Abstract
To redirect the tropism of the vaccine strain of measles virus (MV), Edmonston B, to a targeted cell population, we displayed on the viral hemagglutinin (H) a single-chain antibody (scAb) specific for the tumor-associated carcinoembryonic antigen (CEA). We generated H fusion proteins with three forms of the scAb appended, differing in the lengths of the linkers separating the VH and VL domains and thus in the oligomerization states of the scAbs. All proteins were stable, appeared properly folded, and were transported to the cell surface, but only H displaying the long-linker form of scAb was functional in supporting cell-cell fusion. This protein induced extensive syncytia in cells expressing the normal virus receptor CD46 and also in CD46-negative cells expressing the targeted receptor, human CEA. Replication-competent MV with H replaced by H displaying the long-linker form of scAb was recovered and replicated efficiently in both CD46-positive and CD46-negative, CEA-positive cells. Thus, MV not only tolerates the addition of a scAb on its H protein but also infects cells via a novel interaction between the scAb and its targeted receptor.
Engineering viral tropism is the goal of many gene therapy-based strategies. For cytoreductive therapies which aim to eliminate deleterious cells, developing retargeted viruses that possess inherent cytotoxicity circumvents the delivery of a cytotoxic product. In this case, targeting restricts the viral cytoreductive property to the desired cell type, which is thereby specifically eliminated. Many viral envelope glycoproteins, including those of type-C retroviruses, lentiviruses, and paramyxoviruses, induce extensive cell-cell fusion, recruiting many uninfected cells into large multinucleated syncytia, which ultimately die (12). Retargeted fusogenic membrane glycoproteins are thus a promising novel class of cytotoxic genes (1, 7).
We aimed to retarget the Edmonston B vaccine strain of the paramyxovirus measles virus (MV) to a specific subset of tumor cells by displaying on its hemagglutinin (H) envelope glycoprotein a single-chain antibody (scAb) specific for a tumor-associated antigen. scAbs are particularly desirable targeting ligands, since their conserved structural framework should facilitate the modular exchange of specificity determinants. The ability to incorporate scAbs as extensions to viral envelope glycoproteins was first demonstrated with retroviral vectors; however, these vectors are rarely able to efficiently transduce target cells (18, 27). This block to gene transfer is reportedly due to the inability of the scAb-displaying envelope protein to undergo the post-receptor-binding conformational changes necessary for fusion (35).
MV H is a type II transmembrane glycoprotein responsible for the interaction of the virus with its cellular receptor(s). For the Edmonston strain, two receptors are known: the ubiquitously expressed regulator of complement activation CD46 (6, 19) and the T- and B-cell-specific protein of the immunoglobulin (Ig) superfamily, SLAM (signaling lymphocyte activation molecule [33]). H is postulated to exist at the viral or infected-cell surface as a tetramer of two covalently linked dimers (15) which interacts with the trimeric viral envelope fusion (F) glycoprotein and supports its ability to mediate virus-cell or cell-cell fusion (14). Since MV differs from retroviruses in distributing receptor-binding and fusion functions on two separately encoded proteins (14), we envisaged that scAb display on MV H may result in successful gene transfer.
The targeted antigen in this study, carcinoembryonic antigen (CEA), is highly overexpressed on the surfaces of a number of cancerous cells, particularly colorectal, gastric, lung, pancreatic, and breast carcinomas (20). Its expression in normal adult tissue is restricted to selected epithelial cells (9), and the anti-CEA (αCEA) scAb we used, MFE-23, has little cross-reactivity to nonmalignant human tissue (5). Three forms of a disulfide-stabilized version of this scAb (8) were previously generated which differ in the lengths of the linkers separating the VH and VL domains, and as demonstrated for other scAbs (21), the oligomeric state depended on the linker length, with the zero linker form being trimeric, the short form being dimeric, and the long linker form being monomeric (J. Zhang and S. J. Russell, unpublished data).
We displayed these three forms of the αCEA scAb at the extracellular C terminus of H. All three chimeric proteins were expressed, were stable, and were transported to the cell surface. Furthermore, the long-linker form induced extensive syncytia in both CD46-positive and CD46-negative, CEA-positive cells. A replicating MV expressing this chimeric protein in place of H was generated. Significantly, this virus replicated not only with the efficiency of parental MV in CD46-positive cells but almost as efficiently in nonhuman cells expressing CEA, which the parental MV failed to infect.
MATERIALS AND METHODS
Plasmid construction.
cDNAs encoding the three forms of the scAb were transferred to a pCG-H vector (4) containing a factor Xa (New England Biolabs) cleavage site 3′ to the H open reading frame from retroviral expression vectors (J. Zhang, unpublished data) by PCR amplification (primer sequences, 5′-GCGCGCTGGCCCAGGTG-3′ and 5′-TGCGGCCGCCCGTTTC-3′ [the BssHII and NotI sites are underlined]). For detection purposes, an amino-terminal Flag tag (DYKDDDDK) was inserted downstream of the ATG start codon of each H using the Quick-Change system (Stratagene). The integrity of all constructs was confirmed by DNA sequencing.
The cDNA encoding nontagged HXL was transferred from the pCG construct into a full-length infectious molecular clone of the MV Edmonston strain, p(+)MV-NSe (31), using the restriction enzymes PacI and SpeI and adhering to the reported paramyxovirus “rule of six” requirement (3). The virus was recovered as previously described (24).
Cell culture, transfection, and syncytium formation assay.
Vero, HeLa, HeLa-CEA, and MC38 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, penicillin, and streptomycin at 37°C and 5% CO2. MC38-CEA cells (clone MC38-CEA 2 [25]) were maintained in DMEM containing 10% fetal bovine serum, 0.5% G418, penicillin, and streptomycin at 37°C and 5% CO2.
Cells were transiently transfected using Superfect (Qiagen) and analyzed 18 to 24 h posttransfection. For syncytium formation assays, target cells (5 × 105/well in 35-mm-diameter wells) were cotransfected in duplicate with 1.5 μg of plasmid DNA encoding F and 1.5 μg of plasmid DNA encoding the appropriate H. The syncytia in 20 representative fields (20% of a 35-mm-diameter tissue culture well) were counted at various times, and the number of syncytia per well was calculated.
Preparation of MV stocks, purification of viral particles, and infection.
To prepare MV stocks, Vero cells (80% confluent in 10-cm-diameter dishes) were infected at a multiplicity of infection (MOI) of 0.1 PFU/cell and incubated at 37°C until approximately 90% of the cells were found in syncytia. The cells were resuspended in 3 ml of low-serum medium (Opti-MEM; Gibco), and particles were released by three repeated freeze-thaw cycles. Stock titers were determined by 50% tissue culture infective dose (TCID50) titration on Vero cells, using the Spearman-Karber method.
To purify viral particles, supernatant was harvested from infected cells, cell debris was removed by low-speed centrifugation (20,000 × g; 20 min; 4°C), and virus particles were concentrated at the interphase of a two-step 20 and 60% sucrose gradient in TNE buffer (10 mM Tris [pH 7.8], 100 mM sodium chloride, 1 mM EDTA) by centrifugation at 100,000 × g and 4°C for 90 min. The virus-containing fraction was diluted with TNE buffer to less than 30% sucrose, and particles were pelleted at 100,000 × g and 4°C for 90 min and resuspended in lysis buffer.
For infection, target cells (5 × 105/well in 35-mm-diameter wells) were incubated with cell-associated virus diluted to the appropriate MOI in Opti-MEM for 2 h. The viruses were removed and replaced with DMEM containing 10% fetal bovine serum for the specified duration of infection. For proteolytic digestion of the displayed scAb, viruses in clarified cell extracts (MOI, 1) were pretreated with 10 μg of factor Xa for 2 h at 23°C prior to the adsorption step, and the levels of protease were maintained at 10 μg/ml by replacing the medium with fresh medium containing factor Xa every 12 h. For antibody adsorption of cell surface CEA, cells were pretreated with 10 μg of COL1 (LabVision Corp.) for 2 h prior to infection. The level of antibody was maintained at 10 μg/ml by replacing the medium with fresh medium containing COL1 every 12 h.
Western blot analysis.
For Western blot analysis of MV proteins, transfected or infected cells were lysed for 5 min at 4°C in lysis buffer (50 mM Tris [pH 8.0], 62.5 mM EDTA, 0.4% deoxycholate, 1% Igepal [Sigma]), protease inhibitors (complete mix [Boehringer] and 1 mM phenylmethylsulfonyl fluoride [PMSF]) were added, and the supernatant was clarified by centrifugation at 5,000 × g for 10 min at 4°C. The resulting postnuclear supernatant was mixed with an equal volume of urea buffer (200 mM Tris [pH 6.8], 8 M urea, 5% sodium dodecyl sulfate [SDS], 0.1 mM EDTA, 0.03% bromphenol blue) containing 1.5% dithiothreitol (DTT) and incubated for 25 min at 50°C in a thermomixer. Samples were fractionated on SDS-polyacrylamide gels as indicated, blotted to polyvinyl difluoride membranes (Millipore), probed with antibody, and subjected to enhanced-chemiluminescence detection (Amersham Pharmacia Biotech). Chimeric H-αCEA proteins were detected with an anti-Flag antibody (M2; Sigma), and MV particles were analyzed with an MV-specific goat antiserum (kindly provided by S. Udem).
Metabolic labeling.
Vero cells transfected with various constructs were incubated for 30 min in labeling medium lacking cysteine, methionine, and ammonium sulfate and then metabolically labeled with [35S]-methionine (Amersham Pharmacia Biotech) at a final concentration of 100 μCi/ml for 45 min at 37°C. Subsequently, the labeling medium was replaced by chase medium containing 5% fetal calf serum, and the cells were incubated at 37°C for various period.
The cells were lysed in radioimmunoprecipitation assay buffer (10 mM Tris [pH 7.4], 1% deoxycholate, 1% Triton X-100, 0.1% SDS, 150 mM sodium chloride, protease inhibitors [complete mix and 1 mM PMSF]) for 15 min at 4°C, and the lysates were subjected to centrifugation for 30 min at 20,000 × g. Proteins were precipitated from the cell lysates with agarose-conjugated anti-Flag antibody (M2) by incubation for 1 to 2 h at 4°C. The precipitates were washed four times in lysis buffer prior to resuspension in urea buffer containing 1.5% DTT for 25 min at 50°C and fractionation on polyacrylamide gels. The dried gels were exposed to high-sensitivity film (Kodak Biomax).
Analysis of H dimerization.
For analysis of H complex formation, cells were metabolically labeled as described above. After 1 h of chase time, the cells were lysed (50 mM HEPES [pH 7.3], 100 mM sodium chloride, 10 mM n-dodecyl β-d-maltoside, protease inhibitors [complete mix] and 1 mM PMSF]), and Flag-tagged proteins were precipitated from the cell lysates with agarose-conjugated anti-Flag antibody (M2). For nonreducing gel electrophoresis, the precipitates were incubated in urea buffer lacking DTT.
Fluorescence-activated cell sorter (FACS) analysis.
Target cells (5 × 105/reaction) were incubated on ice in phosphate-buffered saline–fetal calf serum–azide for 30 min to prevent internalization. Primary antibody was incubated with the cell suspension for 1 h at 4°C. The cells were washed, incubated with secondary antibody, repeatedly washed, fixed in 0.4% paraformaldehyde, and analyzed using a Becton-Dickinson FACSCalibur and CellQuest software. For detection of virus binding to the cell surface, the cells were preincubated with virus at an MOI of 3 for 2 h at 4°C.
The 11/88 monoclonal antibody (MAb) (29) was used to detect surface CD46, COL1 antibody was used to detect CEA, and the I29 MAb (30) was used to detect surface H protein in transiently transfected cells and to detect virus bound to the cell surface. All primary antibodies were revealed with an anti-mouse antibody-fluorescein isothiocyanate conjugate (Sigma).
RESULTS
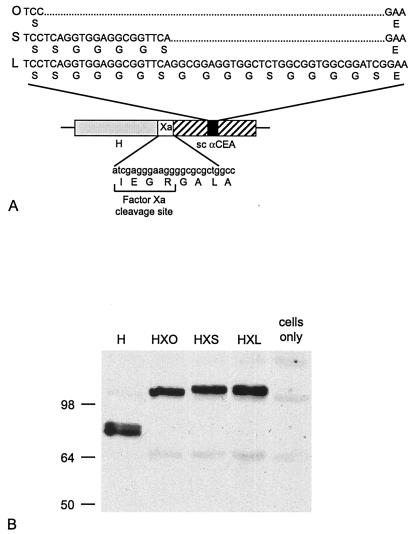
We generated constructs expressing three forms of the αCEA MFE-23 scAb as C-terminal fusions of H (Fig. 1A). The scAb forms differed in the length of the linker separating the VH and VL domains and were designated zero (0), short (S), and long (L), corresponding to linker lengths of 0, 6, and 16 amino acids, respectively. In each construct, the C terminus of H was separated from the scAb by an 8-amino-acid spacer, including a factor Xa cleavage site to facilitate removal of the displayed ligand. The chimeric H-αCEA proteins were designated HX0, HXS, and HXL, accordingly.
FIG. 1.
(A) Schematic diagram showing the relative positions of MV H (shaded), the 8-amino-acid spacer incorporating the factor Xa cleavage site (open), and the appended scAb domain (hatched). The linker sequences separating VH and VL in the three forms of the scAb (solid), indicated as 0, S, and L, are also given. (B) All three chimeric proteins are expressed at the expected molecular weights. Western blot analysis of Vero cells transfected with the indicated Flag-tagged H constructs or mock transfected as a control was performed using an anti-Flag antibody. The numbers correspond to the molecular weights in thousands.
Expression, stability, oligomerization, and cell surface localization of chimeric H-αCEA proteins.
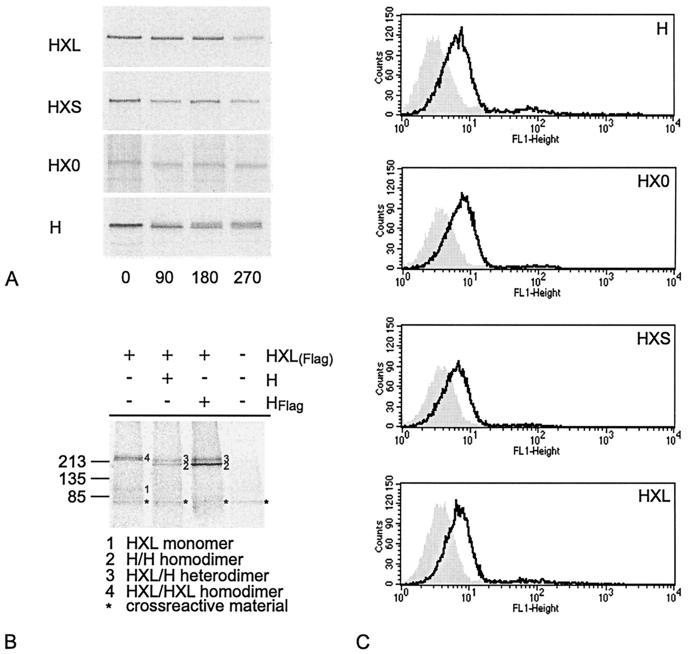
Expression of HX0, HXS, and HXL proteins at the expected molecular weights in all cell lines used was confirmed by Western blot analysis (shown in Fig. 1B for Vero cells; data not shown for HeLa, HeLa-CEA, MC38, and MC38-CEA cells). Furthermore, pulse-chase analyses demonstrated similar stabilities of expression for the chimeric H-αCEA proteins and for unmodified H (Fig. 2A). The half-lives for all proteins were greater than 3 h, as determined by band densitometry with a phosphorimager.
FIG. 2.
(A) All H-αCEA proteins are as stable as unmodified MV H. Pulse-chase analysis of Vero cells transfected with the indicated H constructs is shown. Antigenic material was precipitated after the indicated chase times (in minutes). (B) HXL protein dimerizes with itself and with unmodified H. Vero cells cotransfected with the indicated constructs were radiolabeled and lysed. Material immunoprecipitated with αFlag MAb was dissolved under nonreducing conditions. The numbers on the left refer to molecular weights in thousands. +, present; −, absent. (C) HX0, HXS, and HXL are localized at the cell surface. H was detected at the surfaces of MC38 cells, transfected with the indicated H constructs, by FACS analysis using an antibody directed against the extracellular region of MV H (open curve) or no primary antibody (shaded curve).
The H-like conformation of the HXL protein was verified by its ability to form covalently linked dimers with itself and with unmodified H. Following cotransfection of Flag-tagged HXL with unmodified, untagged H, or with empty plasmid or Flag-tagged H as a control, cells were metabolically labeled. Using an αFlag antibody, tagged proteins and any interacting untagged H proteins were immunoprecipitated, and the dimerization status was analyzed by gel electrophoresis under nonreducing conditions (Fig. 2B). Both homotypic dimers (HXL-HXL) and heterotypic dimers (HXL-H) were identified. In addition, and consistent with the postulated tetrameric nature of MV H, we observed the presence of untagged H-H dimers in the presence of tagged HXL, presumably resulting from the interaction of H-H with HXL-HXL dimers. Under conditions in which both homotypic and heterotypic complexes could form, the heterotypic HXL-H complex predominated, suggesting that dimerization of HXL with unmodified H was more efficient than that with itself. However, we cannot formally exclude the possibility that an excess of H was expressed compared to HXL and that this may bias the efficiency of dimer formation. Assuming that this is not the case, the efficiencies of both HXL-HXL and HXL-H complex formation were reduced compared with that of H-H dimerization. Thus, display of the scAb on H may reduce the efficiency of, but does not prevent, dimerization of the underlying H molecule.
Since the formation of covalently linked dimers is a prerequisite for efficient H transport (22), our data suggested that the HXL protein should be efficiently transported. Indeed, cell surface expression of not only HXL but all H-αCEA proteins was confirmed by FACS analysis of transfected cells to be similar to that of unmodified H (Fig. 2C), indicating efficient transport for all H-αCEA proteins.
HXL supports syncytium formation in both CD46-positive and CD46-negative, CEA-positive cells.
Although display of a scAb on MV H did not affect its proper folding or transport, its receptor-binding and fusion support functions may have been disrupted. We assessed the functionality of the H-αCEA proteins by measuring syncytium formation following coexpression with MV F.
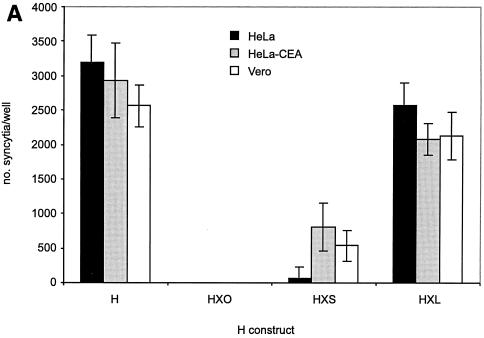
In three CD46-positive cell lines (HeLa, HeLa-CEA, and Vero [Fig. 3A]), expression of HXL with F induced extensive syncytium formation, to a degree similar to that with unmodified H. The HXS protein supported syncytium formation at a lower level, while no cell-cell fusion was observed in cells expressing HX0. No significant difference was observed in the numbers of syncytia in HeLa versus HeLa-CEA cells expressing any of the chimeric proteins. Thus, addition of the long-linker form of the scAb did not impair MV H-induced cell-cell fusion via CD46. We found these three cell lines to be negative for surface SLAM by FACS analysis (data not shown); thus, receptor usage was limited in this case to CD46 and, where expressed, CEA.
FIG. 3.
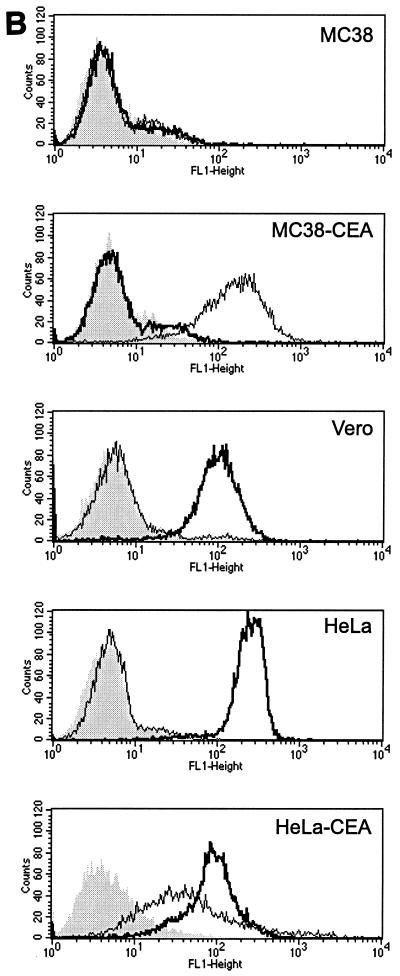
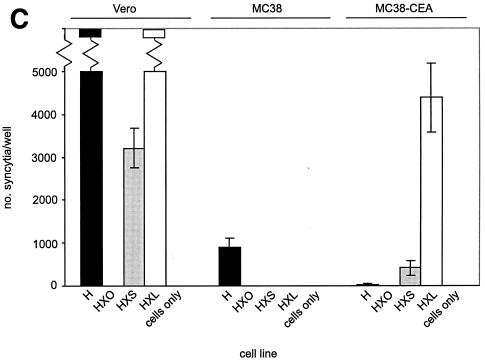
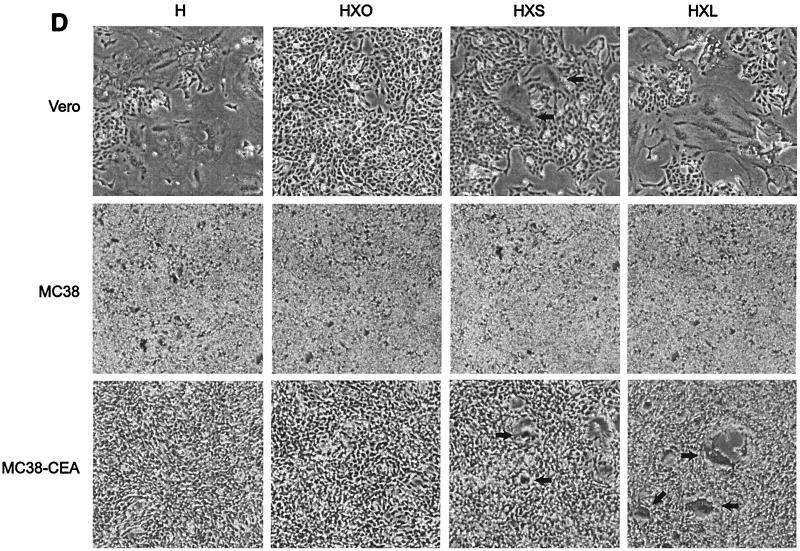
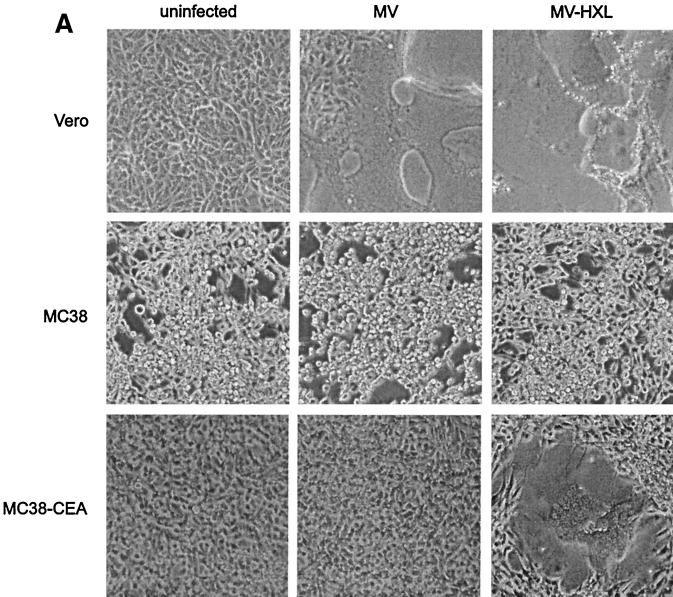
(A) HXL protein efficiently induces syncytium formation in CD46-positive cells. Vero, HeLa, and HeLa-CEA cells were cotransfected with MV F and the indicated H constructs, and syncytia were scored 48 h posttransfection. The error bars indicate standard deviations. (B) Expression levels of CD46 and CEA on all cell lines used in this study. Shading, no primary antibody as a negative control; thin line, CEA; thick line, CD46. (C and D) HXL protein efficiently induces syncytium formation in CD46-negative, CEA-positive cells. Vero, MC38, and MC38-CEA cells were cotransfected with MV F and the indicated H constructs, and syncytia were scored (C) and photographed (D) 48 h posttransfection. For synctium scoring, H and HXL on Vero cells were not countable, as >90% of the cells were in syncytia. The arrows in panel D indicate isolated syncytia.
To assess the contribution of the scAb-CEA interaction in the fusion process, we analyzed CEA-dependent syncytium formation in a CD46-negative background. For this we used MC38-CEA cells, a mouse cell line stably expressing high levels of cell surface human CEA (25), and their CEA-negative parent cell line, MC38. FACS analysis (Fig. 3B) demonstrated levels of CD46 to be similarly high on Vero, HeLa, and HeLa-CEA cells and undetectable on MC38 and MC38-CEA cells. As expected, expression of CEA was high only on HeLa-CEA and MC38-CEA cells. Fusions in MC38, MC38-CEA, and Vero cells were compared (Fig. 3C and D). In MC38 and MC38-CEA cells, coexpression of F with unmodified H induced a low level of syncytium formation, presumably reflecting an inefficient, CD46-independent fusion mechanism for MV H. Strikingly, coexpression of F with chimeric HXL in MC38-CEA cells led to extensive syncytium formation. Although at a reduced level compared with that in Vero cells, the numbers of syncytia were over 100-fold greater than those seen with unmodified H. The HXS protein supported a reduced level of syncytium formation in both Vero and MC38-CEA cells, while coexpression of HX0 with F yielded no detectable cell-cell fusion in any cell line. Thus, MV H displaying the long-linker form of the scAb initiated cell-cell fusion via a novel receptor.
Recovery of replication-competent MV containing chimeric HXL protein in place of H.
The ability of chimeric HXL to functionally replace unmodified H in the context of replicating virus was assessed. In a full-length infectious MV Edmonston cDNA, the H gene was replaced with that encoding HXL, and using the MV recovery protocol (24), virus was isolated from individual syncytia formed in Vero cells.
The authenticity of the recovered MV-HXL was confirmed by Western blot analysis of purified particles (Fig. 4A). Consistent with the sizes of transiently expressed HXL and H proteins (Fig. 1B), purified MV-HXL particles expressed an H protein of ∼110 kDa, in contrast to that of ∼80 kDa expressed from unmodified MV. Furthermore, treatment of purified MV-HXL virious with factor Xa protease demonstrated specific cleavage of the appended scAb, generating an 80-kDa protein corresponding to unmodified H (Fig. 4B). As expected, factor Xa treatment of unmodified MV did not affect the size of the antigenic material detectable as H.
FIG. 4.
(A) Recombinant MV-HXL expresses an H protein of the expected molecular weight. The protein compositions of MV and MV-HXL were revealed by Western blotting with an MV-specific antiserum. The numbers refer to molecular weights in thousands. (B) The displayed scAb can be specifically cleaved from MV H by factor Xa protease treatment. Both viruses were treated with factor Xa for 2 h at 23°C, and H was detected by Western blot analysis with an anti-MV serum. The number refers to the molecular weight in thousands. +, present; −, absent. (C) MV-HXL binds the surfaces of CD46-negative, CEA-positive cells. MC38, MC38-CEA, and Vero cells were incubated with either MV or MV-HXL at an MOI of 3, and bound virus was detected by FACS analysis using an antibody directed against an extracellular epitope of MV H. Shading, no virus as a negative control; thin line, MV-HXL; thick line, MV.
MV-HXL binds to the surfaces of CD46-positive and CD46-negative, CEA-positive cells.
The abilities of MV and MV-HXL to bind cells expressing either CD46 or CEA at the surface were next compared by flow cytometry (Fig. 4C). Neither virus was able to bind the surface of CD46-negative, CEA-negative MC38 cells. In contrast, both viruses bound CD46-positive, CEA-negative Vero cells, with small but significant shifts observed. Thus, the addition of the scAb did not negate the interaction of MV-HXL with cell surface CD46, consistent with the ability of the HXL protein to induce cell-cell fusion in CD46-positive cells. Importantly, MV-HXL bound the surface of the CD46-negative, CEA-positive MC38-CEA cell line, while binding of unmodified MV was negligible.
MV-HXL replicates in CEA-positive cells in the absence of CD46.
Given that MV-HXL bound cells expressing CEA, we assessed its ability to infect both CD46-positive and CD46-negative, CEA-positive cells. We first compared the infectivities of MV and MV-HXL for Vero, MC38, and MC38-CEA cells by observing syncytium formation in the inoculated cells (Fig. 5A). Consistent with our previous results, Vero cells were infectable by either virus. Significantly, infection of MC38-CEA cells with MV-HXL resulted in extensive syncytium formation. In contrast, infection of MC38-CEA cells with unmodified MV and infection of MC38 cells with either virus were undetectable.
FIG. 5.
(A) MV-HXL infects and induces cell-cell fusion in CD46-negative, CEA-positive cells. MV, MV-HXL, or no virus was incubated with Vero, MC38, and MC38-CEA cells at an MOI of 3. Representative fields of view were photographed 72 h postinfection. (B) MV-HXL replicates in CD46-negative, CEA-positive cells to titers approaching those reached in CD46-positive cells. The infectivities of MV and MV-HXL were quantified for HeLa, HeLa-CEA, Vero, MC38, and MC38-CEA cells by TCID50 titration using each of the cell lines as a target.
We next compared the replicative ability of MV-HXL with that of parental MV by determining the viral titers achieved in Vero, HeLa, HeLa-CEA, MC38, and MC38-CEA cells by TCID50 assays using each of the cell lines as a target (Fig. 5B shows one typical example). MV-HXL replicated to titers almost indistinguishable from those obtained with parental MV in all three CD46-positive cell lines tested (7 × 105 to 5 × 107 PFU/ml, depending on the cell line). Thus, the ability of MV-HXL to replicate in a CD46-dependent manner was not affected by display of the scAb, consistent with our previous data.
Remarkably, MV-HXL reached similar titers on CD46-negative, CEA-positive MC38-CEA cells and on CD46-positive cells (from independent experiments, an average of 6.2 × 105 ± 1.3 × 105 PFU/ml), demonstrating that its ability to replicate in a CEA-dependent manner was about as efficient as its CD46-dependent replication. Negligible infection (titers of <102 PFU/ml) was detected in MC38-CEA cells with parental MV and in CEA-negative MC38 cells with either virus. The fact that parental MV H induced a low level of syncytium formation in MC38 cells while viral titers were negligible may be accounted for by differences in the requirements for cell-cell and virus-cell fusion.
We also measured the infectivities of MV and MV-HXL for all cell lines by infecting each at an MOI of 3 and quantifying cell-associated virus by TCID50 titration 72 h postinfection using Vero cells as targets. Since no intrinsic differences in replication of the two viruses in Vero cells existed (Fig. 5B), no bias would be introduced by using this approach. This method gave a pattern of virus titers reproducibly similar to that from the previous assay, but the absolute values were 1 to 2 log units lower, with the titer of MV-HXL on MC38-CEA cells reaching 3.1 × 104 ± 5.1 × 103 PFU/ml (data not shown). This assay was used for subsequent blocking experiments.
Replication of MV-HXL in CD46-negative, CEA-positive cells depends on a specific interaction between the displayed scAb and CEA.
Our data suggested a specific interaction between MV-HXL and CEA. To test this, we assessed the infectivities of MV and MV-HXL for MC38-CEA and Vero cells following incubation of the virus with factor Xa protease to cleave the displayed scAb or pretreatment of the cells with an αCEA MAb (COL1) to block cell surface CEA. In both cases, virus was quantified from the cells 72 h postinfection by TCID50 titration using Vero cells as targets (Fig. 6).
FIG. 6.
MV-HXL infectivity for MC38-CEA cells is inhibited by cleavage of the displayed scAb or antibody preadsorption of cell surface CEA. Vero cells (as a control) and MC38-CEA cells were infected with MV or MV-HXL (MOI, 1), untreated or pretreated with 10 μg of factor Xa/ml. Also, cells were pretreated with 10 μg of αCEA COL1 antibody/ml and then infected with MV or MV-HXL (MOI, 1). Virus released from the cells by freeze-thaw 72 h postinfection was quantified by TCID50 titration on Vero cells.
On Vero cells, neither treatment significantly affected the titer of either MV or MV-HXL; similarly, on MC38-CEA cells, the titer of unmodified MV was unaffected by either treatment. Strikingly, cleavage of the displayed scAb by factor Xa protease reduced the titer of MV-HXL on MC38-CEA cells by greater than 100-fold (from independent experiments, an average of 177-fold ± 2-fold). Inhibition of MV-HXL by pretreating MC38-CEA cells with the αCEA MAb COL1 was less drastic but still significant, with an inhibition of greater than 10-fold. The less pronounced inhibition seen with COL1 may be explained by multivalent virus attachment being inefficiently inhibited by monomeric ligands. The inability of COL1 to inhibit MV infectivity for either cell line demonstrates the specificity of its inhibition for MV-HXL. These data confirm that the ability of MV-HXL to infect CEA-positive cells independently of CD46 depends on a specific interaction between the displayed scAb and the targeted antigen.
DISCUSSION
We describe the generation of a replicating MV with tropism expanded by virtue of a scAb displayed on its H protein. Previously, we demonstrated that compact epidermal growth factor (EGF) and insulin-like growth factor (IGF) domains displayed on the H protein enable virus entry via both the natural and the cognate receptors (28). Despite the complex fusion mechanism of MV and our more radical modification of the H protein with a 244-amino-acid scAb comprising two Ig domains, we report a virus which retains its ability to replicate on cells expressing the viral receptor CD46 while, significantly, it gains the ability to replicate on nonprimate cells expressing human CEA, the targeted receptor. Furthermore, we define a length of the linker between VH and VL of the scAb which is critical for functionality of the underlying H protein. Given the common features of scAb structure, our ability to display one scAb suggests that MV will tolerate the addition of many scAbs as C-terminal fusions of H, a property highly desirable for targeted vectors. In addition, generating an MV with tropism for the clinically relevant tumor-associated antigen CEA establishes a precedent for the development of chimeric MV-scAb vectors as useful cytoreductive therapeutic agents.
Since the folding and transport kinetics of MV H carrying C-terminally appended ligands are crucial for their functionality, we first characterized the chimeric H-αCEA molecules at the protein level. Remarkably, the presence of two independently folding domains in these H-αCEA molecules did not affect intracellular stability, suggesting their correct folding, since many misfolded secretory proteins are subjected to rapid degradation in the early secretory pathway (23). More evidence that the conformation of the underlying H molecule was unaffected came from the ability of HXL to dimerize with itself and with unmodified H and from the similar cell surface expression of all H-αCEA proteins compared with that of unmodified MV H. Significantly, only H displaying the long-linker form of the αCEA scAb was able to initiate efficient cell-cell fusion in cells expressing CD46 and also in CEA-positive cells independently of CD46. In contrast, addition of the zero and short forms of the scAb ablated or reduced the fusogenicity of the molecule, respectively. Since the scAbs of the HX0 and HXS proteins may be oligomeric, they may sterically prevent fusion either by blocking interaction with the cellular receptor or a second factor or by a more complex interference in the oligomerization of H or the interaction between H and F oligomers. The HXL protein, however, is predicted to display a monomeric scAb, which apparently does not obstruct any essential complex formations.
Following these findings, we generated infectious MV expressing HXL in place of H. The two appended Ig-like domains did not appear to interfere with efficient particle assembly, despite an increase in the molecular mass of the H protein by 40%. Furthermore, the displayed scAb did not impair entry and replication competence in CD46-positive cells and, significantly, enabled infection of cells lacking all human proteins other than the targeted receptor, human CEA. Moreover, the CD46-independent infectivity of MV-HXL for cells expressing CEA relied on a specific and inhibitable interaction between the scAb displayed on the virus and CEA.
Our finding that extension of H with a large, independently folding scAb enables it to efficiently function through a receptor of the Ig superfamily protein class coupled with the demonstration that similar display of EGF and IGF allows fusion through the cognate tyrosine kinase receptors (28) is consistent with a rather flexible receptor usage for MV, suggesting that a high-affinity interaction is sufficient to trigger the subsequent fusion process. Indeed, recent evidence suggests that different MV strains infect with different efficiencies via at least two receptors. The wild-type strain KA infects cells efficiently via the Ig superfamily protein SLAM and binds CD46 weakly, whereas the vaccine strains possess stronger CD46-dependent infectivity yet maintain entry via SLAM (10, 16, 32, 33). We propose that binding of the displayed scAb to CEA facilitates fusion by bringing the virus close to the cell surface (this initial step is normally mediated by CD46 or SLAM binding), possibly inducing conformational changes in the H protein, and finally triggering extrusion of the F fusion peptide and membrane mixing. Consistent with this hypothesis, our observation that extension of H with a 244-amino-acid scAb does not impair the fusion process suggests that the position of the attachment site on the H protein is not critical for efficient fusion. It is, however, possible that entry does not occur directly through the CEA molecule and instead depends on the interaction with CEA to facilitate an enhanced interaction with an as-yet-unidentified MV coreceptor molecule, through which the virus enters. Whichever mechanism MV-HXL enters by, the flexibility of the membrane fusion system is not unlimited, since display of a reportedly oligomeric scAb domain impairs fusion triggered by both CD46 and CEA and extension of CD46 by 12 nm ablates fusion (2). Given the inability of the long-linker form of scAb to obstruct the H-CD46 interaction, we suggest, in agreement with others (17), that the CD46 binding site on MV H may be well exposed and highly accessible rather than buried in a canyon or hydrophobic “pocket,” as is the case for certain other viruses (13, 26, 34).
CEA-dependent infection by MV-HXL can be described as positive retargeting of MV, since binding to CEA is followed by CEA-dependent entry. This cannot be achieved with most retroviral vectors, since binding to the targeted receptor does not trigger infection via this molecule and only a low level of transduction is achieved through the normal viral receptor (11, 35). Our findings may suggest the general applicability of MV as a vector which can be positively retargeted to many other cell surface antigens by the display of appropriate scAbs. Our system is limited at present by the maintenance of natural MV receptor usage by the chimeric virus, and we are investigating strategies to eliminate this interaction while maintaining fusion through the targeted receptor. In addition to its inherent cytotoxicity, developing retargeted vectors based on the safe and effective MV Edmonston vaccine strain supports the therapeutic use of replication-competent virus. The combination of potent syncytium induction and replication competence may culminate in a more extensive spread among the target cell population. Indeed, recent in vivo data suggest unmodified, replicating MV Edmonston B causes regression of human lymphoma and myeloma xenografts in a SCID mouse model after intratumoral injection (D. Grote, T. I. Cornu, R. Cattaneo, S. J. Russell, and A. K. Fielding, submitted for publication; K. W. Peng and S. J. Russell, personal communication).
In summary, we have demonstrated that MV not only tolerates the addition of a 244-amino-acid independently folding scAb on its H protein without impairing its natural route of entry, it can also infect cells via a novel interaction between a displayed scAb and its targeted receptor. This chimeric virus provides new opportunities for restricting the inherent cytotoxicity of the virus to specific subsets of cells and thus for the use of MV-scAb chimeras in cytoreductive therapy and is also a valuable tool for understanding the determinants of MV entry.
ACKNOWLEDGMENTS
We thank Jeff Schlom (NIH) for cell lines, Greg Winter (MRC, Cambridge, United Kingdom) for cell lines and MFE-23 scAb, Frances Bullough (Cambridge Genetics, Cambridge, United Kingdom) for plasmids, and S. Udem and J. Schneider-Schaulies for antibodies. We thank Adele Fielding for valuable discussion and Sompong Vongpunsawad for excellent technical assistance.
This work was supported by grants from the Siebens and Mayo Foundations to R.C. and by a career development award from the Deutsche Forschungsgemeinschaft (DFG) to R.K.P.
REFERENCES
- 1.Bateman A, Bullough F, Murphy S, Emiliusen L, Lavillette D, Cosset F L, Cattaneo R, Russell S J, Vile R G. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res. 2000;60:1492–1497. [PubMed] [Google Scholar]
- 2.Buchholz C J, Schneider U, Devaux P, Gerlier D, Cattaneo R. Cell entry by measles virus: long hybrid receptors uncouple binding from membrane fusion. J Virol. 1996;70:3716–3723. doi: 10.1128/jvi.70.6.3716-3723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calain P, Roux L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J Virol. 1993;67:4822–4830. doi: 10.1128/jvi.67.8.4822-4830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cathomen T, Buchholz C J, Spielhofer P, Cattaneo R. Preferential initiation at the second AUG of the measles virus F mRNA: a role for the long untranslated region. Virology. 1995;214:628–632. doi: 10.1006/viro.1995.0075. [DOI] [PubMed] [Google Scholar]
- 5.Chester K A, Begent R H, Robson L, Keep P, Pedley R B, Boden J A, Boxer G, Green A, Winter G, Cochet O, Hawkins R E. Phage libraries for generation of clinically useful antibodies. Lancet. 1994;343:455–456. doi: 10.1016/s0140-6736(94)92695-6. [DOI] [PubMed] [Google Scholar]
- 6.Dorig R E, Marcil A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 7.Fielding A K, Chapel-Fernandes S, Chadwick M P, Bullough F J, Cosset F L, Russell S J. A hyperfusogenic gibbon ape leukemia envelope glycoprotein: targeting of a cytotoxic gene by ligand display. Hum Gene Ther. 2000;11:817–826. doi: 10.1089/10430340050015437. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald K, Holliger P, Winter G. Improved tumour targeting by disulphide stabilized diabodies expressed in Pichia pastoris. Protein Eng. 1997;10:1221–1225. doi: 10.1093/protein/10.10.1221. [DOI] [PubMed] [Google Scholar]
- 9.Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 10.Hsu E C, Sarangi F, Iorio C, Sidhu M S, Udem S A, Dillehay D L, Xu W, Rota P A, Bellini W J, Richardson C D. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J Virol. 1998;72:2905–2916. doi: 10.1128/jvi.72.4.2905-2916.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasahara N, Dozy A M, Kan Y W. Tissue-specific targeting of retroviral vectors through ligand-receptor interactions. Science. 1994;266:1373–1376. doi: 10.1126/science.7973726. [DOI] [PubMed] [Google Scholar]
- 12.Klasse P J, Bron R, Marsh M. Mechanisms of enveloped virus entry into animal cells. Adv Drug Deliv Rev. 1998;34:65–91. doi: 10.1016/S0169-409X(98)00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamb R A. Paramyxovirus fusion: a hypothesis for changes. Virology. 1993;197:1–11. doi: 10.1006/viro.1993.1561. [DOI] [PubMed] [Google Scholar]
- 15.Malvoisin E, Wild T F. Measles virus glycoproteins: studies on the structure and interaction of the haemagglutinin and fusion proteins. J Gen Virol. 1993;74:2365–2372. doi: 10.1099/0022-1317-74-11-2365. [DOI] [PubMed] [Google Scholar]
- 16.Manchester M, Eto D S, Valsamakis A, Liton P B, Fernandez-Munoz R, Rota P A, Bellini W J, Forthal D N, Oldstone M B. Clinical isolates of measles virus use CD46 as a cellular receptor. J Virol. 2000;74:3967–3974. doi: 10.1128/jvi.74.9.3967-3974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manchester M, Naniche D, Stehle T. CD46 as a measles virus receptor: form follows function. Virology. 2000;274:5–10. doi: 10.1006/viro.2000.0469. [DOI] [PubMed] [Google Scholar]
- 18.Martin F, Neil S, Kupsch J, Maurice M, Cosset F, Collins M. Retrovirus targeting by tropism restriction to melanoma cells. J Virol. 1999;73:6923–6929. doi: 10.1128/jvi.73.8.6923-6929.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obrink B. CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr Opin Cell Biol. 1997;9:616–626. doi: 10.1016/S0955-0674(97)80114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perisic O, Webb P A, Holliger P, Winter G, Williams R L. Crystal structure of a diabody, a bivalent antibody fragment. Structure. 1994;2:1217–1226. doi: 10.1016/s0969-2126(94)00123-5. [DOI] [PubMed] [Google Scholar]
- 22.Plemper R K, Hammond A L, Cattaneo R. Characterization of a region of the measles virus hemagglutinin sufficient for its dimerization. J Virol. 2000;74:6485–6493. doi: 10.1128/jvi.74.14.6485-6493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plemper R K, Wolf D H. Retrograde protein translocation: ERADication of secretory proteins in health and disease. Trends Biochem Sci. 1999;24:266–270. doi: 10.1016/s0968-0004(99)01420-6. [DOI] [PubMed] [Google Scholar]
- 24.Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dotsch C, Christiansen G, Billeter M A. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robbins P F, Kantor J A, Salgaller M, Hand P H, Fernsten P D, Schlom J. Transduction and expression of the human carcinoembryonic antigen gene in a murine colon carcinoma cell line. Cancer Res. 1991;51:3657–3662. [PubMed] [Google Scholar]
- 26.Rossmann M G. The canyon hypothesis. Hiding the host cell receptor attachment site on a viral surface from immune surveillance. J Biol Chem. 1989;264:14587–14590. [PubMed] [Google Scholar]
- 27.Russell S J, Cosset F L. Modifying the host range properties of retroviral vectors. J Gene Med. 1999;1:300–311. doi: 10.1002/(SICI)1521-2254(199909/10)1:5<300::AID-JGM59>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 28.Schneider U, Bullough F, Vongpunsawad S, Russell S J, Cattaneo R. Recombinant measles viruses efficiently entering cells through targeted receptors. J Virol. 2000;74:9928–9936. doi: 10.1128/jvi.74.21.9928-9936.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider-Schaulies J, Dunster L M, Schwartz-Albiez R, Krohne G, ter Meulen V. Physical association of moesin and CD46 as a receptor complex for measles virus. J Virol. 1995;69:2248–2256. doi: 10.1128/jvi.69.4.2248-2256.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheshberadaran H, Chen S N, Norrby E. Monoclonal antibodies against five structural components of measles virus. I. Characterization of antigenic determinants on nine strains of measles virus. Virology. 1983;128:341–353. doi: 10.1016/0042-6822(83)90261-1. [DOI] [PubMed] [Google Scholar]
- 31.Singh M, Cattaneo R, Billeter M A. A recombinant measles virus expressing hepatitis B virus surface antigen induces humoral immune responses in genetically modified mice. J Virol. 1999;73:4823–4828. doi: 10.1128/jvi.73.6.4823-4828.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tatsuo H, Okuma K, Tanaka K, Ono N, Minagawa H, Takade A, Matsuura Y, Yanagi Y. Virus entry is a major determinant of cell tropism of Edmonston and wild-type strains of measles virus as revealed by vesicular stomatitis virus pseudotypes bearing their envelope proteins. J Virol. 2000;74:4139–4145. doi: 10.1128/jvi.74.9.4139-4145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 34.Weis W, Brown J H, Cusack S, Paulson J C, Skehel J J, Wiley D C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988;333:426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Zhu L, Lee S, Li L, Chang E, Soong N W, Douer D, Anderson W F. Identification of the block in targeted retroviral-mediated gene transfer. Proc Natl Acad Sci USA. 1999;96:4005–4010. doi: 10.1073/pnas.96.7.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]