Abstract
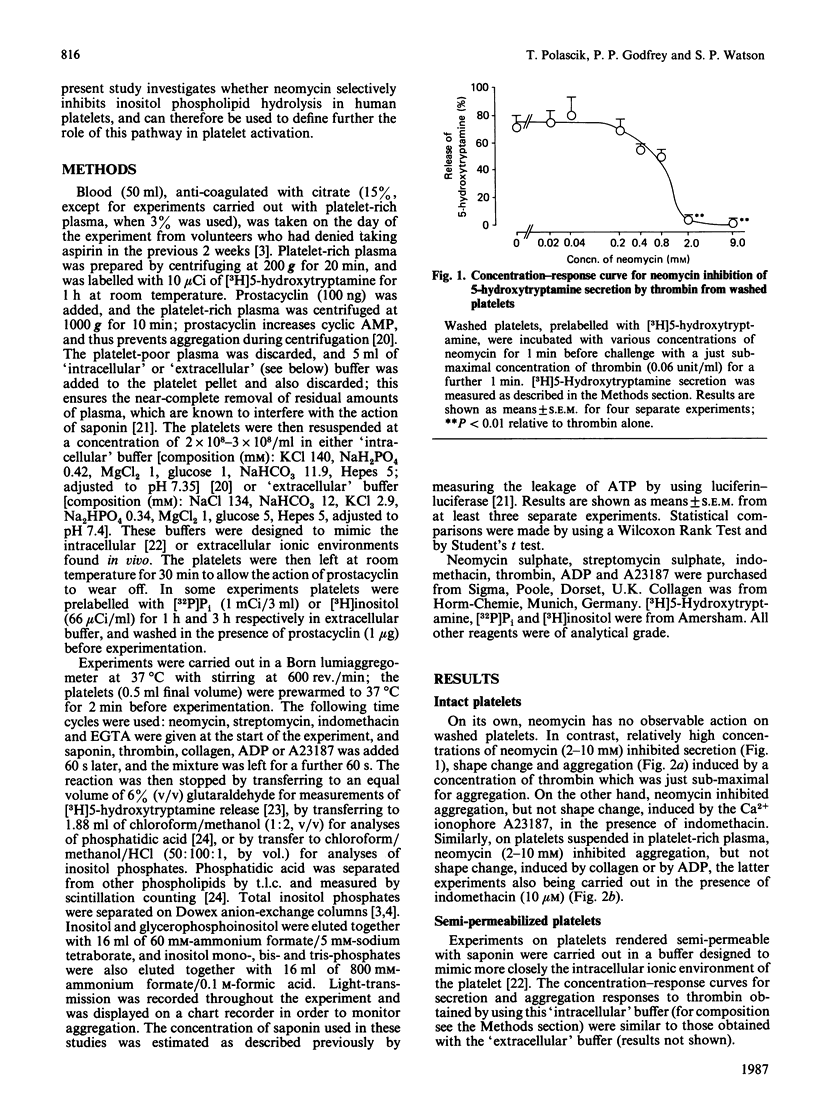
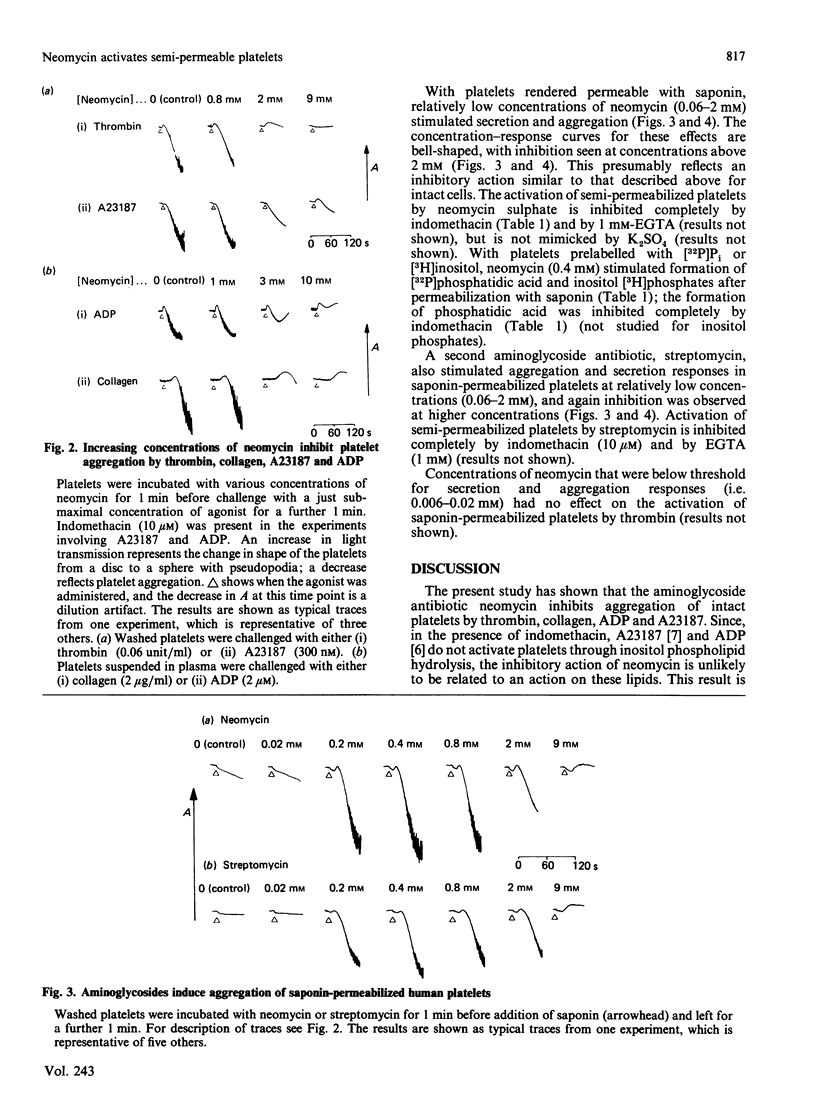
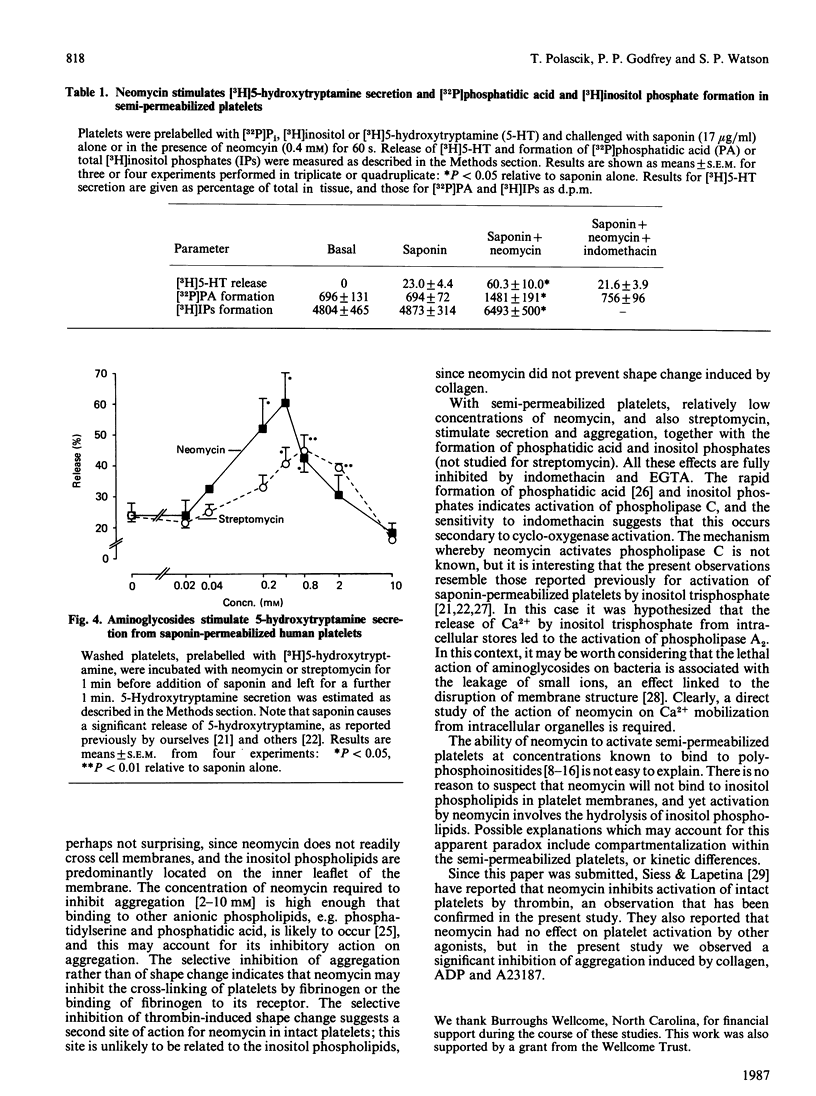
High concentrations of neomycin (2-10 mM) inhibited aggregation, but not shape change, of intact platelets by collagen, ADP and the Ca2+ ionophore, A23187, the last two studies being carried out in the presence of the cyclo-oxygenase inhibitor indomethacin. In contrast, over the same range of concentrations neomycin inhibited both aggregation and shape change induced by thrombin. Under these conditions activation of platelets by collagen and by thrombin, but not by A23187 or by ADP, is believed to be dependent on the hydrolysis of membrane inositol phospholipids. These data therefore suggest that the inhibitory action of neomycin on intact platelets is not related to its previously reported inhibitory effect on phosphoinositide metabolism. The selective inhibition of thrombin-induced shape change indicates a second site of action of neomycin on intact platelets. On platelets rendered semi-permeable with saponin, neomycin and a second aminoglycoside antibiotic, streptomycin (each 0.06-2 mM), stimulated secretion and aggregation responses. These effects were inhibited by indomethacin and by EGTA. Activation of semi-permeabilized platelets by neomycin is associated with the formation of inositol phosphates and phosphatidic acid, indicating activation by phospholipase C. This effect is also inhibited by indomethacin, implying that it is secondary to the formation of prostaglandins and endoperoxides. These results are discussed in the context of the use of neomycin as a selective inhibitor of polyphosphoinositide metabolism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Authi K. S., Evenden B. J., Crawford N. Metabolic and functional consequences of introducing inositol 1,4,5-trisphosphate into saponin-permeabilized human platelets. Biochem J. 1986 Feb 1;233(3):707–718. doi: 10.1042/bj2330707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Brass L. F., Joseph S. K. A role for inositol triphosphate in intracellular Ca2+ mobilization and granule secretion in platelets. J Biol Chem. 1985 Dec 5;260(28):15172–15179. [PubMed] [Google Scholar]
- Carney D. H., Scott D. L., Gordon E. A., LaBelle E. F. Phosphoinositides in mitogenesis: neomycin inhibits thrombin-stimulated phosphoinositide turnover and initiation of cell proliferation. Cell. 1985 Sep;42(2):479–488. doi: 10.1016/0092-8674(85)90105-9. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Fisher G. J., Bakshian S., Baldassare J. J. Activation of human platelets by ADP causes a rapid rise in cytosolic free calcium without hydrolysis of phosphatidylinositol-4,5-bisphosphate. Biochem Biophys Res Commun. 1985 Jun 28;129(3):958–964. doi: 10.1016/0006-291x(85)91984-9. [DOI] [PubMed] [Google Scholar]
- Griffin H. D., Sykes M., Hawthorne J. N. Effects of neomycin on calcium and polyphosphoinositide metabolism of guinea pig synaptosomes. J Neurochem. 1980 Mar;34(3):750–752. doi: 10.1111/j.1471-4159.1980.tb11209.x. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Lang V., Pryhitka G., Buckley J. T. Effect of neomycin and ionophore A23189 on ATP levels and turnover of polyphosphoinositides in human erythrocytes. Can J Biochem. 1977 Sep;55(9):1007–1012. doi: 10.1139/o77-150. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Cuatrecasas P. Stimulation of phosphatidic acid production in platelets precedes the formation of arachidonate and parallels the release of serotonin. Biochim Biophys Acta. 1979 May 25;573(2):394–402. doi: 10.1016/0005-2760(79)90072-9. [DOI] [PubMed] [Google Scholar]
- Lipsky J. J., Lietman P. S. Aminoglycoside inhibition of a renal phosphatidylinositol phospholipase C. J Pharmacol Exp Ther. 1982 Feb;220(2):287–292. [PubMed] [Google Scholar]
- Lodhi S., Weiner N. D., Schacht J. Interactions of neomycin with monomolecular films of polyphosphoinositides and other lipids. Biochim Biophys Acta. 1979 Oct 19;557(1):1–8. doi: 10.1016/0005-2736(79)90084-1. [DOI] [PubMed] [Google Scholar]
- Orsulakova A., Stockhorst E., Schacht J. Effect of neomycin on phosphoinositide labelling and calcium binding in guinea-pig inner ear tissues in vivo and in vitro. J Neurochem. 1976 Feb;26(2):285–290. doi: 10.1111/j.1471-4159.1976.tb04478.x. [DOI] [PubMed] [Google Scholar]
- Packham M. A., Guccione M. A., Greenberg J. P., Kinlough-Rathbone R. L., Mustard J. F. Release of 14C-serotonin during initial platelet changes induced by thrombin, collagen, or A23187. Blood. 1977 Nov;50(5):915–926. [PubMed] [Google Scholar]
- Rittenhouse S. E. Activation of human platelet phospholipase C by ionophore A23187 is totally dependent upon cyclo-oxygenase products and ADP. Biochem J. 1984 Aug 15;222(1):103–110. doi: 10.1042/bj2220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastrasinh M., Knauss T. C., Weinberg J. M., Humes H. D. Identification of the aminoglycoside binding site in rat renal brush border membranes. J Pharmacol Exp Ther. 1982 Aug;222(2):350–358. [PubMed] [Google Scholar]
- Schacht J. Inhibition by neomycin of polyphosphoinositide turnover in subcellular fractions of guinea-pig cerebral cortex in vitro. J Neurochem. 1976 Nov;27(5):1119–1124. doi: 10.1111/j.1471-4159.1976.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Schacht J. Purification of polyphosphoinositides by chromatography on immobilized neomycin. J Lipid Res. 1978 Nov;19(8):1063–1067. [PubMed] [Google Scholar]
- Schibeci A., Schacht J. Action of neomycin on the metabolism of polyphosphoinositides in the guinea pig kidney. Biochem Pharmacol. 1977 Oct 1;26(19):1769–1774. doi: 10.1016/0006-2952(77)90344-6. [DOI] [PubMed] [Google Scholar]
- Schwertz D. W., Kreisberg J. I., Venkatachalam M. A. Effects of aminoglycosides on proximal tubule brush border membrane phosphatidylinositol-specific phospholipase C. J Pharmacol Exp Ther. 1984 Oct;231(1):48–55. [PubMed] [Google Scholar]
- Siess W., Lapetina E. G. Neomycin inhibits inositol phosphate formation in human platelets stimulated by thrombin but not other agonists. FEBS Lett. 1986 Oct 20;207(1):53–57. doi: 10.1016/0014-5793(86)80011-4. [DOI] [PubMed] [Google Scholar]
- Vargas J. R., Radomski M., Moncada S. The use of prostacyclin in the separation from plasma and washing of human platelets. Prostaglandins. 1982 Jun;23(6):929–945. doi: 10.1016/0090-6980(82)90135-6. [DOI] [PubMed] [Google Scholar]
- Watson S. P., Ganong B. R., Bell R. M., Lapetina E. G. 1,2-Diacylglycerols do not potentiate the action of phospholipases A2 and C in human platelets. Biochem Biophys Res Commun. 1984 May 31;121(1):386–391. doi: 10.1016/0006-291x(84)90734-4. [DOI] [PubMed] [Google Scholar]
- Watson S. P., McConnell R. T., Lapetina E. G. The rapid formation of inositol phosphates in human platelets by thrombin is inhibited by prostacyclin. J Biol Chem. 1984 Nov 10;259(21):13199–13203. [PubMed] [Google Scholar]
- Watson S. P., Reep B., McConnell R. T., Lapetina E. G. Collagen stimulates [3H]inositol trisphosphate formation in indomethacin-treated human platelets. Biochem J. 1985 Mar 15;226(3):831–837. doi: 10.1042/bj2260831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. P., Ruggiero M., Abrahams S. L., Lapetina E. G. Inositol 1,4,5-trisphosphate induces aggregation and release of 5-hydroxytryptamine from saponin-permeabilized human platelets. J Biol Chem. 1986 Apr 25;261(12):5368–5372. [PubMed] [Google Scholar]
- Whitaker M., Aitchison M. Calcium-dependent polyphosphoinositide hydrolysis is associated with exocytosis in vitro. FEBS Lett. 1985 Mar 11;182(1):119–124. doi: 10.1016/0014-5793(85)81167-4. [DOI] [PubMed] [Google Scholar]


