Abstract
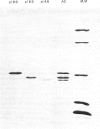
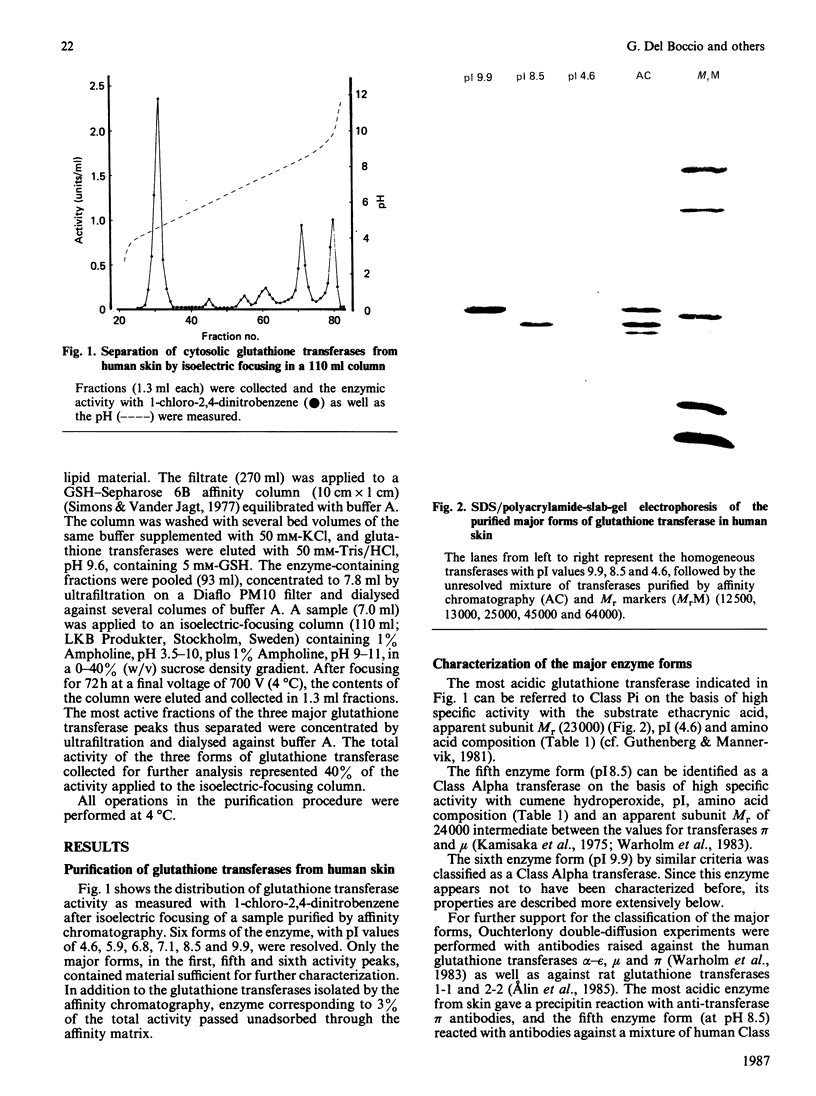
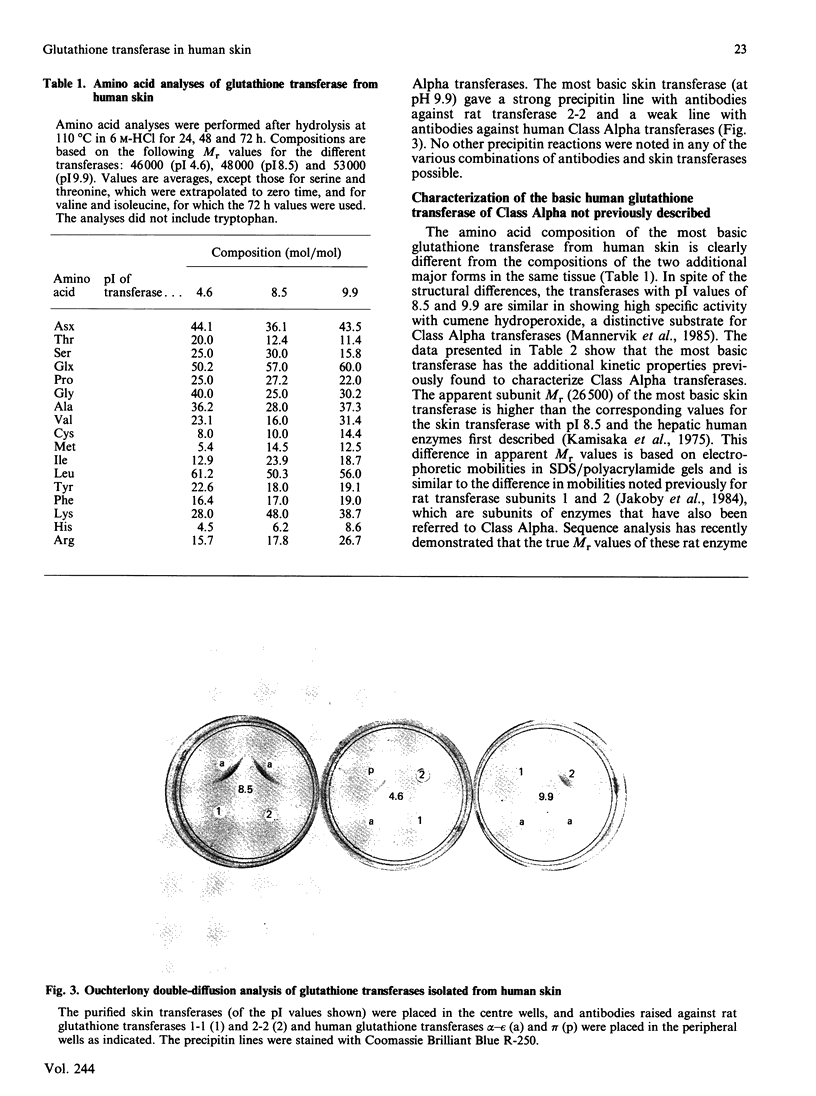
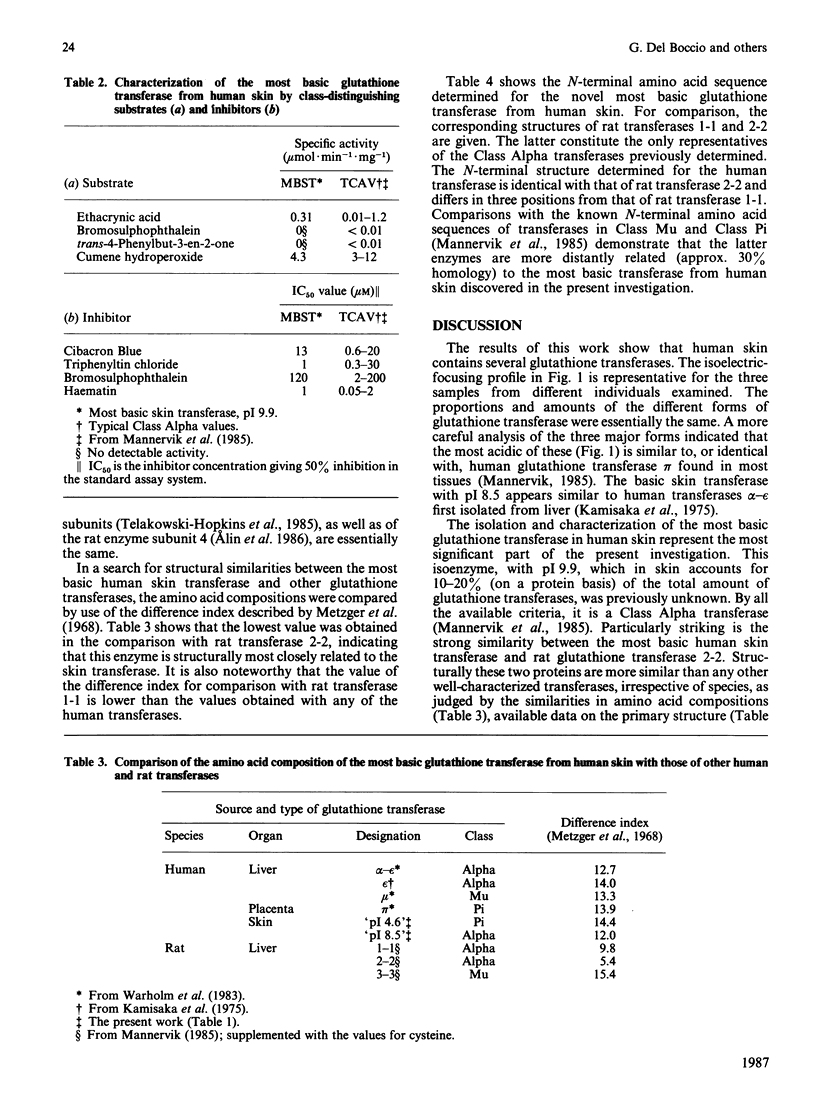
Six forms of glutathione transferase with pI values of 4.6, 5.9, 6.8, 7.1, 8.5 and 9.9 have been isolated from the cytosol fraction of normal skin from three human subjects. The three most abundant enzymes were an acidic Class Pi transferase (pI 4.6; apparent subunit Mr 23,000), a basic Class Alpha transferase (pI 8.5; apparent subunit Mr 24,000) and an even more basic glutathione transferase of Class Alpha (pI 9.9; apparent subunit Mr 26,500). The last enzyme, which was previously unknown, accounts for 10-20% of the glutathione transferase in human skin. The novel transferase showed greater similarities with rat glutathione transferase 2-2, another Class Alpha enzyme, than with any other known transferase irrespective of species. The most striking similarities included reactions with antibodies, amino acid compositions and identical N-terminal amino acid sequences (16 residues). The close relationship between the human most basic and the rat glutathione transferase 2-2 supports the classification of the transferases previously proposed and indicates that the similarities between enzymes isolated from different species are more extensive than had been assumed previously.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alin P., Jensson H., Guthenberg C., Danielson U. H., Tahir M. K., Mannervik B. Purification of major basic glutathione transferase isoenzymes from rat liver by use of affinity chromatography and fast protein liquid chromatofocusing. Anal Biochem. 1985 May 1;146(2):313–320. doi: 10.1016/0003-2697(85)90545-7. [DOI] [PubMed] [Google Scholar]
- Alin P., Mannervik B., Jörnvall H. Cytosolic rat liver glutathione transferase 4-4. Primary structure of the protein reveals extensive differences between homologous glutathione transferases of classes alpha and mu. Eur J Biochem. 1986 Apr 15;156(2):343–350. doi: 10.1111/j.1432-1033.1986.tb09588.x. [DOI] [PubMed] [Google Scholar]
- Ding G. J., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. Nucleotide sequence analysis of a Yb1 cDNA clone and prediction of the complete amino acid sequence of the Yb1 subunit. J Biol Chem. 1985 Oct 25;260(24):13268–13271. [PubMed] [Google Scholar]
- Guthenberg C., Mannervik B. Glutathione S-transferase (transferase pi) from human placenta is identical or closely related to glutathione S-transferase (transferase rho) from erythrocytes. Biochim Biophys Acta. 1981 Oct 13;661(2):255–260. doi: 10.1016/0005-2744(81)90012-7. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B., Ketterer B., Mannervik B. Glutathione transferases: nomenclature. Biochem Pharmacol. 1984 Aug 15;33(16):2539–2540. doi: 10.1016/0006-2952(84)90621-x. [DOI] [PubMed] [Google Scholar]
- Kamisaka K., Habig W. H., Ketley J. N., Arias M., Jakoby W. B. Multiple forms of human glutathione S-transferase and their affinity for bilirubin. Eur J Biochem. 1975 Dec 1;60(1):153–161. doi: 10.1111/j.1432-1033.1975.tb20987.x. [DOI] [PubMed] [Google Scholar]
- Lai H. C., Li N., Weiss M. J., Reddy C. C., Tu C. P. The nucleotide sequence of a rat liver glutathione S-transferase subunit cDNA clone. J Biol Chem. 1984 May 10;259(9):5536–5542. [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Metzger H., Shapiro M. B., Mosimann J. E., Vinton J. E. Assessment of compositional relatedness between proteins. Nature. 1968 Sep 14;219(5159):1166–1168. doi: 10.1038/2191166a0. [DOI] [PubMed] [Google Scholar]
- Pickett C. B., Telakowski-Hopkins C. A., Ding G. J., Argenbright L., Lu A. Y. Rat liver glutathione S-transferases. Complete nucleotide sequence of a glutathione S-transferase mRNA and the regulation of the Ya, Yb, and Yc mRNAs by 3-methylcholanthrene and phenobarbital. J Biol Chem. 1984 Apr 25;259(8):5182–5188. [PubMed] [Google Scholar]
- Simons P. C., Vander Jagt D. L. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal Biochem. 1977 Oct;82(2):334–341. doi: 10.1016/0003-2697(77)90169-5. [DOI] [PubMed] [Google Scholar]
- Telakowski-Hopkins C. A., Rodkey J. A., Bennett C. D., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. Construction of a cDNA clone complementary to a Yc mRNA and prediction of the complete amino acid sequence of a Yc subunit. J Biol Chem. 1985 May 10;260(9):5820–5825. [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B. Molecular and catalytic properties of glutathione transferase mu from human liver: an enzyme efficiently conjugating epoxides. Biochemistry. 1983 Jul 19;22(15):3610–3617. doi: 10.1021/bi00284a011. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]