associated with Mycoplasma pneumoniae pneumonia. BMC Pediatr.23(1), 370. 10.1186/s12887-023-04188-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freund, Y., Cohen-Aubart, F. & Bloom, B. Acute pulmonary embolism: A review. JAMA328(13), 1336–1345. 10.1001/jama.2022.16815 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Sheng, C-Q., Yang, C-F., Ao, Y., Zhao, Z-Y. & Li, Y-M. Mycoplasma pneumoniae pneumonia with pulmonary embolism: A study on pediatric cases in Jilin Province of China. Exp. Therap. Med.21(3), 201. 10.3892/etm.2021.9634 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han, C. et al. Analysis of the risk factors and clinical features of Mycoplasma pneumoniae pneumonia with embolism in children: a retrospective study. Ital. J. Pediatr.48(1), 153. 10.1186/s13052-022-01344-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maughan, B. C., Jarman, A. F., Redmond, A., Geersing, G-J. & Kline, J. A. Pulmonary embolism. BMJ (Clinical research ed.), 384: p. e071662. 10.1136/bmj-2022-071662 (2024). [DOI] [PubMed]
- 21.Konstantinides, S. V. et al. ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J.41(4), 543–603. (2019). 10.1093/eurheartj/ehz405 [DOI] [PubMed]
- 22.Hennelly, K. E. et al. Detection of pulmonary embolism in high-risk children. J. Pediatr.178, 214–218. 10.1016/j.jpeds.2016.07.046 (2016). .e3. [DOI] [PubMed] [Google Scholar]
- 23.Gu, H., Li, B., Han, Y., Yang, S. & Wang, X. Risk factors for suspected pulmonary embolism in children: Complication of Mycoplasma pneumoniae pneumonia. Eur. J. Radiol.176, 111474. 10.1016/j.ejrad.2024.111474 (2024). [DOI] [PubMed] [Google Scholar]
- 24.Zhu, Y. et al. Immune response plays a role in Mycoplasma pneumoniae pneumonia. Front. Immunol.14, 1189647. 10.3389/fimmu.2023.1189647 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen, I. S., Baeten, D. L. P. & Dunnen, J. The inflammatory function of human IgA. Cell. Mol. Life Sci.76(6), 1041–1055. 10.1007/s00018-018-2976-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang, Y. et al. Serum amyloid a, C-reactive protein, and procalcitonin levels in children with Mycoplasma pneumoniae infection. J. Clin. Lab. Anal.36(3), e24265. 10.1002/jcla.24265 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, X., Sun, R., Jia, W., Li, P. & Song, C. Clinical characteristics of lung consolidation with Mycoplasma pneumoniae pneumonia and risk factors for mycoplasma pneumoniae necrotizing pneumonia in children. Infect. Dis. Therapy10.1007/s40121-023-00914-x (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang, J-J., Yang, X-Q., Zhuo, Z-Q. & Yuan, L. Clinical characteristics of plastic bronchitis in children: A retrospective analysis of 43 cases. Respir. Res.23(1), 51. 10.1186/s12931-022-01975-1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang, L. et al. Clinical features and risk factors of plastic bronchitis caused by Mycoplasma pneumoniae pneumonia in children. BMC Pulm. Med.23(1), 468. 10.1186/s12890-023-02766-0 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura, S. et al. Histological characteristics of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinases-1 in asthmatic murine model during A(H1N1)pdm09 infection. Pathol. Int.72(10), 506–518. 10.1111/pin.13268 (2022). [DOI] [PubMed] [Google Scholar]
- 31.Berlot, J. R., Mrvič, T., Košnik, M. & Keše, D. The association between Mycoplasma pneumoniae genotype and cutaneous disease. Microorganisms. 11 (1). 10.3390/microorganisms11010205 (2023). [DOI] [PMC free article] [PubMed]
- 32.Sauteur, P. M. M. et al. Frequency and clinical presentation of mucocutaneous disease due to Mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol.. 156 (2), 144–150. 10.1001/jamadermatol.2019.3602 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haseeb, A. et al. Ocular involvement in mycoplasma induced rash and mucositis: A systematic review of the literature. Ocul. Surf.28, 1–10. 10.1016/j.jtos.2022.11.007 (2023). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due the hospital’s policies or confidentiality agreements but are available from the corresponding author on reasonable request.