Abstract
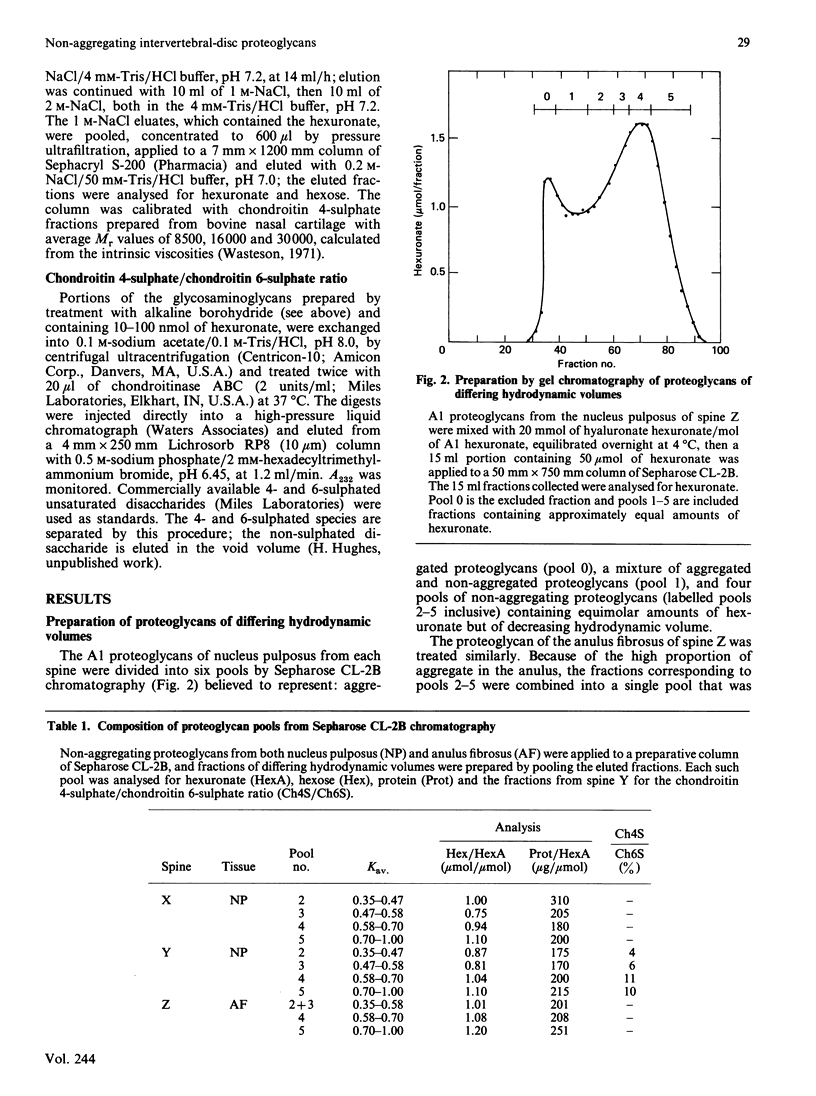
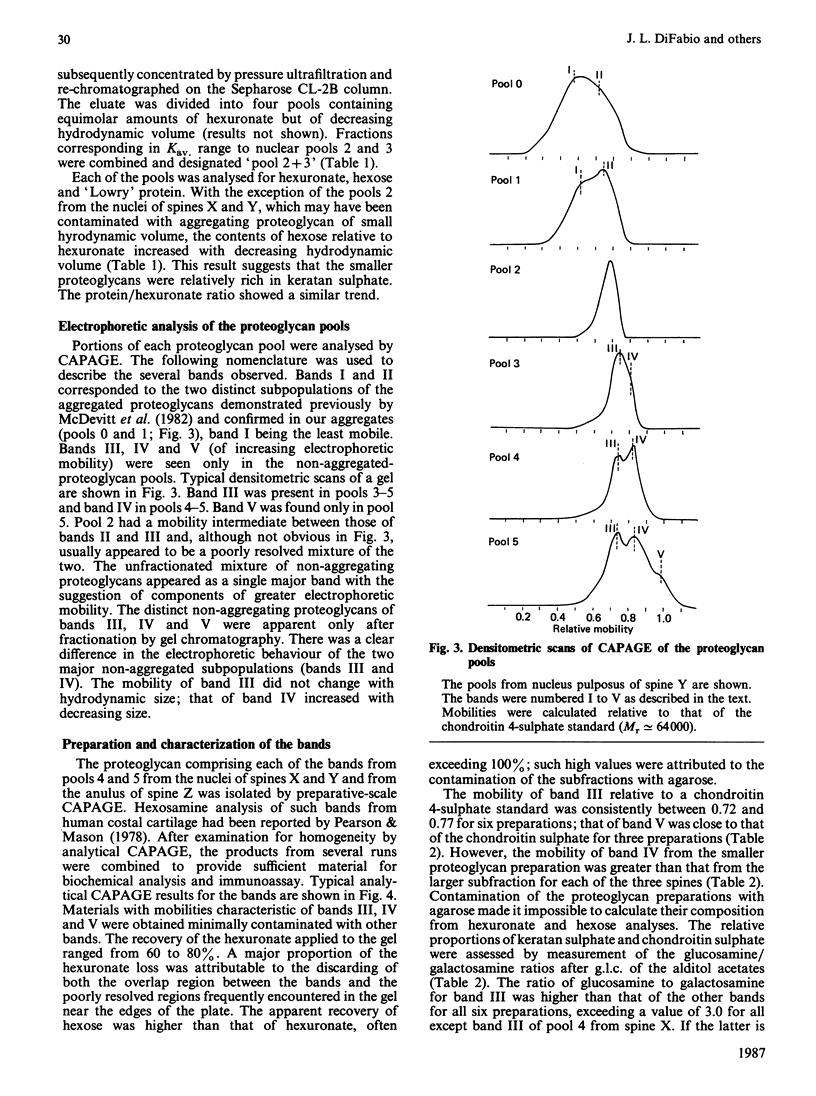
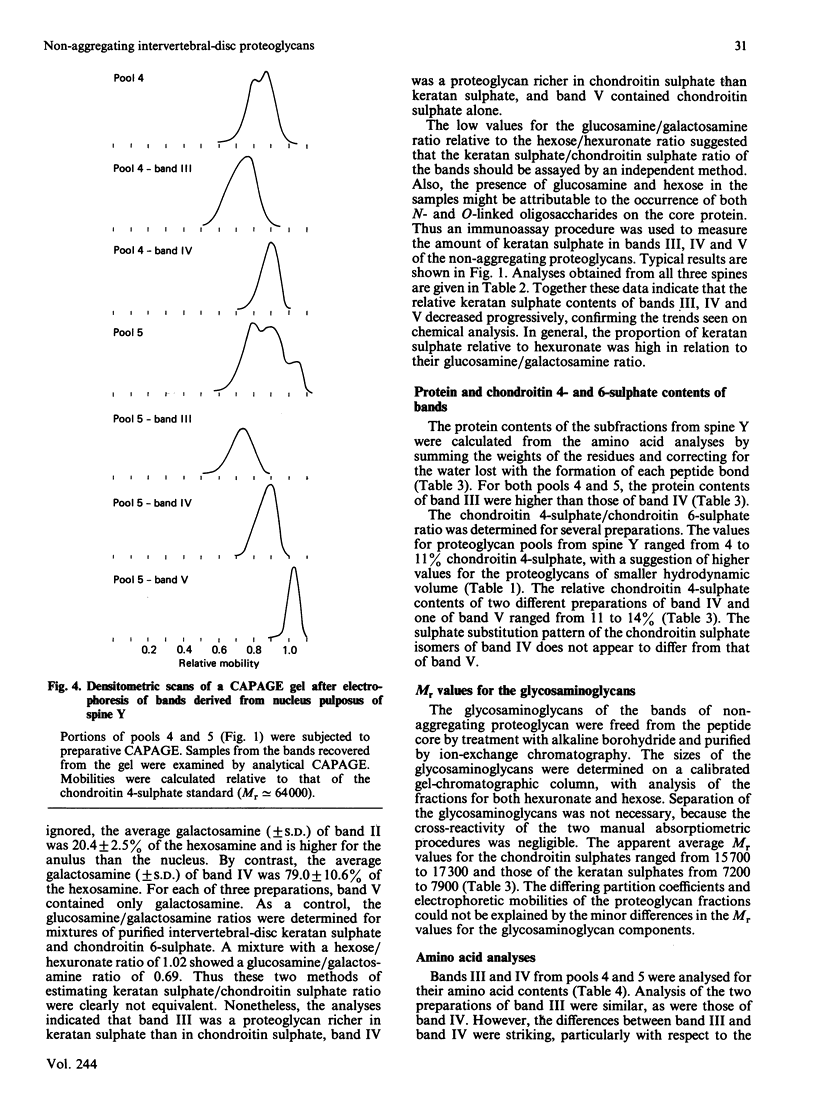
Non-aggregating proteoglycans of differing average hydrodynamic volumes were prepared from nuclei pulposi and anuli fibrosi of three human lumbar spines and characterized by biochemical and immunochemical analyses. The hexose-to-hexuronate and protein-to-hexuronate ratios increased with decreasing hydrodynamic volume. Analysis by composite agarose/polyacrylamide-gel electrophoresis has demonstrated two aggregating subpopulations [McDevitt, Jahnke & Green (1982) Trans. Annu. Meet. Orthop. Res. Soc. 7, 50]. In the present study, electrophoresis of the non-aggregating pools has shown three additional subpopulations, here named bands III, IV and V. The two smallest proteoglycan pools from each tissue contained two and three components respectively. These components were isolated by preparative electrophoresis and analysed. Band III was a proteoglycan richer in keratan sulphate than in chondroitin sulphate; band IV was a proteoglycan richer in chondroitin sulphate than in keratan sulphate; band V contained only chondroitin sulphate. Unsaturated disaccharides prepared from the chondroitin sulphate of all bands were predominantly 6-sulphated, with only 5-15% 4-sulphated. The molecular masses of the chondroitin sulphate and keratan sulphate did not differ between the bands. The amino acid composition of band III differed from that of band IV. Thus three distinct subpopulations of non-aggregating proteoglycan were demonstrated in the human intervertebral disc.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P., Muir H. Qualitative changes with age of proteoglycans of human lumbar discs. Ann Rheum Dis. 1976 Aug;35(4):289–296. doi: 10.1136/ard.35.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bray B. A., Lieberman R., Meyer K. Structure of human skeletal keratosulfate. The linkage region. J Biol Chem. 1967 Jul 25;242(14):3373–3380. [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
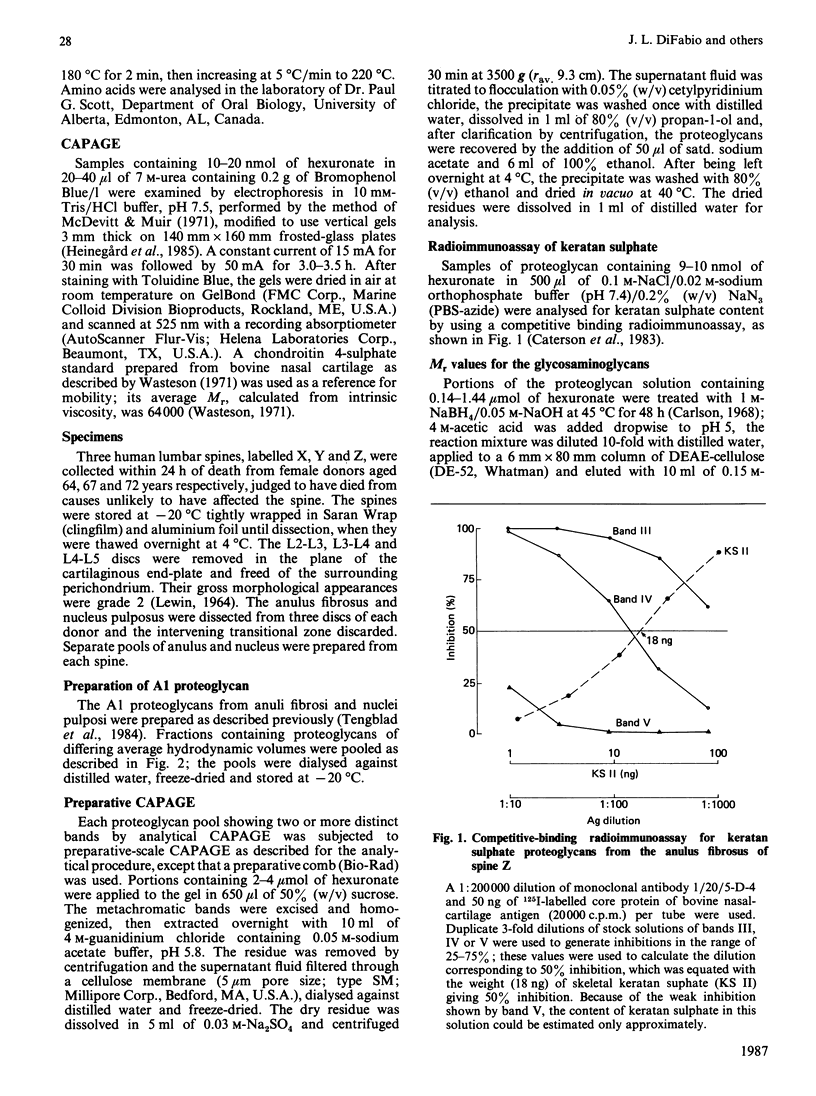
- Caterson B., Christner J. E., Baker J. R. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J Biol Chem. 1983 Jul 25;258(14):8848–8854. [PubMed] [Google Scholar]
- Emes J. H., Pearce R. H. The proteoglycans of the human intervertebral disc. Biochem J. 1975 Mar;145(3):549–556. doi: 10.1042/bj1450549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Axelsson I. Distribution of keratan sulfate in cartilage proteoglycans. J Biol Chem. 1977 Mar 25;252(6):1971–1979. [PubMed] [Google Scholar]
- Heinegård D., Paulsson M., Inerot S., Carlström C. A novel low-molecular weight chondroitin sulphate proteoglycan isolated from cartilage. Biochem J. 1981 Aug 1;197(2):355–366. doi: 10.1042/bj1970355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Wieslander J., Sheehan J., Paulsson M., Sommarin Y. Separation and characterization of two populations of aggregating proteoglycans from cartilage. Biochem J. 1985 Jan 1;225(1):95–106. doi: 10.1042/bj2250095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIN T. OSTEOARTHRITIS IN LUMBAR SYNOVIAL JOINTS. A MORPHOLOGIC STUDY. Acta Orthop Scand Suppl. 1964:SUPPL 73–8112. doi: 10.3109/ort.1964.35.suppl-73.01. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McDevitt C. A., Muir H. Gel electrophoresis of proteoglycans and glycosaminoglycans on large-pore composite polyacrylamide-agarose gels. Anal Biochem. 1971 Dec;44(2):612–622. doi: 10.1016/0003-2697(71)90250-8. [DOI] [PubMed] [Google Scholar]
- Mehmet H., Scudder P., Tang P. W., Hounsell E. F., Caterson B., Feizi T. The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve sulphated hepta- or larger oligosaccharides of the poly(N-acetyllactosamine) series. Eur J Biochem. 1986 Jun 2;157(2):385–391. doi: 10.1111/j.1432-1033.1986.tb09680.x. [DOI] [PubMed] [Google Scholar]
- Park C. M., Reid P. E., Applegarth D. A., Wong L. T., MacDonald I. B. Comparison of the carbohydrate composition of alpha 2-macroglobulin from patients with cystic fibrosis and normal controls. Pediatr Res. 1985 Apr;19(4):344–346. doi: 10.1203/00006450-198519040-00005. [DOI] [PubMed] [Google Scholar]
- Pearce R. H., Grimmer B. J. The chemical constitution of the proteoglycan of human intervertebral disc. Biochem J. 1976 Sep 1;157(3):753–763. doi: 10.1042/bj1570753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. P., Mason R. M. Heterogeneity of proteoglycans from adult human costal cartilage [proceedings]. Biochem Soc Trans. 1978;6(1):244–246. doi: 10.1042/bst0060244. [DOI] [PubMed] [Google Scholar]
- Stanescu V., Maroteaux P., Sobczak E. Proteoglycan populations of baboon (Papio papio) cartilages from different anatomical sites: gel electrophoretic analysis of dissociated proteoglycans and of fractions obtained by density gradient centrifugation. Biochim Biophys Acta. 1980 May 7;629(2):371–381. doi: 10.1016/0304-4165(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Ewins R. J., Revell P. A., Muir H. Proteoglycans of the intervertebral disc. Homology of structure with laryngeal proteoglycans. Biochem J. 1979 Jun 1;179(3):561–572. doi: 10.1042/bj1790561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengblad A., Pearce R. H., Grimmer B. J. Demonstration of link protein in proteoglycan aggregates from human intervertebral disc. Biochem J. 1984 Aug 15;222(1):85–92. doi: 10.1042/bj2220085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A. Properties of fractionated chondroitin sulphate from ox nasal septa. Biochem J. 1971 May;122(4):477–485. doi: 10.1042/bj1220477. [DOI] [PMC free article] [PubMed] [Google Scholar]


