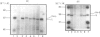Abstract
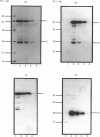
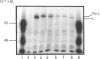
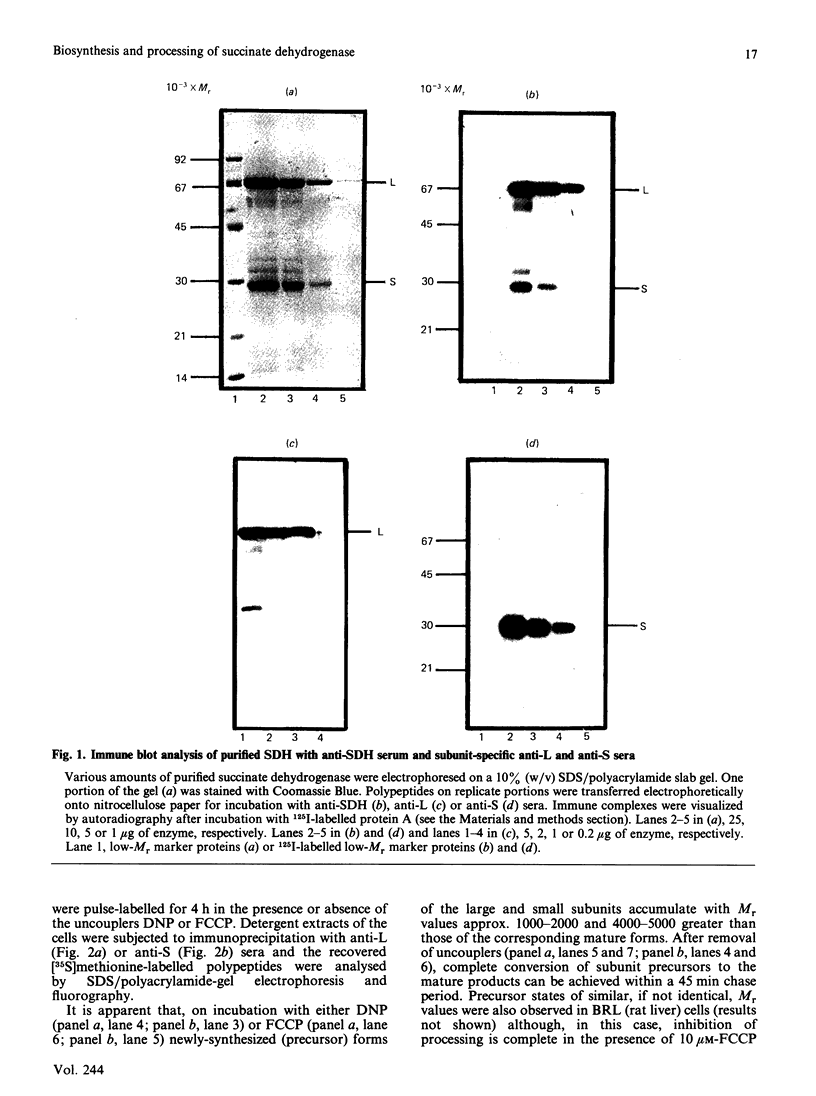
Monospecific polyclonal antisera have been raised to purified bovine heart succinate dehydrogenase and to the individual large and small subunits of this enzyme. These antisera exhibit cross-reactivity with the corresponding polypeptides in rat liver (BRL), pig kidney (PK-15) and bovine kidney (NBL-1) cell lines, and were employed to investigate some of the events involved in the biogenesis of succinate dehydrogenase in the PK-15 cell line. Newly-synthesized forms of the large and small subunits of succinate dehydrogenase were detected in cultured PK-15 and BRL cells labelled with [35S]methionine in the presence of uncouplers of oxidative phosphorylation. In PK-15 cells, the precursor forms of the large and small subunits exhibit Mr values approx. 1000-2000 and 4000-5000 greater than those of the corresponding mature forms. When the uncoupler is removed in pulse-chase experiments, complete conversion of the precursors to the mature forms occurs within 45 min. Studies on the kinetics of processing and stability of the large subunit precursor revealed that reversal of precursor accumulation is rapid, with processing occurring with a half-time of 5-7.5 min, and that the accumulated precursor exhibits long-term stability when PK-15 cells are maintained in the presence of 2,4-dinitrophenol.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrell B. A., Ball M. B., Kearney E. B. Peptides from complex II active in reconstitution of succinate-ubiquinone reductase. J Biol Chem. 1980 Apr 10;255(7):2761–2769. [PubMed] [Google Scholar]
- Barber B. H., Delovitch T. L. The identification of actin as a major lymphocyte component. J Immunol. 1979 Jan;122(1):320–325. [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Sweetland J., Merli A. Polypeptides in the succinate-coenzyme Q reductase segment of the respiratory chain. Biochemistry. 1977 Dec 27;16(26):5707–5710. doi: 10.1021/bi00645a009. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y. Succinate dehydrogenase. I. Purification, molecular properties, and substructure. Biochemistry. 1971 Jun 22;10(13):2509–2516. doi: 10.1021/bi00789a014. [DOI] [PubMed] [Google Scholar]
- De Marcucci O. L., Hunter A., Lindsay J. G. Low immunogenicity of the common lipoamide dehydrogenase subunit (E3) of mammalian pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase multienzyme complexes. Biochem J. 1985 Mar 1;226(2):509–517. doi: 10.1042/bj2260509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton W. A., Hack A. M., Helfgott D., Rosenberg L. E. Biogenesis of the mitochondrial enzyme methylmalonyl-CoA mutase. Synthesis and processing of a precursor in a cell-free system and in cultured cells. J Biol Chem. 1984 May 25;259(10):6616–6621. [PubMed] [Google Scholar]
- Freeman K. B., Chien S. M., Litchfield D., Patel H. V. Synthesis in vitro of rat brown adipose tissue 32 000 Mr protein. FEBS Lett. 1983 Jul 25;158(2):325–330. doi: 10.1016/0014-5793(83)80606-1. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Daum G., Schatz G. Import of proteins into mitochondria. Energy-dependent uptake of precursors by isolated mitochondria. J Biol Chem. 1982 Nov 10;257(21):13034–13041. [PubMed] [Google Scholar]
- Girdlestone J., Bisson R., Capaldi R. A. Interaction of succinate--ubiquinone reductase (complex II) with (arylazido)phospholipids. Biochemistry. 1981 Jan 6;20(1):152–156. doi: 10.1021/bi00504a025. [DOI] [PubMed] [Google Scholar]
- Hatalová I., Kolarov J. Synthesis and intracellular transport of cytochrome oxidase subunit IV and ADP/ATP translocator protein in intact hepatoma cells. Biochem Biophys Res Commun. 1983 Jan 14;110(1):132–139. doi: 10.1016/0006-291x(83)91270-6. [DOI] [PubMed] [Google Scholar]
- Hunter A., Lindsay J. G. Immunological and biosynthetic studies on the mammalian 2-oxoglutarate dehydrogenase multienzyme complex. Eur J Biochem. 1986 Feb 17;155(1):103–109. doi: 10.1111/j.1432-1033.1986.tb09464.x. [DOI] [PubMed] [Google Scholar]
- Jaussi R., Sonderegger P., Flückiger J., Christen P. Biosynthesis and topogenesis of aspartate aminotransferase isoenzymes in chicken embryo fibroblasts. The precursor of the mitochondrial isoenzyme is either imported into mitochondria or degraded in the cytosol. J Biol Chem. 1982 Nov 25;257(22):13334–13340. [PubMed] [Google Scholar]
- Klingenberg M. Localization of the glycerol-phosphate dehydrogenase in the outer phase of the mitochondrial inner membrane. Eur J Biochem. 1970 Apr;13(2):247–252. doi: 10.1111/j.1432-1033.1970.tb00924.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Merli A., Capaldi R. A., Ackrell B. A., Kearney E. B. Arrangement of complex II (succinate-ubiguinone reductase) in the mitochondrial inner membrane. Biochemistry. 1979 Apr 17;18(8):1393–1400. doi: 10.1021/bi00575a001. [DOI] [PubMed] [Google Scholar]
- Mori M., Morita T., Ikeda F., Amaya Y., Tatibana M., Cohen P. P. Synthesis, intracellular transport, and processing of the precursors for mitochondrial ornithine transcarbamylase and carbamoyl-phosphate synthetase I in isolated hepatocytes. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6056–6060. doi: 10.1073/pnas.78.10.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley K., Pratt P., Robertson J., Lilly M., Douglas M. G. Selection of the nuclear gene for the mitochondrial adenine nucleotide translocator by genetic complementation of the op1 mutation in yeast. J Biol Chem. 1982 Feb 25;257(4):2097–2103. [PubMed] [Google Scholar]
- Ono H., Tuboi S. Translocation of proteins into rat liver mitochondria. The precursor polypeptides of a large subunit of succinate dehydrogenase and ornithine aminotransferase and their imports into their own locations of mitochondria. Eur J Biochem. 1986 Mar 17;155(3):543–549. doi: 10.1111/j.1432-1033.1986.tb09522.x. [DOI] [PubMed] [Google Scholar]
- Raymond Y., Shore G. C. Processing of the precursor for the mitochondrial enzyme, carbamyl phosphate synthetase. Inhibition by rho-aminobenzamidine leads to very rapid degradation (clearing) of the precursor. J Biol Chem. 1981 Mar 10;256(5):2087–2090. [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Schleyer M., Schmidt B., Neupert W. Requirement of a membrane potential for the posttranslational transfer of proteins into mitochondria. Eur J Biochem. 1982 Jun 15;125(1):109–116. doi: 10.1111/j.1432-1033.1982.tb06657.x. [DOI] [PubMed] [Google Scholar]
- Singer T. P., Johnson M. K. The prosthetic groups of succinate dehydrogenase: 30 years from discovery to identification. FEBS Lett. 1985 Oct 14;190(2):189–198. doi: 10.1016/0014-5793(85)81282-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. H., Singer T. P. Identification of the covalently bound flavin of succinate dehydrogenase as 8-alpha-(histidyl) flavin adenine dinucleotide. J Biol Chem. 1970 Aug 25;245(16):4224–4225. [PubMed] [Google Scholar]