Abstract
To investigate the effect and safety of recanalization of stenosed or occluded venous sinuses for dural arteriovenous fistulas (DAVFs) and possible mechanism of DAVF formation, patients with DAVF accompanied by venous sinus stenosis or occlusion treated with balloon angioplasty and/or stenting were retrospectively enrolled. The clinical data, treatment outcomes and complications were analyzed. In 7 patients enrolled, the DAVF was Cognard IIa grade in 3 (42.9% or 3/7) and IIa + b in 4 (57.1% or 4/7) patients, including complex DAVFs in 2 (28.6% or 2/7). The angioplasty procedure was successful in all (100%) patients, including complete cure in 3 (42.9% or 3/7) patients with the initial Cognard grade IIa, transformation from Cognard grade IIa + b to I in 2 (28.6% or 2/7) patients, and unchanged Cognard grade IIa + b in 2 (28.6% or 2/7) patients. The symptoms were all improved. At 3-month angiographic follow-up, 5 (71.4% or 5/7) cases were cured, whereas 2 cases still had grade I fistulas but no clinical symptoms. Staged embolization in 2 patients resulted in significant symptom improvement. At the last follow-up of a median 4 years, no clinical symptoms were present in 5 (71.4% or 5/7) patients, intermittent headache in 1 (14.3% or 1/7), and death from trauma in 1 (14.3% or 1/7). In conclusion, endovascular recanalization of occluded or stenosed venous sinuses using balloon angioplasty and stenting is able to induce occlusion of DAVFs and is a safe and efficient treatment approach for DAVFs which are possibly caused by significant pressure increase.
Keywords: dural arteriovenous fistula, endovascular angioplasty, fluid dynamics, occlusion, venous sinus
1. Introduction
Dural arteriovenous fistulas (DAVFs) are abnormal acquired pathological shunts located in the dural wall of intracranial venous structures between dural arteries and dural venous sinuses, meningeal veins or cortical veins.[1–8] Being relatively uncommon, these fistulas represent approximately 10% to 15% of all intracranial arteriovenous shunts.[1,8–11] Patients with DAVFs of the transverse and sigmoid sinuses often experience lateralized pulsatile bruit combined with retroauricular pulsation and pain, severe neurological impairment and dementia in case of intracranial venous hypertension, and intracranial hemorrhage associated with cortical vein reflux. Most DAVFs are treated with either endovascular embolization or surgical treatment. Endovascular treatment focuses on embolization of the feeding arteries and occlusion of the fistula, whereas the surgical approaches comprise arterial ligation, direct resection of the fistulous sinuses, or isolation of the involved sinuses.[1,9,11–13] The disadvantages of these treatment approaches lie in the invasiveness or definitive occlusion of a sinus which could remain functional. Moreover, de novo formation of DAVFs has been reported many times after previous DAVFs have been successfully treated or occluded.[14–19] The true etiology of DAVFs is unclear although the common mechanism is thought to be venous hypertension,[1,2,10–12,20] which may be caused by obstruction of the venous outflow because of venous or sinus thrombosis, stenosis, or occlusion or because of increased blood hypercoagulation states or viscosity. Animal experiments in rats have demonstrated that artificial creation of chronic venous hypertension for 2 to 3 months without associated venous or sinus thrombosis can induce new DAVFs similar to those in humans.[21–26] Moreover, relief of the venous hypertension via balloon angioplasty and stent deployment in the involved sinuses has been shown to be able to resolve the DAVFs.[1,2,27–31] Endovascular recanalization of the involved sinuses in DAVFs using balloon angioplasty and stent deployment may thus be considered a better alternative therapy because endovascular recanalization of the involved sinuses is able to eliminate the venous hypertension caused by sinus stenosis, occlusion, or increased blood hypercoagulation states or viscosity. This study was thus performed to investigate the effect of endovascular angioplasty and stent placement on DAVFs and the possible mechanism of DAVF formation.
2. Materials and methods
This retrospective single-center study was approved by the ethics committee of Henan Provincial People’s Hospital, and informed consent was waived by the same ethics committee because of the retrospective study design. All methods were conducted in accordance with the relevant guidelines and regulations. Between January 2009 and January 2023, patients with DAVFs accompanied by venous sinus stenosis or occlusion who were treated with venous sinus balloon angioplasty or stenting were retrospectively enrolled. The inclusion criteria were patients with DAVF accompanied by venous sinus stenosis or occlusion, with resultant intracranial hypertension, cerebral edema and/or cerebral hemorrhage caused by stasis of intracranial venous reflux, and treated with venous sinus recanalization or venous sinus balloon angioplasty or stenting. The exclusion criteria were patients who did not have DAVFs or were not treated with the above approaches.
3. Endovascular procedures
The endovascular procedure was performed under general anesthesia with the patient being in a supine position with endotracheal intubation. During the procedure, systematic heparin was performed to keep an activated coagulation time of 250 to 300 seconds. The right femoral artery was punctured with the traditional Seldinger technique before a 5F arterial sheath was inserted for navigating an angiographic catheter to the main supply artery of the DAVF. The femoral vein was canalized with a 6F 90 cm long sheath (Codis, Johnson&Johnson, Bridgewater) before a 6F 100 cm MPD guiding catheter (Johnson&Johnson, Bridgewater) and a 4F VER 125 cm single-curved catheter (Termo, Tokyo, Japan) were guided by a loach guide wire to the proximal site of the venous sinus occlusion or stenosis. The venous sinus was opened along the direction of the venous sinus. After the guide wire was successfully passed through the occluded segment, the VER catheter was navigated to the distal end of the occluded segment along the guide wire. The diameter of the distal venous sinus in the occluded segment was measured. A 300 cm Asachi guide wire (Intek, Aichi, Japan) was exchanged and sent in place before a suitable balloon catheter (Sterling Balloon Catheter, Boston Scientific Corporation, Boston, MA) was selected according to the diameter of the distal venous sinus to dilate the occluded segment of blood vessels. After satisfactory expansion, a Precise stent (Johnson&Johnson, Bridgewater) was deployed at the stenosis or occlusion. Angiography after venous sinus recanalization was conducted to clarify the condition of the DAVF, and the endovascular procedure was terminated if the DAVF was cured or significantly improved. Immediately after stent placement, antiplatelet aggregation therapy with tirofiban (Xinvinin) was administered at a rate of 0.4µg/kg/min for the initial 30 minutes. After the initial infusion volume was completed, the infusion was continued at a rate of 0.1µg/kg/min. If significant improvement was obtained in the DAVF, no further embolization of the DAVF was performed. However, DAVF embolization was necessary in case of no significant improvement of the DAVF after stenting, or a secondary embolization surgery plan was prepared in case of a complex DAVF. After the endovascular procedure, aspirin 100mg/day and clopidogrel 75mg/day were administered for antiplatelet aggregation therapy for 6 months. The criteria for successful endovascular treatment were clinical symptoms relieved and the Cognard classification scores decreased.[7]
4. Follow-up
Follow-up digital subtraction angiography (DSA) was conducted 1 to 3 months after the procedure, and the patients’ conditions were recorded including the symptoms, signs and classification of the DAVF. The grade of DAVF was evaluated with the Cognard classification system.[7] Further treatment plan was made based on the angiographic outcomes. Six months later, the patients were followed up clinically.
5. Statistical analysis
The SPSS software (version 21.0, IBM, Chicago) was used for statistical analysis in this study. Continuous measurement data were recorded as mean and standard deviations if in the normal distribution and tested with the t test or as medians and interquartile ranges if not in the normal distribution and tested with the Mann–Whitney U test. Categorical data were presented in frequency and percentage. The significant P value was set at <.05.
6. Results
Seven patients with DAVF accompanied by venous sinus stenosis or occlusion were enrolled and treated with balloon angioplasty and/or stent deployment in the sinuses (Table 1), including 5 male and 2 female patients aged 14 to 75 (mean 48.4 ± 20.5) years. The disease course ranged from 3 months to 12 years (median 5, interquartile range 3–120 months), including 10 years in 1 (14.3% or 1/7) patient and 12 years in another (14.3% or 1/7). The DAVF was Cognard grade IIa in 3 (42.9% or 3/7) and IIa + b in 4 (57.1% or 4/7) patients, including complex DAVFs in 2 (28.6% or 2/7). The location of fistula was in the transverse sinus in 2 (28.6% or 2/7) patients, sigmoid sinus in 2 (28.6% or 2/7), both sigmoid and transverse sinuses in 2 (28.6% or 2/7), and the junction of the right transverse sinus and sigmoid sinus in 1 (14.3% or 1/7). Bilateral transverse sinus occlusion was present in 1 (14.3% or 1/7) patient, occlusion of 1 sigmoid sinus and 1 transverse sinus in 2 (28.6% or 2/7), occlusion of right sigmoid sinus and right jugular vein plus severe stenosis of the left sigmoid sinus in 1 (14.3% or 1/7), occlusion of left transverse sinus and severe stenosis of right sigmoid sinus in 1 (14.3% or 1/7), and occlusion of right transverse sinus and junction of left sigmoid and transverse sinuses in 1 (14.3% or 1/7).
Table 1.
Demography, DAVFs features, endovascular treatment and prognosis.
| No/sex/age | Symptoms | Disease course | Treatment | DAVF | Gognard grade | Fistula location | Prognosis |
|---|---|---|---|---|---|---|---|
| 1/F/55 | Intermittent headache and blurred vision | 6 m | Balloon and stent angioplasty | Occlusion of both transverse sinuses | IIa | Right transverse sinus | Cured with no symptoms |
| 2/M/75 | Memory decline, headache and right limb weakness for over 3 months and occipital lobe edema | 3 m | Balloon and stent angioplasty | Occlusion of right sigmoid and left transverse sinuses | IIa | Right sigmoid sinus | Cured with no symptoms |
| 3/M/58 | Memory decline, headache and right limb weakness for over 3 months and Occipital lobe edema | 3 m | Balloon and stent angioplasty | Occlusion of right sigmoid sinus and right jugular vein and severe stenosis of left transverse sinus | IIa + b | Right transverse sinus | Improved to grade I with no symptoms |
| 4/M/53 | Blurred vision and right temporal -occipital subcortical hemorrhage | 4 m | Balloon and stent angioplasty | Occlusion of right sigmoid sinus and right jugular vein and severe stenosis of left sigmoid sinus | IIa | Junction of the right transverse and sigmoid sinuses | Cured with slight headache |
| 5/F/28 | Headache, dizziness, tinnitus, decreased memory, right exophthalmos, and diplopia | 12 yr | Balloon dilation and embolization with coil and Onyx glue | Severe stenosis of right sigmoid sinus and occlusion of left transverse sinus | IIa + b with complex structures | Superior sagittal and transverse sinuses | Improved (Ⅳ) with improved exophthalmos |
| 6/M/14 | Dizziness, tinnitus, intracranial murmurs, and left limb numbness | 10 yr | Left jugular foramen-sigmoid sinus opening, balloon dilation, and soft membrane fistula embolization | Occlusion of left sigmoid and right transverse sinuses | IIa + b with complex structures | Superior sagittal and transverse sinuses | Improved (IV) with no tinnitus. |
| 7/M/56 | Intellectual decline, difficulty walking, and left temporal lobe hemorrhage | 3 m | Balloon and stent angioplasty | Occlusion of right transverse sinus and junction occlusion of left sigmoid and sigmoid sinuses | IIa + b | Left sigmoid sinus | Improved to grade I with no symptoms |
Note: DAVF = dural arteriovenous fistula.
The endovascular procedure was successful in all (100%) patients (Figs. 1 and 2), including balloon angioplasty and stent deployment in 5 (71.4% or 5/7) patients and balloon angioplasty plus coil and Onyx embolization in 1 (14.3% or 1/7). The last patient was treated with recanalization of the left sigmoid sinus, balloon angioplasty, and embolization of soft membrane fistula. Significant improvement was achieved in the DAVF signs and symptoms in all patients after angioplasty, including complete cure in 3 (42.9% or 3/7) patients with the initial Cognard grade IIa, disease course of 3 to 6 (median 5) months, and no symptoms after angioplasty. Two (28.6% or 2/7) patients were transformed from Cognard grade IIa + b to I after angioplasty, and 2 (28.6% or 2/7) patients had the same unchanged Cognard grade IIa + b. The Cognard grades were significantly improved compared with those before endovascular treatment (P = .02). In the 2 patients without changes in the Cognard grade, the clinical symptoms were significantly improved after angioplasty, with significant relief in the intracranial hypertension, exophthalmos, and intracranial murmurs.
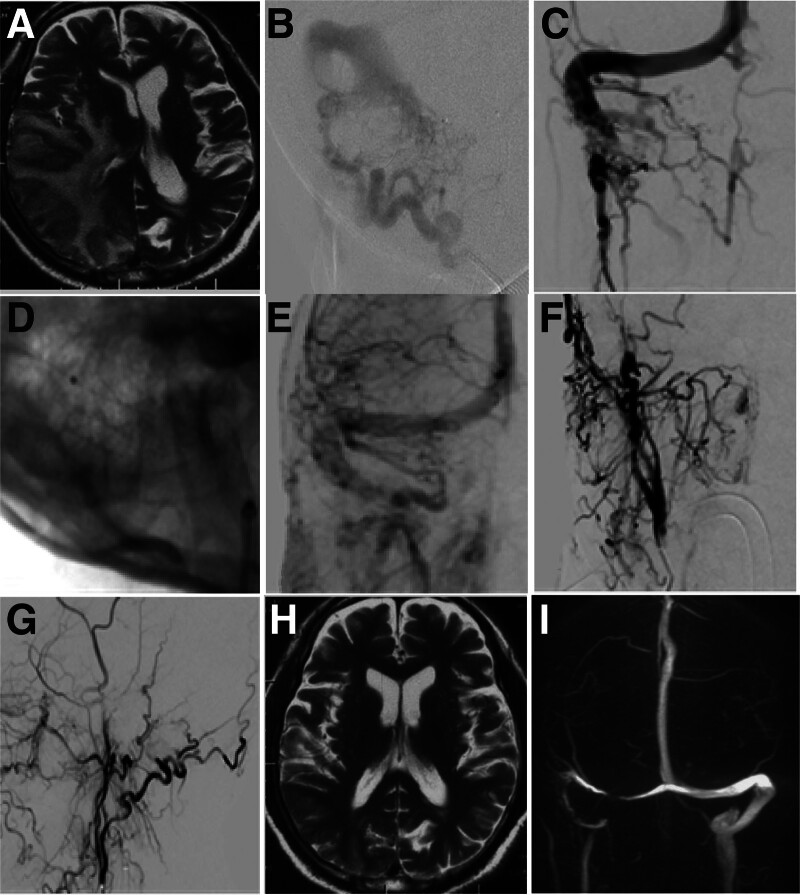
Figure 1.
Occlusion of right sigmoid and left transverse sinuses treated with endovascular angioplasty in a 75-year-old male patient (case 2). (A) pretreatment magnetic resonance imaging (MRI) showed abnormal cerebral parenchyma. (B) Right sigmoid sinus dural arteriovenous fistula (DAVF) was shown before treatmnet. (C) Right sigmoid and left transverse sinuses were occluded, with the occlusion location at the right sigmoid sinus. (D) The right sigmoid sinus was recanalized with deployment of a stent. (E) The right sigmoid sinus was patent. F&G. After recanalization of the venous sinuses, the DAVF disappeared in the anterior-posterior view (F) and lateral view (G). H&I. Follow-up MRI revealed normal cerebral parenchyma, disappearance of DAVF, and normal sinuses.
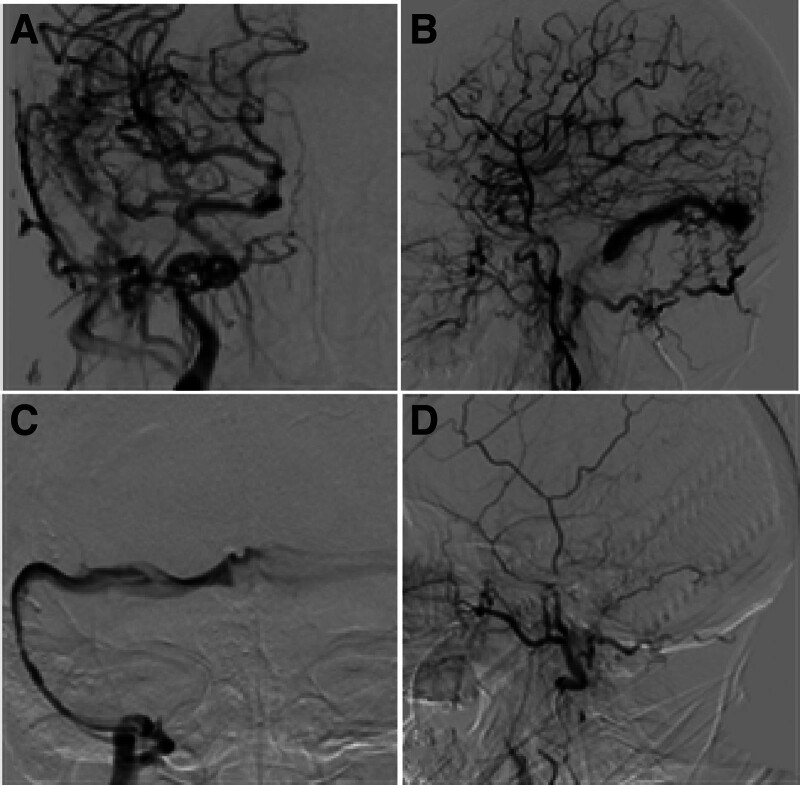
Figure 2.
A dural arteriovenous fistula (DAVF) was present in a male patient (case 4) at the right sigmoid sinus treated with endovascular angioplasty. (A and B) The DAVF was present at the right sigmoid sinus, with occlusion of the right sigmoid and right internal jugular vein in the anterior-posterior view (A) and lateral view (B). (C) After the right sigmoid sinus and right internal jugular vein, the left sigmoid sinus was dilated, and venous sinus angiography was performed. (D) Angiography through the right internal carotid artery revealed disappearance of the right sigmoid sinus DAVF.
Followed up with DSA 3 months after the procedure, 5 (71.4% or 5/7) cases were cured, whereas the other 2 cases with Cognard grade I and over 10 years of disease course still had fistulas but no clinical symptoms. The Cognard grades were significantly improved compared with those before endovascular treatment (P < .0001). These 2 patients with Cognard grade I were treated with 3 and 4 therapies, respectively, using coils plus Onyx glue embolization through staged arterial-access endovascular treatment, with significant improvement in clinical symptoms. At the last follow-up 6 months to 6 years (median 4 years), all patients (100%) had imaging examinations, including magnetic resonance imaging angiography in 2 (28.6%) and DSA in the rest 5 (71.4%). No clinical symptoms were present in 5 (71.4% or 5/7) patients, intermittent headache was present in 1 (14.3% or 1/7), and death from trauma in the last 1 (14.3% or 1/7). Among the 5 patients treated with stents, 1 (20%, case 3) developed in-stent restenosis (65%) but without significant clinical symptoms during follow-up (Fig. 3). In this patient, DSA 6 months after endovascular treatment showed DAVF and in-stent restenosis. The DAVF was cured after onyx embolization and in-stent dilatation was performed concurrently to relieve the stenosis. No other patients experienced repeated treatment.
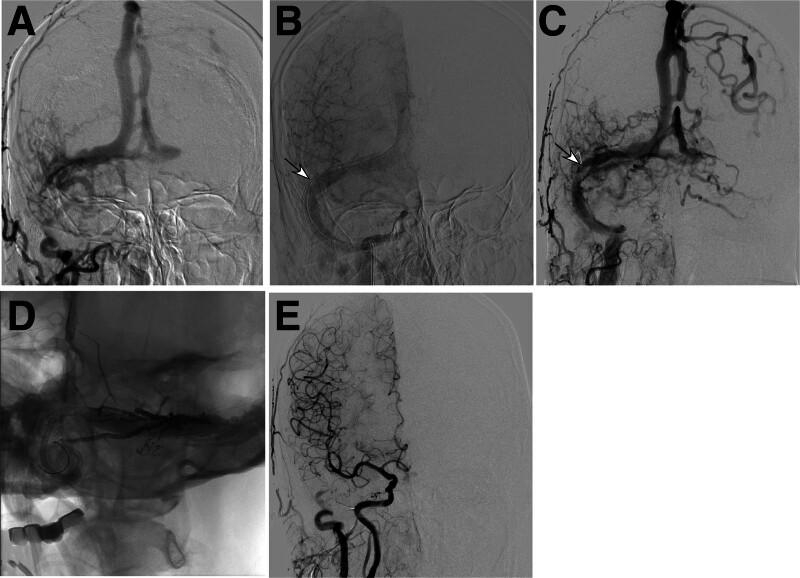
Figure 3.
A dural arteriovenous fistula (DAVF) was present in a male patient (case 3) at the right transverse sinus treated with endovascular angioplasty. (A) pretreatment digital subtraction angiography (DSA) revealed occlusion of the right sigmoid sinus, right jugular vein and left transverse sinus. (B) A stent (arrow) was deployed in the right transverse sinus. (C) Follow-up DSA demonstrated in-stent restenosis (arrow). (D) Percutaneous transluminal coronary angioplasty was performed to relieve the in-stent restenosis. (E) After onyx embolization, the DAVF was completely occluded.
7. Discussion
Our study investigated the effect and safety of endovascular recanalization of occluded venous sinuses for the treatment of DAVF. It was found that endovascular recanalization of the involved occluded or stenotic venous sinuses was safe and efficient in inducing occlusion or relief of the DAVFs which were probably caused by significant pressure increase.
Similar outcomes have been reported in ther literature. In 1 study of 10 patients with transverse-sigmoid DAVF combined with sinus thrombosis in 2 patients and sinus stenosis in the other 8 patients,[1] stent angioplasty had led to complete DAVF occlusion in 4 patients, significant flow reduction in 4, and significant clinical symptom improvement in 2 at a mean follow-up time of 7.5 months. This study indicates that stent angioplasty can result in a cure or significant clinical improvement in all patients harboring a DAVF, serving as a promising technique for the treatment of DAVFs. This study also suggests that embolization alone of the DAVF may not be an effective measure if the stenosed or occluded sinuses have not been resolved in the first place. In the same study,[1] 1 patient with a type I DAVF in the stenosed transverse sinus initially experienced transarterial embolization of the fistula, but the type I DAVF was transformed to type IV ten years later with left temporal lobe hematoma. Stent angioplasty of the stenosed transverse sinus immediately led to dramatic improvement in the symptoms and complete healing 6 months later.
Surgical removal or endovascular occlusion of the fistulous sinus may eliminate the fistula as a cure for the shunt, but it may also cause recruitment of other feeding arteries to the shunt besides some rare and severe complications.[1] Transvenous sinus occlusion has been reported as an appealing approach to deal with the DAVF. Nonetheless, occlusion of the involved sinus may impair the normal functioning of the sinus because of the drainage of cortical veins to the involved sinus. Thus, occlusion of the sinus is not a better approach than recanalization of the sinus in case of blood drainage from the cortical veins.
Liebig et al had reported use of transvenous angioplasty and stent deployment for reconstruction of DAVFs of the transverse and sigmoid sinuses in 4 patients who had been previously treated with repeated transarterial embolization of the fistulas.[2] Despite repeated embolization, the shunt flow was only temporarily decreased. In 2 patients, the involved sigmoid sinuses were thrombosed and required recanalization, and in the other 2 patients, the involved sinus wall was irregular and stenosed. In 1 patient, although balloon dilation of the involved sinus was sufficient to induce occlusion of the fistula, a stent was deployed to maintain this effect. In 3 patients, the fistula was completely occluded by transvenous stent deployment in combination with transarterial embolization. Therefore, the authors suggested that angioplasty and stent deployment should be considered as a first-line therapy for DAVFs. The authors also recommended that treatment of DAVFs should be focused on reestablishment of the sinus patency in case of occluded or severely stenosed sinuses, patent functional venous pathways should not be sacrificed, and treatment itself should not induce redirection of blood flow to other venous structures.[2]
Studies have found that the essential abnormality of DAVFs is not a newly created direct arterivenous shunt to the sinus lumen but innative connections of small venules of approximately 30 μm in diameter between the dural arteries and dural veins within the venous sinus wall.[32,33] These studies believed that sinus hypertension triggered development of fistulous connections between arteries and veins inside the dural wall, which is an essential part of the pathogenesis of DAVFs. Sinus thrombosis is not an essential factor for DAVF formation. Sinus hypertension may lead to increasingly dilated venules inside the dural mater wall and finally formation of DAVFs. The theory of sinus hypertension to trigger DAVFs is further supported by animal experiments in rats whose developed DAVFs were positively correlated with surgically-induced venous hypertension.[21,22] Development of DAVFs was positively correlated with both venous hypertension and angiogenic activity, which may indicate that venous hypertension may induce angiogenic activity either indirectly or directly via decreasing cerebral perfusion and increasing ischemia. De novo development of DAVFs following transvenous therapy and coil embolization of the involved sinus segment has been reported,[14–16] which may support a role for venous hypertension in the pathogenesis of DAVFs.
Research has confirmed that 1 of the most important hemodynamic features of DAVF is the imbalance of local blood flow velocity gradient and pressure gradient.[3,6,7,34–36] Thrombosis, vascular inflammation, infection, and trauma in any blood vessels or venous sinus will cause blood pressure increase inside the vascular lumen, and the high “kinetic or potential energy” will be released from high to low pressure regions to reduce the pressure gradient, thereby reducing the risk of diseases caused by gradient changes, which may be the self-adjustment ability of the body itself. However, due to the fact that the geometric structure of the dural mater is a 2-dimensional membranous plane, the distribution of blood vessels on the dural mater is limited to the plane itself. Because the brain parenchyma is a 3-dimensional tissue structure, its distribution of blood vessels is in 3-dimensional or different directions, and the “kinetic or potential energy” can be quickly spread in all directions, thus being able to significantly decrease the energy gradient and subsequent formation of DAVFs. Because the dural blood vessels only exist in the dural mater membranous plane, the high kinetic or potential energy in the dural blood vessels cannot be quickly spread to other locations through the blood vessels like it does in the brain parenchyma as a 3-dimensional tissue structure, and a high gradient of energy will be formed between dural arteries and veins, leading to formation of DAVFs. This can explain that after venous sinus occlusion, although there is significant congestion and dilation of the vascular system in the brain parenchyma, arteriovenous fistulas rarely occur in the brain parenchyma, whereas arteriovenous fistulas frequently form in the dura mater.
In our study, the DAVFs in patients 1, 2, and 4 were cured immediately following endovascular recanalization of the occluded or stenosed sinuses. These patients had a short disease course (3–6 months), which may not be long enough to cause significant changes or damages to the small venules connecting the dural arteries and dural veins within the venous sinus wall to form an arteriovenous fistula.[32,33] At imaging follow-up 3 months later, 2 additional patients were cured, whereas 2 cases with Cognard grade I and a disease course of over 10 years still had fistulas but no clinical symptoms even though the venous sinus stenosis or occlusion had been successfully resolved. Long-term disease courses in these 2 patients may have significantly damaged the small venules within the venous sinus wall which cannot be restored to the initial status in morphology and function in a short time even after the venous hypertension had been resolved through endovascular recanalization. Venous sinus stenosis or occlusion will cause cease or stasis of blood flow and subsequently increase the pressure of blood within the venous sinus. Because the blood cannot be smoothly exported from the arterial system into the venous sinuses due to venous hypertension, the pressure of blood in the arterial system will also rise, and when the pressure increase exceeds a certain level or the threshold, the small venules of approximately 30 μm in diameter within the venous sinus wall[32,33] will be opened or dilated, leading to formation of arteriovenous fistula. Resolution of venous hypertension will significantly decrease the venous blood pressure while increase the pressure gradient between the arterial system and venous sinuses, leading to smooth export of blood, and the small venules within the dural mater wall or venous sinus wall will be closed. However, long-term existence of arteriovenous fistulas will greatly damage the small venules and will not restore them to their initial status even after the venous hypertension has been eliminated. In these cases with a long disease course, embolizing the arteriovenous fistula in combination with resolving the sinus stenosis or occlusion may be the best solution. Recurrence or de novo formation of arteriovenous fistulas after successful fistula closure may also be caused by the pressure increase in the arterial segment because of difficult blood discharge into the venous system. Decrease of blood pressure in the arterial segment will subsequently decrease the pressure difference or gradient between the arterial and venous ends, beneficial to relieving the fistula.
Some limitations existed in our study, including the retrospective and single-center study design, a small cohort of patients, no randomization, no control, and no further pathological exploration, which may all affect the generalization of the outcome. Future studies will have to resolve these issues for better outcomes.
In conclusion, recanalization of occluded or stenosed venous sinuses using balloon angioplasty and stent deployment is able to induce occlusion of DAVFs and is a safe and efficient treatment approach for DAVFs which are possibly caused by significant pressure increase.
Author contributions
Conceptualization: Tong-Yuan Zhao, Jiang-Yu Xue.
Data curation: Tong-Yuan Zhao, Gang-Qin Xu, Zhong-Can Chen, Jiang-Yu Xue, Dong-Yang Cai, Bo-Wen Yang, Tian-Xiao Li, Bu-Lang Gao.
Formal analysis: Tong-Yuan Zhao, Gang-Qin Xu, Zhong-Can Chen, Jiang-Yu Xue, Dong-Yang Cai, Bo-Wen Yang, Tian-Xiao Li, Bu-Lang Gao.
Funding acquisition: Zhong-Can Chen, Jiang-Yu Xue, Bo-Wen Yang, Bu-Lang Gao.
Investigation: Tong-Yuan Zhao, Gang-Qin Xu, Zhong-Can Chen, Jiang-Yu Xue, Dong-Yang Cai, Bo-Wen Yang, Tian-Xiao Li, Bu-Lang Gao.
Methodology: Tong-Yuan Zhao, Gang-Qin Xu, Zhong-Can Chen, Jiang-Yu Xue, Dong-Yang Cai, Bo-Wen Yang, Tian-Xiao Li.
Project administration: Jiang-Yu Xue.
Resources: Dong-Yang Cai, Tian-Xiao Li.
Software: Dong-Yang Cai, Tian-Xiao Li.
Supervision: Tong-Yuan Zhao, Gang-Qin Xu, Zhong-Can Chen, Jiang-Yu Xue, Bo-Wen Yang, Tian-Xiao Li, Bu-Lang Gao.
Validation: Tong-Yuan Zhao, Gang-Qin Xu, Zhong-Can Chen, Jiang-Yu Xue, Dong-Yang Cai, Bo-Wen Yang, Tian-Xiao Li, Bu-Lang Gao.
Visualization: Tong-Yuan Zhao, Gang-Qin Xu, Zhong-Can Chen, Jiang-Yu Xue, Dong-Yang Cai, Bo-Wen Yang, Bu-Lang Gao.
Writing – original draft: Bu-Lang Gao.
Writing – review & editing: Bu-Lang Gao.
Abbreviations:
- CCF
- carotid-cavernous fistula
- DAVFs
- dural arteriovenous fistulas
- ICA
- internal carotid artery
This study was supported by funding from Henan Center for Outstanding Overseas Scientists (No. GZS2022019), the 13th 5-year Plan of China for Research and Development (2016YFC1300702), and Scientific and Technology Plan of Henan Province (162102310268, 172102310509),
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Zhao T-Y, Xu G-Q, Chen Z-C, Xue J-Y, Cai D-Y, Yang B-W, Li T-X, Gao B-L. Dural arteriovenous fistula may be occluded through recanalization of impaired venous sinuses. Medicine 2024;103:41(e40097).
Contributor Information
Tong-Yuan Zhao, Email: zhaotongyuan@163.com.
Gang-Qin Xu, Email: xugangqin@163.com.
Zhong-Can Chen, Email: chenzhongcan@163.com.
Dong-Yang Cai, Email: caidongyang@163.com.
Bo-Wen Yang, Email: yangbowen@163.com.
Tian-Xiao Li, Email: litianxiaod@163.com.
Bu-Lang Gao, Email: browngao@163.com.
References
- [1].Levrier O, Metellus P, Fuentes S, et al. Use of a self-expanding stent with balloon angioplasty in the treatment of dural arteriovenous fistulas involving the transverse and/or sigmoid sinus: functional and neuroimaging-based outcome in 10 patients. J Neurosurg. 2006;104:254–63. [DOI] [PubMed] [Google Scholar]
- [2].Liebig T, Henkes H, Brew S, Miloslavski E, Kirsch M, Kühne D. Reconstructive treatment of dural arteriovenous fistulas of the transverse and sigmoid sinus: transvenous angioplasty and stent deployment. Neuroradiology. 2005;47:543–51. [DOI] [PubMed] [Google Scholar]
- [3].Chen L, Mao Y, Zhou LF. Local chronic hypoperfusion secondary to sinus high pressure seems to be mainly responsible for the formation of intracranial dural arteriovenous fistula. Neurosurgery. 2009;64:973–83; discussion 983. [DOI] [PubMed] [Google Scholar]
- [4].Noguchi K, Kubo M, Kuwayama N, et al. Intracranial dural arteriovenous fistulas with retrograde cortical venous drainage: assessment with cerebral blood volume by dynamic susceptibility contrast magnetic resonance imaging. AJNR Am J Neuroradiol. 2006;27:1252–6. [PMC free article] [PubMed] [Google Scholar]
- [5].Oh JT, Chung SY, Lanzino G, et al. Intracranial dural arteriovenous fistulas: clinical characteristics and management based on location and hemodynamics. J Cerebrovasc Endovasc Neurosurg. 2012;14:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Meadows CA, Carlson A, Vorobieff P. Reversal of cortical venous reflux in dural arteriovenous fistula with change in blood pressure. Clin Neuroradiol. 2019;29:375–8. [DOI] [PubMed] [Google Scholar]
- [7].Kosinepalli SS, Das SK, B G, Chittaragi K. The gridlock between chronic cerebral venous thrombosis and dural arteriovenous fistulas. Cureus. 2023;15:e35035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alkhaibary A, Alnefaie N, Alharbi A, et al. Intracranial dural arteriovenous fistula: a comprehensive review of the history, management, and future prospective. Acta Neurol Belg. 2023;123:359–66. [DOI] [PubMed] [Google Scholar]
- [9].Abdalkader M, Nguyen TN, Diana F, et al. Intracranial dural arteriovenous fistulas. Semin Neurol. 2023;43:388–96. [DOI] [PubMed] [Google Scholar]
- [10].Zyck S, De Jesus O, Gould GC. Dural Arteriovenous Fistula. In: StatPearls. Treasure Island (FL). Ineligible companies. Disclosure: Orlando De Jesus declares no relevant financial relationships with ineligible companies. Disclosure: Grahame Gould declares no relevant financial relationships with ineligible companies; 2023. [Google Scholar]
- [11].Akdag R, Soylu U, Daglioglu E, Akmangit I, Açik V, Belen AD. Management and outcome of intracranial dural arteriovenous fistulas that have caused a hemorrhage in the posterior fossa: a clinical study. J Korean Neurosurg Soc. 2023;66:672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kang YS, Cho WS, Lee SH, Kim K, Kang H-S, Kim JE. Role of surgery in management of intracranial dural arteriovenous fistulas. J Cerebrovasc Endovasc Neurosurg. 2023;25:117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Darsaut TE, Magro E, Bojanowski MW, et al. TOBAS Collaborative Group. Surgical treatment of brain arteriovenous malformations: clinical outcomes of patients included in the registry of a pragmatic randomized trial. J Neurosurg. 2023;138:891–9. [DOI] [PubMed] [Google Scholar]
- [14].Kiyosue H, Tanoue S, Okahara M, Yamashita M, Nagatomi H, Mori H. Recurrence of dural arteriovenous fistula in another location after selective transvenous coil embolization: report of two cases. AJNR Am J Neuroradiol. 2002;23:689–92. [PMC free article] [PubMed] [Google Scholar]
- [15].Kubo M, Kuwayama N, Hirashima Y, Kurimoto M, Takaku A, Endo S. Dural arteriovenous fistulae developing at different locations after resolution of previous fistulae: report of three cases and review of the literature. AJNR Am J Neuroradiol. 2002;23:787–9. [PMC free article] [PubMed] [Google Scholar]
- [16].Kurl S, Vanninen R, Saari T, Hernesniemi J. Development of right transverse sinus dural arteriovenous malformation after embolisation of a similar lesion on the left. Neuroradiology. 1996;38:386–8. [DOI] [PubMed] [Google Scholar]
- [17].Duquette E, Dowlati E, Abdullah T, et al. De Novo dural arteriovenous fistulas after endovascular treatment: case illustration and literature review. Interv Neuroradiol. 2022;15910199221118517:159101992211185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Makita I, Kamio Y, Hiramatsu H, Kurozumi K. Occurrence of de novo dural arteriovenous fistula after transvenous embolization of dural arteriovenous fistula: case reports of two patients. J Korean Neurosurg Soc. 2022;65:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Paramasivam S, Toma N, Niimi Y, Berenstein A. De novo development of dural arteriovenous fistula after endovascular embolization of pial arteriovenous fistula. J Neurointerv Surg. 2013;5:321–6. [DOI] [PubMed] [Google Scholar]
- [20].Takeuchi S, Miyakoshi A, Hawke P. Craniocervical junction dural arteriovenous fistula and pial arteriovenous fistula presenting concomitantly in separate locations with subarachnoid hemorrhage. World Neurosurg. 2023;180:14–6. [DOI] [PubMed] [Google Scholar]
- [21].Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997;87:267–74. [DOI] [PubMed] [Google Scholar]
- [22].Terada T, Higashida RT, Halbach VV, et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994;80:884–9. [DOI] [PubMed] [Google Scholar]
- [23].Bederson JB, Wiestler OD, Brustle O, Roth P, Frick R, Yaşargil MG. Intracranial venous hypertension and the effects of venous outflow obstruction in a rat model of arteriovenous fistula. Neurosurgery. 1991;29:341–50. [DOI] [PubMed] [Google Scholar]
- [24].Li Q, Zhang Q, Huang QH, et al. A pivotal role of the vascular endothelial growth factor signaling pathway in the formation of venous hypertension-induced dural arteriovenous fistulas. Mol Med Rep. 2014;9:1551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Herman JM, Spetzler RF, Bederson JB, Kurbat JM, Zabramski JM. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg. 1995;83:539–45. [DOI] [PubMed] [Google Scholar]
- [26].Sahara Y, Miyachi S, Nagasaka T, et al. Radiological and pathological changes in the sinus of an experimental arteriovenous fistula of the rat. Interv Neuroradiol. 2003;9(Suppl 1):101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hirata E, Higashi T, Iwamuro Y, et al. Angioplasty and stent deployment in acute sinus thrombosis following endovascular treatment of dural arteriovenous fistulae. J Clin Neurosci. 2009;16:725–7. [DOI] [PubMed] [Google Scholar]
- [28].Murphy KJ, Gailloud P, Venbrux A, Deramond H, Hanley D, Rigamonti D. Endovascular treatment of a grade IV transverse sinus dural arteriovenous fistula by sinus recanalization, angioplasty, and stent placement: technical case report. Neurosurgery. 2000;46:497–500; discussion 500. [DOI] [PubMed] [Google Scholar]
- [29].Osuki T, Ikeda H, Hayashi T, et al. Gradual dilatation of an occluded transverse sinus associated with dural arteriovenous fistula after balloon angioplasty with sinus packing: a case report. Neuroradiol J. 2022;35:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ohara N, Toyota S, Kobayashi M, Wakayama A. Superior sagittal sinus dural arteriovenous fistulas treated by stent placement for an occluded sinus and transarterial embolization. A case report. Interv Neuroradiol. 2012;18:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang K, Gao BL, Zhu LF, Xue J-Y, Yang B-W, Li T-X. Endovascular recanalization of occluded dural sinus in patient with dural arteriovenous fistulas: case report and literature review. World Neurosurg. 2018;114:269–73. [DOI] [PubMed] [Google Scholar]
- [32].Hamada Y, Goto K, Inoue T, et al. Histopathological aspects of dural arteriovenous fistulas in the transverse-sigmoid sinus region in nine patients. Neurosurgery. 1997;40:452–6; discussion 456. [DOI] [PubMed] [Google Scholar]
- [33].Nishijima M, Takaku A, Endo S, et al. Etiological evaluation of dural arteriovenous malformations of the lateral and sigmoid sinuses based on histopathological examinations. J Neurosurg. 1992;76:600–6. [DOI] [PubMed] [Google Scholar]
- [34].Liu P, Shi Y, Li S, et al. Pathology and protein changes of the spinal dural arteriovenous fistula arterial draining vein under sustained high vascular pressure. Front Neurol. 2021;12:713355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Prosperini L, Gentile M, Ricci M, et al. Dural arteriovenous fistula as a reversible cause of progressive parkinsonism and dementia: a case report. Neurohospitalist. 2022;12:559–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cheng HC, Bodani VP, Chung E, Mosimann PJ. Transvenous retrograde pressure cooker technique for embolization of an ethmoidal dural arteriovenous fistula. J Neurointerv Surg. 2023;16:742–742. [DOI] [PubMed] [Google Scholar]