Abstract
Background:
Chronic obstructive pulmonary disease (COPD) and heart failure are often coexisting conditions that can severely impact patients’ cardiopulmonary function and quality of life. Pulmonary rehabilitation programs, particularly those based on empowerment theory, may improve clinical outcomes by enhancing self-efficacy and promoting patient engagement.
Methods:
A total of 70 patients with COPD and heart failure admitted to our hospital’s respiratory department from January 1, 2023, to April 31, 2024, were randomly assigned to either a control group (n = 35) or an observation group (n = 35). The control group received routine care, while the observation group underwent an empowerment-based pulmonary rehabilitation program in addition to routine care for 4 weeks. Lung function (forced vital capacity, forced expiratory volume in 1 second, maximum voluntary ventilation), arterial blood gas levels (partial pressure of carbon dioxide, partial pressure of oxygen, and arterial oxygen saturation), cardiac function (left ventricular ejection fraction and serum brain natriuretic peptide), cardiopulmonary function (heart rate, respiratory rate, and 6-minute walk test), self-efficacy, and rehabilitation compliance were measured before and after the intervention.
Results:
There were no significant differences between the groups before the intervention (P > 0.05). After the intervention, the observation group exhibited significant improvements in lung function, arterial blood gas levels, cardiac and cardiopulmonary function, and self-efficacy scores compared with the control group (P < 0.05). Rehabilitation compliance was also significantly higher in the observation group (P < 0.05).
Conclusion:
An empowerment-based pulmonary rehabilitation program effectively improves rehabilitation compliance, lung and heart function, and self-efficacy in COPD patients with heart failure, suggesting it has strong potential for clinical application.
Keywords: chronic obstructive pulmonary disease, empowerment theory, heart failure, pulmonary rehabilitation training, self-efficacy
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a prevalent chronic respiratory condition characterized by airflow obstruction and alveolar abnormalities, leading to airflow limitation. Due to its insidious onset and recurrent symptoms, COPD contributes to high mortality and disability rates worldwide.[1,2] In 2019, studies reported approximately 455 million COPD patients globally, with 3.9 million deaths attributed to the disease.[3] COPD has become the third leading cause of death worldwide,[4] and projections suggest that by 2030, over 4.5 million people will die from COPD or its complications, further increasing its contribution to global mortality.[5] Although COPD is incurable and progressive, requiring long-term and continuous treatment, it is highly preventable and can be managed effectively through pulmonary rehabilitation.[6] Chronic obstructive pulmonary infection can lead to left heart failure, making COPD combined with heart failure a common clinical scenario. Improving the cardiopulmonary function of these patients through pulmonary rehabilitation is crucial to reduce mortality and enhance patient outcomes. Pulmonary rehabilitation, a form of respiratory rehabilitation, is the most effective non-pharmacological treatment for COPD patients. It involves comprehensive interventions, including exercise, health education, behavior modification, psychosocial support, oxygen therapy, and nutritional support, aiming to improve lung function, enhance physical endurance and cardiopulmonary health, and slow disease progression.[7] Current national and international studies primarily focus on developing exercise programs and comparing pulmonary rehabilitation methods for COPD and heart failure patients. However, these studies often overlook patient compliance during exercise interventions, potentially limiting the benefits of exercise therapy and resulting in suboptimal symptom control. Research indicates that some COPD and CHF patients experience accelerated breathing and heart rate during pulmonary rehabilitation, leading to reluctance to participate and exacerbating their symptoms, creating a vicious cycle.[8] For instance, a study by Jing et al involving 31 eligible patients found that 24 (77.42%) refused pulmonary rehabilitation, and only 6 (19.35%) completed the program, with training times below the expected 30 to 40 minutes.[9] This highlights the low acceptance of pulmonary rehabilitation among COPD patients with heart failure. Improving exercise compliance, consolidating the effectiveness of exercise therapy, and promoting the rehabilitation of COPD patients with CHF have become a focal point of research for scholars worldwide.
Empowerment theory is a management practice emphasizing decentralized decision-making, where individuals are given the appropriate rights to participate in organizational decision-making. This approach increases perceptions of effort and expectations, fostering self-motivation.[10] The theory aims to enhance autonomy, control, and self-determination in individuals or groups through behavioral interventions across 4 stages: pre-intention, intention, action, and maintenance. These stages increase self-efficacy, self-esteem, and autonomy, improving the capacity to participate in society and achieve personal goals. In the medical field, empowerment theory involves granting patients and their families decision-making rights during diagnosis and treatment, stimulating patients’ potential, and improving their self-efficacy through health knowledge and skill training.[11] It is primarily applied to chronic disease management, enhancing adherence, and offering new patient treatment and rehabilitation approaches.[12] For instance, Halpin et al developed a home exercise program based on self-efficacy and empowerment concepts for older adults at risk of falls, implemented through a mobile app.[13] The results demonstrated that the program effectively reduced the incidence of falls and improved adherence to home exercise. Despite these advancements, no studies have applied empowerment theory to pulmonary rehabilitation programs for COPD and heart failure patients. Therefore, this study aims to apply empowerment theory to a pulmonary rehabilitation program for patients with COPD and heart failure to improve pulmonary rehabilitation effectiveness, cardiopulmonary function, and quality of life. The following report details our findings.
2. Objects and methods
2.1. Study subjects
Patients with COPD combined with heart failure admitted to the Respiratory Medicine Department of our hospital from January 1, 2023 to April 31, 2024 were selected for the study. Inclusion criteria: ① meeting the relevant diagnostic criteria for COPD and CHF[14]; ② age > 18 years; ③ normal cognitive function; ④ hospitalization duration > 4 weeks; ⑤ consent to participate in this study. Exclusion criteria: (1) hearing impairment, inability to cooperate in completing the interview; (2) combined with severe liver and kidney dysfunction and malignant tumor patients; (3) severe joint deformity, unable to exercise. The sample size was calculated according to the formula[15]: n1 = n2 = {(Zɑ/2 + Zβ)×σ/δ}2, taking ɑ = 0.05, β = 0.1, two-sided test, checking the table to get Zɑ/2 = 1.96, Zβ = 1.282. Checking the relevant literature,[16] we took σ = 4.02, δ = 3.49. Substituting the formula to calculate n1 = n2 = 28, the sample size should be 56 cases, but considering the 10% of lost visits, the final sample size was 70 cases. Patients were divided into a control group and an observation group, with 35 patients in each group, using opaque sealed envelope allocation. The hospital’s ethics committee approved this study.
2.2. Methods
The control group adopts the routine nursing intervention program, including the following: ① assessment of respiratory and circulatory status and symptoms: monitoring the patient’s respiratory rate, depth of breathing, oxygen saturation, cough symptoms, heart rate, and rhythm, etc, and timely detection and treatment of relevant abnormalities. ② Oxygen therapy: if there are symptoms of hypoxemia or heart failure, it is necessary to give oxygen inhalation therapy or noninvasive ventilator CPAP mode assisted respiration as prescribed by the doctor to maintain the patient’s oxygen saturation at 90% or above. ③ Give drugs for symptomatic supportive treatment: give drugs such as bronchodilators, steroids, recombinant human brain natriuretic peptide (BNP), and other drugs for symptomatic supportive treatment, used to relieve respiratory symptoms and heart failure symptoms and reduce the inflammatory response. ④ Nutritional support therapy: after assessing the nutritional risk of patients, provide appropriate nutritional support as prescribed by the physician and, if necessary, provide an indwelling gastrointestinal tube for enteral nutritional support therapy to meet the energy and nutritional needs of patients. ⑤ Prevention of respiratory tract infection: control the greenhouse degree in the ward within the appropriate range, keep the patient’s respiratory tract clean and moist, and tell the patient to keep warm to avoid respiratory tract infection caused by cold. ⑥ Prevention of venous thrombosis: for patients who have been bedridden for a long time, instruct patients to do ankle pump exercises, foot massage, wear elastic stockings, and other measures to prevent venous thrombosis. ⑦ Health education and rehabilitation counseling: providing health education and rehabilitation counseling to patients and their families, providing appropriate rehabilitation programs and relevant knowledge to help patients recover as soon as possible and prevent disease recurrence.
2.2.1. Composition of the research team
The research team comprised 11 people, including 1 respiratory physician, 1 cardiologist, 1 rehabilitation physician, 2 rehabilitation therapists, 1 senior respiratory nurse, 1 senior cardiology nurse, and 2 respiratory and 2 cardiology nurses. The senior respiratory and cardiology nurse acted as the project leader, responsible for the research design and organizational coordination of the whole project, as well as driving the project forward and supervising the implementation of the interventions; the 4 respiratory and cardiology nurses were responsible for overseeing the pulmonary rehabilitation training of the patients, empowering the patients and collecting data. The respiratory physician and cardiologist were accountable for the training and supervision of COPD combined with heart failure, the rehabilitation physician was responsible for the theoretical training of pulmonary rehabilitation, and the rehabilitation therapist was responsible for supervising the pulmonary rehabilitation training. Before the official start of the study, all researchers underwent uniform training, which included knowledge of COPD combined with heart failure, empowerment theory, pulmonary rehabilitation training, and other related knowledge. They also passed the examination before being allowed to participate in this study.
2.2.2. Intervention methodology
Using empowerment theory as a framework, the intervention was implemented according to the 4 stages and 5 steps of empowerment theory. The 4 stages are pre-intention, intention, action, and maintenance, and the 5 steps are clarifying the problem, expressing feelings, setting goals, making plans, and evaluating effects.
2.2.2.1. Pre-intention stage
This stage focuses on assessment and analysis. The main focus is to conduct a comprehensive evaluation of the patient to understand the patient’s cardiopulmonary function, physical function, and willingness to undergo cardiopulmonary rehabilitation. For patients who are unwilling and unmotivated to undergo rehabilitation, we will understand the main reasons why patients refuse to undergo pulmonary rehabilitation, clarify the reasons for refusal through face-to-face communication, and communicate the benefits of pulmonary rehabilitation through brochures, multimedia, and other forms of communication to share successful cases and health care knowledge to increase patients’ willingness to participate in pulmonary rehabilitation. For patients who are willing to participate in pulmonary rehabilitation, we provide positive encouragement and reassurance and explain the relevant contents of pulmonary rehabilitation training (the importance of pulmonary rehabilitation training, methods, and precautions), etc, so that they understand the impact of pulmonary rehabilitation training on the disease and increase their confidence and motivation to participate.
2.2.2.2. Intention stage
This stage focuses on physician-nurse-patient collaboration to increase the patient’s intention to participate. The patient is seen as the center of empowerment, encouraged to say whether there are any barriers to pulmonary rehabilitation training, helped to analyze the causes of the problem, and channeled ambivalence to increase confidence in the training; for those who do not comply with the training, listing the impact of the disease on themselves and the economic and emotional burden on their families, so that the patient can consider the need for their training using these factors; and constructing the rehabilitation goals with their families and giving the patient a home-made health guide and guiding the introduction of its contents to stimulate the patient’s firm intention to participate in the training. We will construct rehabilitation goals with family members and present patients with home-made health guides and introduce their contents to stimulate patients’ firm intention to train, increase their subjective initiative to participate in rehabilitation and increase the degree of cooperation and motivation for intervention by achieving material rewards through the milestones of the goals.
2.2.2.3. Action phase
This phase aims to improve patients’ self-efficacy and promote their deliberate adherence to pulmonary rehabilitation exercises. The 5 steps of empowerment theory were implemented in the intervention process. The details are as follows:
-
①
Clear question: to assess the current status of COPD combined with heart failure and to understand the patient’s pulmonary rehabilitation exercise training. Elicit the patient’s experience of pulmonary rehabilitation exercise training by asking open-ended questions, such as “Have you been compliant with pulmonary rehabilitation exercise training?” “How confident do you feel about doing pulmonary rehabilitation exercises?” “What difficulties have you encountered during your pulmonary rehabilitation exercise program?” “Why are you unable to keep up with your pulmonary rehabilitation exercises?” (a) To clarify the patient’s problems with the rehabilitation exercise process.
-
②
Expression of emotions: after clarifying the problems, the researcher first understood the impact caused by the daily life of patients with COPD combined with heart failure through communication and empathized with the patients, which facilitated the establishment of a harmonious and equal nurse-patient relationship and made them feel a sense of trust. The researcher then guided the patients to express their views on pulmonary rehabilitation exercise and encouraged them to vent their emotions to identify their positive and negative attitudes. For those with positive attitudes, patients were given reassurance and support; for those with negative attitudes, positive psychotherapy and peer education were used, successful cases were shared, confidence in pulmonary rehabilitation exercise was increased, and patients were encouraged to take responsibility for self-management of their disease.
-
③
Setting goals: in response to the patient’s obstruction of pulmonary rehabilitation exercise, intensity, exercise time, and exercise frequency by the patient to set their own goals, the researcher combined the interpretation of the pulmonary rehabilitation exercise prescription guidelines and jointly set practical goals.[17] Establishing achievable goals together. Specifically: (a) Exercise intensity: It is recommended to be selected based on patient endurance. High-intensity exercise targets 60% to 80% of the maximum heart rate, moderate-intensity exercise targets 41% to 59% of the maximum, and low-intensity exercise targets 30% to 40%. This study begins with low-intensity exercise and gradually increases intensity, with patients progressing to moderate-to-high intensity if tolerated. (b) Exercise duration: It is recommended to accumulate 30 to 60 minutes of exercise per day (60 minutes being more effective), ensuring each session lasts at least 10 minutes, totaling 150 to 300 minutes per week. This study sets a minimum of 40 minutes per day and at least 200 minutes per week. (c) Exercise frequency: the recommended exercise frequency is 3 to 5 times per week. This study sets a minimum of 3 pulmonary rehabilitation sessions per week.
-
④
Formulation and implementation of the plan: a simple and easy-to-use pulmonary rehabilitation training program was formulated based on the characteristics of the patient’s condition and in combination with the exercise program recommended by the pulmonary rehabilitation guidelines.
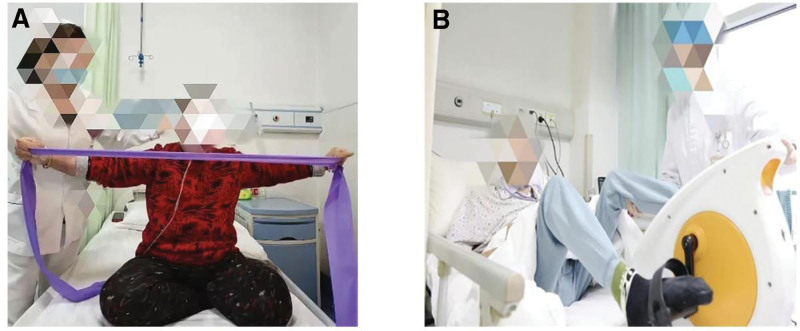
Patients with COPD combined with heart failure have more severe symptoms in the acute phase, and it is not appropriate to start pulmonary rehabilitation training immediately after hospital admission. The symptoms of an acute exacerbation of COPD combined with heart failure usually last for 2 to 3 days, and the course of treatment takes 3 to 5 days. The symptoms are effectively controlled after 1 week and then gradually enter the stabilization phase. This study proposes a phased approach to address patients’ concerns about exacerbating symptoms and reluctance to engage in limb exercises during pulmonary rehabilitation. During periods of severe symptoms, patients will initially undergo respiratory-focused pulmonary rehabilitation. Once they transition to a stable phase, they will proceed to a three-week limb exercise program as part of a comprehensive month-long pulmonary rehabilitation regimen. The specific pulmonary rehabilitation program is outlined as follows (Table 1). Selected pictures of selected pulmonary rehabilitation interventions are shown in Figure 1.
Table 1.
Pulmonary rehabilitation training programme.
| Modalities | Phased | Company-specific programs |
|---|---|---|
| Pulmonary rehabilitation respiratory training | Severe symptomatic phase (admission – week 1) | 1. Lip-contraction breathing training method: tightly closed lips nostrils after inhalation, with puffed cheeks, lip-contraction, such as whistling, slowly exhaling gas, exhalation time as long as possible, at least 4 seconds, 30 min/times, practice 3 times/d, the frequency and depth of breathing and lip-contraction movements to the best effortless, after proficiency can be increased as appropriate. 2. Abdominal breathing training methods: position can be lying down, sitting, standing position; try to keep the chest motionless, one hand on the chest, the other on the abdomen, relax the upper body, tightly close lips, inhale through the nose, natural contraction of the abdomen, the diaphragm down, the hand can be used to give the abdominal pressure; to contract the lips and slowly exhale the gas, diaphragm upward, the exhalation time for the inhalation of time 2 to 3 times; 15 min/times, the initial practice 3 times/d, skilled can be increased as appropriate. Exercise 3 times/d can be increased as appropriate after proficiency. At this stage, 30% to 40% of the maximum heart rate of low-intensity exercise. 3. Breathing exercises, Action 1: Inhale sincerely as you raise your arms in adduction and exhale as you lower them for 5 seconds. Action 2: Move the hands alternately up and down along the side of the body, inhaling as you move up and exhaling as you move down. Do this exercise about 15 times a day, or more or less according to your situation: Lift the knee, alternately lift the lower limb, bend it at 90°, inhaling as you raise it and exhaling as you lower it, practice this exercise about 15 times a day, or more or less according to your situation. Breathe in as you lift and out as you lower; do this about 15 times a day, or more or less, depending on your situation. 4. Increase aerobic exercise, strengthen upper limbs, and improve activity endurance; borrow fitness equipment (see Figure 1A), such as toner, exercise, and pay attention to breathing rhythm. 30 min/d, 5 times/week, can be increased or decreased as appropriate. At this stage, moderate-intensity exercise is 41% to 59% of the maximum heart rate. If the patient can tolerate it, high-intensity exercise can be taken at 60% to 80% of the maximum heart rate. |
| Pulmonary rehabilitation physical training | Symptom stabilization (weeks 2–4) | 1. Instruct the patient to perform passive limb joint activities to maintain normal joint mobility at least once a day for 15 minutes. Assist the patient in a semi-recumbent position of approximately 45 degrees, at a cardiac-adapted height, to maintain or increase joint mobility; continue to perform passive limb joint activities or simple bicycle exercises (see Figure 1B) in bed at least once daily for 15 minutes. 2. Assist the patient in doing posture adjustment training to increase the patient’s cardiorespiratory reserve capacity and airway clearance capacity, to support the regular change of position on the bed, upper and lower limb activities on the bed, sitting up on the bed, sitting up on the bedside, at least 1 time/day, 15 min/time; increase the mobility training of the auxiliary joints of the limbs to prevent the deceleration caused by other complications. At this stage, 30% to 40% of the maximum heart rate of low-intensity exercise is adopted. 3. Instruct the patient to sit and stand at will at the bedside to increase cardiopulmonary reserve capacity and exercise endurance at least 1 time/day, 15 min/times; continue limb resistance training at least 1 time/day, 15 min/times; Instructing and assisting the patient in moving his feet to stand up from a chair with the help of a minimum range of 1 degree to improve peripheral blood flow, increase muscular pumping function and return blood flow to the heart, at least 2 times/day, 15 min/times; walking with a walker to reduce respiratory work, at least 2 times/day, 15 min/times; further instruction in Activities of Daily Living (ADL). 4. Continue the above casual bedside sitting and standing and limb resistance training at least 2 times/day, 15 min/time; move the feet to stand up from the chair by oneself or with assistive devices, at least 2 times/day, 15 min/time; at the same time, be able to walk independently (but need to be supervised); and further instruct the patient to perform daily living skills training. At this stage, moderate-intensity exercise is used at 41% to 59% of the maximum heart rate, and high-intensity exercise can be used at 60% to 80% of the maximum heart rate if tolerated by the patient. |
Figure 1.
(A) Aerobic exercise for pulmonary rehabilitation. (B) Passive joint exercise for pulmonary rehabilitation.
-
⑤
Effective evaluation: help the patient summarize the completion of the plan, confirm the goals that have been achieved, and encourage the patient to continue to adhere to them. We will analyze why goals and plans have not been achieved and work together to solve the problems so that patients have the confidence to complete the following goals and plans. At the time of discharge, we will assess the effectiveness of the intervention in terms of cardiorespiratory function, self-efficacy, and adherence.
2.2.2.4. Maintenance phase
This phase focuses on the joint analysis and adherence to the behavior of the clinician, nurse, and patient. Nurses record the patient’s pulmonary rehabilitation training in the behavioral phase, including the degree of mastery of breathing exercises, exercise time, exercise intensity, breathing, and heart rate, and provide timely feedback to the patient and doctor to adjust the training plan and drug treatment plan; at the same time, they encourage the patient’s behavioral performance in the action phase, assess and prevent the possible risk of behavioral regression, and emphasize the long-term effects of pulmonary rehabilitation training. Emphasize the long-term effects of pulmonary rehabilitation training. If the patient is at risk of behavioral regression or if behavioral regression is detected, analyze the reasons with the patient and family promptly and provide appropriate behavioral interventions. Encourage patients to express their emotions and provide timely guidance to patients with negative emotions; at the same time, mobilize the strength of family members and instruct them to support patients in training properly. As the backbone of supervising the patient’s home exercise, family members should support, cooperate, and be encouraged to lay the foundation for home rehabilitation exercise after discharge.
2.3. Observational indicators
2.3.1. Pulmonary function
Pulmonary function indices were measured using the Japanese GOULD automatic lung function analyzer, including forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and maximal voluntary ventilation (MVV). These 3 indices are commonly used to assess individual clinical lung function. FVC represents the maximum air volume forcefully exhaled after a maximal deep inhalation, indicating the recovery of lung capacity; a higher FVC indicates better recovery. FEV1 measures the volume of air exhaled during the first second of a forced expiration after a maximal deep inhalation, reflecting airway obstruction. MVV measures the maximum volume of air a person can breathe per minute during rapid, deep breathing, indicating recovery of respiratory muscle function.
2.3.2. Respiratory function indices
Arterial blood samples were drawn and analyzed using a Siemens blood gas analyzer. Key parameters included arterial partial pressure of oxygen, arterial partial pressure of carbon dioxide, and arterial oxygen saturation.
2.3.3. Cardiac function indices
Cardiac function was evaluated by a physician trained in echocardiography using a diagnostic ultrasound system (Model: Mindray M9) to assess left ventricular ejection fraction (LVEF). Additionally, fasting venous blood samples were collected, and serum BNP levels were measured using immunoassay electrochemiluminescence.
2.3.4. Cardiopulmonary function indices
Cardiopulmonary function was assessed using the six-minute walk test (6MWT), a measure of functional exercise capacity. The 6MWT evaluates the distance a patient can walk in six minutes and objectively reflects cardiopulmonary function. This test is widely used in clinical research due to its simplicity and practicality.[18] The 6MWT was conducted at admission and discharge to assess changes in functional capacity over treatment.
2.3.5. Self-efficacy
Patient self-efficacy was assessed using the General Self-Efficacy Scale.[19] This scale consists of 10 items, each rated on a scale of 1 to 4, where responses range from “not at all true” to “exactly true.” The total score ranges from 10 to 40, with higher scores indicating higher levels of self-efficacy. The General Self-Efficacy Scale has demonstrated good reliability with Cronbach α of 0.87, test–retest reliability of 0.83, and split-half reliability of 0.82, confirming its validity and consistency.
2.3.6. Rehabilitation compliance
A pulmonary rehabilitation training diary card was designed according to the study objectives, documenting the date and specific exercises completed during pulmonary rehabilitation sessions. Compliance was assessed based on the completion of the pulmonary rehabilitation plan during hospitalization: achieving ≥ 70% was considered complete compliance (scored 3 points), 40% to 70% was regarded as partial compliance (scored 2 points), and <40% was considered noncompliance (scored 1 point). Higher scores indicate higher compliance with the rehabilitation program.
2.4. Data collection and processing
Data collection was conducted by the researchers, with measurements taken at two-time points: before intervention (upon admission) and after 4 weeks of intervention. Data included pulmonary function, respiratory function, cardiac function, cardiopulmonary function, and self-efficacy-related indicators. Compliance with rehabilitation was assessed at week 4. All completed questionnaires and scales were collected immediately after administration and checked for completeness and accuracy to prevent missing data or errors. Data were double-entered and validated before statistical analysis using computer software.
2.5. Statistical analysis
SPSS 22.0 statistical software was used for statistical analysis of the data. Measurement data conforming to normal distribution were expressed as mean ± standard deviation; a t test was used; count data were expressed as several cases and percentages, and the χ2 test was used. The difference was statistically significant at P < .05.
3. Results
3.1. Comparison of general information between the 2 groups of patients
The 2 groups of patients were compared based on general demographic characteristics, including gender, age, education level, family situation, smoking history, pulmonary function classification, and cardiac function classification. The differences were insignificant (P > .05), indicating comparability between the groups. Please refer Table 2 for specific details.
Table 2.
Comparison of the general data of the 2 groups of patients.
| Item | Classification | Control group | Observation group | t/χ2 value | P value |
|---|---|---|---|---|---|
| Sex | Male | 21 (60.0%) | 20 (57.1%) | 0.059 | .808 |
| Female | 14 (40.0%) | 15 (42.9%) | |||
| Age | – | 61.58 ± 6.95 | 62.58 ± 5.79 | −0.875 | .384 |
| Academic qualification | Junior high school and below | 18 (51.4%) | 20 (57.1%) | 0.230 | .631 |
| High school and above | 17 (48.6%) | 15 (42.9%) | |||
| Family type | Spouse | 15 (42.9%) | 14 (40.0%) | 0.255 | .880 |
| Child | 12 (34.3%) | 14 (40.0%) | |||
| Nurse or others | 8 (22.9%) | 7 (20.0%) | |||
| Smoking or not | Yes | 28 | 26 | 0.094 | .759 |
| No | 7 | 9 | |||
| Lung function class | II | 18 | 16 | 0.562 | .765 |
| III | 12 | 15 | |||
| IV | 5 | 4 | |||
| Cardiac function class | II | 16 | 17 | 0.729 | .694 |
| III | 15 | 16 | |||
| IV | 4 | 2 |
3.2. Comparison of lung function between the 2 groups at different time points
Before the intervention, there was no statistically significant difference in FVC, FEV1, and MVV between the 2 groups (P > .05). After the intervention, the observation group showed significantly better pulmonary function indicators than the control group, with statistically significant differences (P < .05). Please refer Table 3 for specific details.
Table 3.
Comparison of lung function at different time points between the 2 groups.
| Group | FVC (L) | FEV1 (L) | MVV (L/min) | |||
|---|---|---|---|---|---|---|
| Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | |
| Control group | 1.06 ± 0.17 | 1.83 ± 0.62 | 0.50 ± 0.43 | 1.05 ± 0.55* | 40.79 ± 7.81 | 50.32 ± 8.41 |
| Observation group | 1.08 ± 0.13 | 2.55 ± 0.71 | 0.48 ± 0.75 | 1.49 ± 0.71* | 40.53 ± 8.42 | 55.48 ± 7.69 |
| t-value | −0.699 | −8.278 | 0.512 | 5.942 | 0.238 | 8.431 |
| P value | .486 | .000 | .394 | .000 | .568 | .000 |
FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, MVV = maximal voluntary ventilation.
P < .05.
3.3. Comparison of arterial blood gas indicators at different time points between the 2 groups of patients
Before the intervention, the 2 groups had no statistically significant difference in arterial blood gas indicators (P > .05). After the intervention, the observation group showed significantly better arterial blood gas indicators than the control group (P < .05). Please refer Table 4 for specific details.
Table 4.
Comparison of respiratory arterial blood gas indices at different time points between the 2 groups of patients.
| Group | PaO2 (mm Hg) | PaCO2 (mm Hg) | SaO2 (%) | |||
|---|---|---|---|---|---|---|
| Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | |
| Control group | 54.53 ± 7.70 | 81.54 ± 8.28 | 75.63 ± 9.59 | 56.94 ± 7.15 | 80.79 ± 10.91 | 90.96 ± 10.72 |
| Observation group | 52.84 ± 8.54 | 89.14 ± 6.28 | 74.65 ± 10.24 | 49.94 ± 9.68 | 80.35 ± 10.52 | 97.96 ± 9.78 |
| t-value | 0.369 | −4.536 | 0.345 | 5.176 | 0.081 | 3.578 |
| P value | .713 | .000 | .876 | .000 | .896 | .002 |
PaCO2 = partial pressure of arterial carbon dioxide, PaO2 = partial pressure of oxygen, SaO2 = oxygen saturation of blood.
3.4. Comparison of dim sum function at different times in 2 groups
Before the intervention, the 2 groups had no statistically significant difference in LVEF and serum BNP levels (P > .05). After the intervention, the observation group showed significantly higher LVEF and lower BNP levels than the control group, with statistically significant differences (P < .05). Please refer Table 5 for specific details.
Table 5.
Comparison of LVEF and serum BNP indices between the 2 groups of patients.
| Group | LVEF (%) | BNP (pg/mL) | ||
|---|---|---|---|---|
| Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | |
| Control group | 39.92 ± 6.84 | 44.78 ± 5.85 | 899.3 ± 70.1 | 224.2 ± 48.8 |
| Observation group | 40.18 ± 5.84 | 49.68 ± 4.92 | 919.3 ± 63.9 | 115.2 ± 52.6 |
| t-value | −0.312 | 2.581 | 0.054 | 8.735 |
| P value | .735 | .003 | .996 | .000 |
BNP = brain natriuretic peptide, LVEF = left ventricular ejection fraction.
3.5. Comparison of cardiopulmonary function at different time points between the 2 groups of patients
Before the intervention, there was no statistically significant difference in HR (heart rate), RR (respiratory rate), and 6MWT between the 2 groups (P > .05). After the intervention, the observation group showed significantly better HR, RR, and 6MWT outcomes than the control group, with statistically significant differences (P < .05). Please refer Table 6 for specific details.
Table 6.
Comparison of HR, RR, and 6MWT between the 2 groups of patients.
| Group | HR (times/min) | RR (times/min) | 6MWT (m) | |||
|---|---|---|---|---|---|---|
| Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | |
| Control group | 121.01 ± 12.68 | 95.45 ± 9.62 | 27.58 ± 5.65 | 26.94 ± 5.15 | 435.53 ± 102.55 | 586.78 ± 135.89 |
| Observation group | 118.84 ± 9.54 | 88.14 ± 7.28 | 20.65 ± 6.24 | 18.46 ± 6.89 | 418.53 ± 108.96 | 635.92 ± 161.87 |
| t-value | 0.559 | 5.556 | 0.452 | 6.176 | 0.126 | 4.682 |
| P value | .413 | .000 | .687 | .000 | .738 | .001 |
6MWT = 6-minute walking test, HR = heart rate, RR = respiratory rate.
3.6. Comparison of self-efficacy at different time points between the 2 groups of patients
Before intervention, the 2 groups had no statistically significant difference in self-efficacy (P > .05). After the intervention, the observation group exhibited significantly higher self-efficacy levels than the control group, with statistically significant differences (P < .05). Please refer Table 7 for specific details.
Table 7.
Comparison of self-efficacy between the 2 groups.
| Group | Pre-intervention | Post-intervention |
|---|---|---|
| Control group | 25.36 ± 8.72 | 30.48 ± 6.51 |
| Observation group | 24.83 ± 9.35 | 38.53 ± 7.25 |
| t-value | 0.345 | 6.212 |
| P value | .549 | .000 |
3.7. Comparison of pulmonary rehabilitation compliance between 2 groups
In the fourth week, the compliance score of the observation group patients (2.68 ± 0.74) was higher than that of the control group (1.83 ± 0.81), with a statistically significant difference (t = 5.215, P < .05).
4. Discussion
4.1. Clinical application significance of pulmonary rehabilitation training program based on empowerment theory
The global initiative for COPD has highlighted the crucial role of pulmonary rehabilitation in enhancing both the physical and mental well-being and the overall quality of life of COPD patients.[20] This non-pharmacological intervention is recognized as an essential component of clinical COPD management. However, despite its importance, adherence to pulmonary rehabilitation remains suboptimal, with only a small fraction of patients completing the program as prescribed. A foreign cohort study following 440 COPD patients over an 8-week pulmonary rehabilitation program revealed that merely 229 patients completed the training.[16] Domestic and international researchers have explored various strategies to address this issue and improve adherence to pulmonary rehabilitation. One approach gaining traction is the application of empowerment theory in clinical practice, which has shown promise in managing and rehabilitating chronic diseases. Empowerment theory fosters a collaborative relationship between healthcare providers and patients, encouraging active patient participation in treatment and building lasting confidence and self-management skills throughout rehabilitation.[21] This study proposes a pulmonary rehabilitation training program grounded in empowerment theory to enhance adherence to rehabilitation exercises in patients with COPD and heart failure, ultimately improving their cardiopulmonary function and self-efficacy.
4.2. An empowerment theory-based pulmonary rehabilitation program improves lung function in COPD and heart failure patients
The results of this study demonstrated that the observation group exhibited significantly better respiratory frequency, lung function indexes, and arterial blood gas analysis indexes than the control group. This indicates that a pulmonary rehabilitation training program based on empowerment theory can enhance lung function and respiratory capacity in patients with COPD and CHF, aligning with findings from previous studies.[22–24] COPD patients frequently experience complications such as heart failure due to the chronic nature of the disease, and the incidence of COPD combined with heart failure is increasing annually in clinical settings. Pulmonary rehabilitation is a critical treatment strategy for COPD patients, involving planned breathing exercises and physical activities that, when adhered to long-term, can improve breathing depth and frequency, promote carbon dioxide expulsion, reduce arterial blood carbon dioxide partial pressure, enhance lung function, and improve physical endurance and cardiopulmonary health. However, the success of pulmonary rehabilitation hinges on patient cooperation and adherence, often resulting in suboptimal outcomes due to poor compliance. Empowerment theory emphasizes individuals’ active involvement and participation in health decisions and behaviors, transforming them from passive recipients to proactive health managers.[25] In this study, the pulmonary rehabilitation training program was developed with the patient as the central figure. A customized training program was co-created by thoroughly assessing the patient’s willingness and behavior, incorporating gradual and systematic respiratory and exercise training to improve lung capacity and function. The empowerment-based approach ensured the patient was actively involved in rehabilitation, particularly during severe symptoms. Various respiratory training methods were employed, including pursed-lip breathing, abdominal breathing, and respiratory exercise linkage, complemented by aerobic exercise training. This patient-centered approach enhanced lung function and improved overall adherence and outcomes of the pulmonary rehabilitation program.
4.3. An empowerment theory-based pulmonary rehabilitation training program improves cardiac function in COPD and heart failure patients
The results of this study showed that the heart rate left ventricular ejection fraction, BNP index, and 6MWT index of patients in the observation group were better than those in the control group, indicating that the pulmonary rehabilitation training program based on the empowerment theory was able to improve the cardiac function of patients with COPD combined with heart failure, which is consistent with the results of the study by Schulté et al.[26] This is because the pulmonary rehabilitation program based on the empowerment theory has 2 phases of rehabilitation according to the severity of the patient’s symptoms. In the symptomatic stability phase, patients are mainly provided with limb training, which improves cardiopulmonary circulation, cardiac function, and motor coordination of the limbs and bones. Relevant studies have also confirmed that moderate exercise training can improve patients’ cardiac function, prevent ventricular remodeling, increase cardiac output, gradually improve cardiac function’s adaptive characteristics, and improve cardiac function.[27] In addition, the pulmonary rehabilitation training program based on the empowerment theory in this study is patient-centered, selecting appropriate exercises for patients according to their preferences and actual conditions, which improves patients’ motivation to participate in exercise training actively, and at the same time provides patients with timely encouragement and guidance to ensure patients’ persistence in exercise training. Long-term exercise training can improve patients’ myocardial contractility, promote cardiac collateral circulation, increase coronary artery blood supply, and improve cardiopulmonary fitness and cardiac function.[28]
4.4. An empowerment theory-based pulmonary rehabilitation program improves self-efficacy and adherence in COPD and heart failure patients
The results of this study showed that the self-efficacy scores and rehabilitation adherence scores of the patients in the observation group were higher than those of the control group, indicating that the pulmonary rehabilitation training program based on the empowerment theory can improve the self-efficacy and rehabilitation adherence of patients with COPD combined with CHF, which is consistent with the results of several studies.[29–31] Self-efficacy reflects an individual’s confidence in completing a specific task, and rehabilitation adherence demonstrates an individual’s willingness to complete rehabilitation training. Currently, adherence to pulmonary rehabilitation in patients with COPD in the UK and abroad is not optimistic. A prospective cohort study conducted in a foreign country showed that patients participating in pulmonary rehabilitation had a high dropout rate, with almost half not completing the entire pulmonary rehabilitation program.[32] Hence, measures need to be taken to improve patients’ adherence to pulmonary rehabilitation. Patients in this study had higher self-efficacy and adherence to a pulmonary rehabilitation training program based on empowerment theory because empowerment theory focuses mainly on the patient’s right to participate in decision-making and has a self-motivating effect on patients.[21] The pulmonary rehabilitation training program based on empowerment theory fully embodies the patient’s right to participate and make decisions in the formulation process, which improves the patient’s subjective initiative and increases the patient’s self-efficacy. A closed loop is formed through 5 steps: clarifying the problem, expressing emotions, setting goals, formulating and implementing a plan, and evaluating the effect. By understanding the patient’s positive and negative thoughts about pulmonary rehabilitation training and using a variety of ways to provide targeted guidance and psychological counseling, as well as targeted communication and encouragement to the patient, nurses enhanced the patient’s knowledge and skills to make correct medical decisions, ensured the patient’s ability and suitable to participate in decision-making, and increased the patient’s self-efficacy and compliance. In addition, rehabilitation training improved patients’ cardiorespiratory function, reduced patients’ discomfort symptoms, and increased their confidence in their ability to exercise and the effects of rehabilitation, thereby increasing their beliefs and self-efficacy to adhere to exercise.[33]
5. Conclusion
The pulmonary rehabilitation training program based on empowerment theory demonstrates significant potential in improving compliance, enhancing cardiopulmonary function, and increasing self-efficacy in patients with COPD and cardiac insufficiency, making it a valuable addition to clinical practice. However, this study has limitations, including a relatively small sample size, a short intervention period, and the absence of post-discharge follow-up. Future research should focus on large-scale, multicenter studies with extended follow-up periods to further validate the efficacy and long-term benefits of the empowerment-based pulmonary rehabilitation program in this patient population.
Author contributions
Conceptualization: Yue Zhang, Huang Hai.
Data curation: Yue Zhang, Chunfang Gu, Lin Sun.
Formal analysis: Yue Zhang.
Methodology: Yue Zhang.
Project administration: Huang Hai.
Writing – original draft: Yue Zhang.
Writing – review & editing: Yue Zhang, Huang Hai.
Abbreviations:
- 6MWT
- six-minute walk test
- BNP
- brain natriuretic peptide
- COPD
- chronic obstructive pulmonary disease
- FEV1
- forced expiratory volume in 1 second
- FVC
- forced vital capacity
- HR
- heart rate
- LVEF
- left ventricular ejection fraction
- MVV
- maximum voluntary ventilation
- RR
- respiratory rate
The First People’s Hospital of Yancheng (2024-K-169).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Zhang Y, Gu C, Sun L, Hai H. The application effect of a pulmonary rehabilitation program based on empowerment theory for patients with COPD combined with heart failure. Medicine 2024;103:41(e40067).
Contributor Information
Yue Zhang, Email: zy145765@163.com.
Chunfang Gu, Email: gcf11987@163.com.
Lin Sun, Email: Gcf112233@163.com.
References
- [1].Neder JA, Rocha A, Alencar MCN, et al. Current challenges in managing comorbid heart failure and COPD. Expert Rev Cardiovasc Ther. 2018;16:653–73. [DOI] [PubMed] [Google Scholar]
- [2].Corbellini C, Rossino E, Massaccesi R, et al. Improvements in perimeter thoracic mobility on patients with COPD after pulmonary rehabilitation: a case series. Electron J Gen Med. 2022;19:em361. [Google Scholar]
- [3].Alsayed AR, Abed A, Khader HA, Hasoun L, Al Maqbali M, Al Shawabkeh MJ. The role of human rhinovirus in COPD exacerbations in Abu Dhabi: molecular epidemiology and clinical significance. Libyan J Med. 2024;19:2307679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Siraj RA. Comorbid cognitive impairment in Chronic Obstructive Pulmonary Disease (COPD): current understanding, risk factors, implications for clinical practice, and suggested interventions. Medicina (Kaunas). 2023;59:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399:2227–42. [DOI] [PubMed] [Google Scholar]
- [6].von Siemens SM, Jörres RA, Behr J, et al. Effect of COPD severity and comorbidities on the result of the PHQ-9 tool for the diagnosis of depression: results from the COSYCONET cohort study. Respir Res. 2019;20:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chambers D, Thompson S. Empowerment and its application in health promotion in acute care settings: nurses’ perceptions. J Adv Nurs. 2009;65:130–8. [DOI] [PubMed] [Google Scholar]
- [8].Wouters EF, Posthuma R, Koopman M, et al. An update on pulmonary rehabilitation techniques for patients with chronic obstructive pulmonary disease. Expert Rev Respir Med. 2020;14:149–61. [DOI] [PubMed] [Google Scholar]
- [9].Jing Y, Ma Y, Zhang H, et al. Pulmonary rehabilitation integrated coached exercise training for patients with COPD: a study protocol for a randomized controlled trial. Trials. 2023;24:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Doherty DP, Hunter Revell SM. Developing nurse leaders: toward a theory of authentic leadership empowerment. Nurs Forum. 2020;55:416–24. [DOI] [PubMed] [Google Scholar]
- [11].Tao Y, Wang Y. Effect of empowerment theory health education on disease control level and compliance of elderly T2DM. Pak J Pharm Sci. 2023;36:643–8. [PubMed] [Google Scholar]
- [12].Park C, Song M, Cho B, et al. Effects of a multi-disciplinary approached, empowerment theory based self-management intervention in older adults with chronic illness. J Korean Acad Nurs. 2015;45:192–201. [DOI] [PubMed] [Google Scholar]
- [13].Halpin DM, Miravitlles M, Metzdorf N, Celli B. Impact and prevention of severe exacerbations of COPD: a review of the evidence. Int J Chron Obstruct Pulmon Dis. 2017;12:2891–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Del Águila MR, González-Ramírez A. Sample size calculation. Allergol Immunopathol (Madr). 2014;42:485–92. [DOI] [PubMed] [Google Scholar]
- [16].Higashimoto Y, Ando M, Sano A, et al. Effect of pulmonary rehabilitation programs including lower limb endurance training on dyspnea in stable COPD: a systematic review and meta-analysis. Respir Investig. 2020;58:355–66. [DOI] [PubMed] [Google Scholar]
- [17].Barreiro E, Bustamante V, Cejudo P, et al. Guidelines for the evaluation and treatment of muscle dysfunction in patients with chronic obstructive pulmonary disease. Arch Bronconeumol. 2015;51:384–95. [DOI] [PubMed] [Google Scholar]
- [18].Toukhsati SR, Mathews S, Sheed A, et al. Confirming a beneficial effect of the six-minute walk test on exercise confidence in patients with heart failure. Eur J Cardiovasc Nurs. 2020;19:165–71. [DOI] [PubMed] [Google Scholar]
- [19].Clavijo M, Yévenes F, Gallardo I, Contreras AM, Santos C. The general self-efficacy scale (GSES): reevaluation of its reliability and validity evidence in Chile. Rev Med Chil. 2020;148:1452–60. [DOI] [PubMed] [Google Scholar]
- [20].Smith SMS, Chaudhary K, Blackstock F. Concordant evidence-based interventions in cardiac and pulmonary rehabilitation guidelines. J Cardiopulm Rehabil Prev. 2019;39:9–18. [DOI] [PubMed] [Google Scholar]
- [21].Li H, Gan L, Sun Y, Yu HT. A randomized controlled study on systematic nursing care based on health empowerment theory and its effect on the self-care and functional abilities of patients with spinal fractures. J Orthop Surg Res. 2023;18:821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Neunhäuserer D, Patti A, Niederseer D, et al. Systemic inflammation, vascular function, and endothelial progenitor cells after an exercise training intervention in COPD. Am J Med. 2021;134:e171–80. [DOI] [PubMed] [Google Scholar]
- [23].Jin L, An W, Li Z, Jiang L, Chen C. Pulmonary rehabilitation training for improving pulmonary function and exercise tolerance in patients with stable chronic obstructive pulmonary disease. Am J Transl Res. 2021;13:8330–6. [PMC free article] [PubMed] [Google Scholar]
- [24].Ma Y, Chen Y, Zhang N, et al. Efficacy and safety of pulmonary rehabilitation training on lung function, quality of life, and T cell immune function in patients with stable chronic obstructive pulmonary disease: a randomized controlled trial. Ann Palliat Med. 2022;11:1774–85. [DOI] [PubMed] [Google Scholar]
- [25].Hua Y, Wang M, Li L, et al. Telephone follow-up based on empowerment theory to improve resilience and quality of life among patients after coronary artery stent implantation: a randomized controlled trial. Front Psychiatry. 2024;15:1248424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schulté B, Nieborak L, Leclercq F, Villafañe JH, Sánchez Romero EA, Corbellini C. The comparison of high-intensity interval training versus moderate-intensity continuous training after coronary artery bypass graft: a systematic review of recent studies. J Cardiovasc Dev Dis. 2022;9:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Slimani M, Ramirez-Campillo R, Paravlic A, Hayes LD, Bragazzi NL, Sellami M. The effects of physical training on quality of life, aerobic capacity, and cardiac function in older patients with heart failure: a meta-analysis. Front Physiol. 2018;9:1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tan GA, Peiris CL, Dennett AM. Cancer survivors maintain health benefits 6 to 12 months after exercise-based rehabilitation: a systematic review and meta-analysis. J Cancer Surviv. 2024;18:651–72. [DOI] [PubMed] [Google Scholar]
- [29].Ji Y, Zhang L. Intervention effect of solution-focused brief therapy based on empowerment theory on loneliness in obese children. Iran J Public Health. 2023;52:1692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Aliakbari F, Alipour FM, Tavassoli E, Sedehi M. The effect of empowerment program based on the social cognitive theory on the activity of daily living in patients with chronic obstructive pulmonary disease. J Educ Health Promot. 2020;9:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jiang R, Liu H, Jiang X, Wang D, Li X, Shang Y. Impact of empowerment theory-based nursing intervention on the quality of life and negative emotions of patients diagnosed with brain metastasis post breast cancer surgery. J Multidiscip Healthc. 2024;17:2303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yohannes AM, Casaburi R, Dryden S, Hanania NA. Predictors of premature discontinuation and prevalence of dropouts from a pulmonary rehabilitation program in patients with chronic obstructive pulmonary disease. Respir Med. 2022;193:106742. [DOI] [PubMed] [Google Scholar]
- [33].Royani Z, Rayyani M, Behnampour N, Arab M, Goleij J. The effect of empowerment program on empowerment level and self-care self-efficacy of patients on hemodialysis treatment. Iran J Nurs Midwifery Res. 2013;18:84–7. [PMC free article] [PubMed] [Google Scholar]