Abstract
Viral encephalitis caused by neuroadapted yellow fever 17D virus (PYF) was studied in parental and gamma interferon (IFN-γ)-deficient (IFN-γ knockout [GKO]) C57BL/6 mice. The T-cell responses which enter the brain during acute fatal encephalitis of nonimmunized mice, as well as nonfatal encephalitis of immunized mice, were characterized for relative proportions of CD4+ and CD8+ cells, their proliferative responses, and antigen-specific expression of cytokines during stimulation in vitro. Unimmunized mice accumulated only low levels of T cells within the brain during fatal disease, whereas the brains of immunized mice contained higher levels of both T-cell subsets in response to challenge, with CD8+ cells increased relative to the CD4+ subset. The presence of T cells correlated with the time at which virus was cleared from the central nervous system in both parental and GKO mice. Lymphocytes isolated from the brains of challenged immunized mice failed to proliferate in vitro in response to T-cell mitogens or viral antigens; however, IFN-γ, interleukin 4 (IL-4), and, to a lesser extent, IL-2 were detectable after stimulation. The levels of IFN-γ, but not IL-2 or IL-4, were augmented in response to viral antigen, and this specificity was detectable in the CD4+ compartment. When tested for the ability to survive both immunization and challenge with PYF virus, GKO and CD8 knockout mice did not differ from parental mice (80 to 85% survival), although GKO mice exhibited a defect in virus clearance. In contrast, CD4 knockout and Igh-6 mice were unable to resist challenge. The data implicate antibody in conjunction with CD4+ lymphocytes bearing a Th1 phenotype as the critical factors involved in virus clearance in this model.
Viruses within the Flavivirus genus of the family Flaviviridae are generally neurotropic, typically causing a fatal encephalitis associated with acute inflammation and widespread neuronal destruction (16, 25, 45, 67). Infected brains of vertebrate species exhibit similar pathologic features, often targeted to specific regions (41). The histologic changes commonly include perivascular mononuclear cell infiltrates and microglial activation within the brain parenchyma (16, 25, 45, 67). In humans, neuropathogenic flaviviruses cause an acute fatal encephalomyelitis (47). This disease has traditionally been modeled in laboratory mice, where the outcome is influenced by both the virulence of the infecting strain and host factors which govern susceptibility to the disease (3, 44, 51, 58). The immunological requirements for protection have been only partially defined, with previous studies demonstrating a dependence, in part, on antiviral T-cell responses (reviewed in reference 47). T-cell-deficient mice fail to generate protective immunity (7, 8, 13, 27), and adoptive transfer of immune spleen cells can prevent fatal encephalitis (31). On the other hand, depletion of lymphocytes from normal mice has been shown to reduce the central nervous system (CNS) inflammatory response to virus challenge and to slightly prolong survival, suggesting that under some circumstances the T-cell responses may be deleterious (27).
The lymphocyte subsets which constitute the protective T-cell response within the CNS have not been fully characterized. T cells with virus-specific cytotoxic activity were isolated from the brains of mice with West Nile virus encephalitis (43), and studies with Japanese encephalitis (JE) virus suggest that both CD8+ cytotoxic T cells and CD4+ T cells are required for protection (49). Activation of virus-specific CD4+ and CD8+ T cells has been demonstrated in humans and laboratory animals after exposure to JE virus antigens (1, 35, 36, 48), and T cells expressing these surface markers have been detected in perivascular infiltrates during encephalitis (32). Collectively, these studies also implicate virus-specific T cells in protection, although the critical effector functions involved in clearance of virus from the CNS are not known. The nature of the functional activities of T cells which respond to viral infection of the brain remains a fundamental question (62). To gain further insight into the immune response which occurs in the CNS during the pathogenesis of flavivirus encephalitis, we characterized the profile and properties of the T cells recruited into the brains of mice during infection with a neuroadapted strain of yellow fever (YF) 17D virus. Studies were conducted under conditions where virus is either cleared or not cleared from the CNS. In addition, gamma interferon (IFN-γ), CD4+, CD8+, and B-cell knockout (Igh-6) strains were used to determine the requirements for protective immunity in this model.
MATERIALS AND METHODS
Cells and viruses.
SW-13 (human adrenal adenocarcinoma) and Vero cells were originally obtained from the American Type Culture Collection (ATCC) and passaged in alpha minimal essential medium plus 10% fetal calf serum. The neuroadapted Porterfield strain of YF 17D virus (PYF), which exhibits high neurovirulence for young adult mice (61), was passaged in SW-13 cells, and titers were determined by plaque assay on Vero cells.
Animal experiments.
C57BL/6J mice (parental) and CD4, CD8, and B-cell knockout mice of this strain (22, 34, 55) were obtained from the Jackson Laboratory. IFN-γ knockout (GKO) mice (14), were originally obtained from R. Mark Buller (St. Louis University, St. Louis, Mo.). Breeding colonies were established, and the genotypes of litters were monitored by PCR assays using appropriate oligonucleotide primer pairs (22, 34, 55; B. Hultgren [Genentech, Inc., San Franscisco, Calif., personal communication). ICR mice were obtained from Harlan (Indianapolis, Ind.). The mice were used at 4 to 5 weeks of age for either acute challenge or immunization. Immunization was done by inoculation of 106 PFU of PYF diluted in sterile phosphate-buffered saline (PBS) plus 10% fetal bovine serum (FBS) by the intraperitoneal (i.p.) route. Virus challenge was done by intracerebral (i.c.) inoculation of anesthetized mice with 104 PFU of virus in the same diluent. Immunized mice were challenged 3 to 4 weeks after the single i.p. inoculation of virus. For experiments measuring the time course of accumulation and clearance of virus from the CNS, brains were harvested at serial intervals after i.c. inoculation and the virus contents were determined by plaque assay of 20% (wt/vol) brain suspensions in PBS plus 10% FBS.
Isolation of brain-associated lymphocytes.
Brain-associated lymphocytes were isolated using a modification of a published protocol (69). Anesthesized mice were perfused with cold PBS (4°C) to deplete the intravascular compartment of circulating cells. Brains were removed and homogenized as 10% solutions (wt/vol) in PBS. The cells were recovered by centrifugation, washed once with RPMI 1640, and resuspended in a solution of 80% Percoll (Pharmacia) in RPMI at 4 ml per brain. Step gradients of Percoll were formed by layers of 100% on the bottom, 80% with resuspended brain cells in the middle, and 40% on top. The gradient was centrifuged at 400 × g for 20 min at 25°C. The interface between the 80 and 40% Percoll layers was collected, and cells in this layer were washed twice with RPMI 1640 and resuspended in RPMI 1640 plus 10% FBS. Viable cells were quantitated by counting in the presence of trypan blue.
Flow cytometry.
Fluorescein isothiocyanate-conjugated anti-CD4 and anti-CD8 antibodies and phycoerythrin-conjugated anti-CD3 antibody (PharMingen) were used to detect lymphocyte cell surface markers. Staining was carried out at 4°C. Aliquots of gradient-purified lymphocytes were washed three times with staining buffer (PBS plus 3% FBS), and the cells were resuspended in 100 μl of staining buffer and incubated with Fc block (anti-CD16-CD32 antibody; PharMingen). Antibodies for surface markers were added for 30 min. The cells were then washed three times with staining buffer and resuspended in 250 μl of staining buffer for fluorescence-activated cell sorter (FACS) analysis. Before analysis, 1 μl of propidium iodide solution (1 mg/ml) was added to each tube. The cells were analyzed with a Becton Dickinson flow cytometer equipped with FACSCalibur and CellQuest software. Dual-fluorescence analysis on the lymphocyte-gated population was performed using propidium iodide positivity to establish a parameter for exclusion of nonviable cells. The percentage of cells staining above background and the mean fluorescence intensity of each marker were determined on each sample.
Cytokine ELISA.
Cytokine production by brain-associated lymphocytes was analyzed using gradient-purified cells harvested on day 4 or 5 postchallenge of immunized mice. Cells from groups of five mice were pooled for these assays. The cells were seeded at a density of 105 cells/well and stimulated with concanavalin A (Con A), anti-CD3 antibody (25 μg per well) (generated from hybridoma 145-2C11 [ATCC]) or viral or mock (SW-13 cell) antigen in various experiments. Viral antigen was prepared from PYF-infected SW-13 cells by Dounce homogenization in PBS plus 10% FBS, followed by centrifugation at 10,000 rpm for 20 min in a Sorvall RC-5B refrigerated centrifuge. The supernatant was collected and stored at −70°C until it was used. Mock-infected SW-13 cell extract was prepared by similar treatment of uninfected cells. Antigen preparations were used at final dilutions of 1:120 to 1:240. Medium from the fourth day of culture was used for cytokine assays. Measurements of interleukin 2 (IL-2), IL-4, and IFN-γ were done using commercially available enzyme-linked immunosorbent assay (ELISA) kits (BioSource). Samples were tested in duplicate for individual experiments, and values were referenced against standard curves generated with the manufacturer's reagents.
IL-2-IL-4 bioassay.
Bioassay for IL-2 and IL-4 was performed using the T-cell growth factor-dependent CTLL-2 cell line (ATCC). Dose dependence and peak levels of IL-2-induced proliferation were determined by serial dilution of IL-2 (recombinant IL-2 [kindly provided by Matthew Thomas, Washington University, St. Louis, Mo.]). Anti-IL-2 and anti-IL-4 antibodies (PharMingen) were used to establish the specifity of the proliferative response to these growth factors (see Results). Brain-associated lymphocytes harvested on day 4 or 5 following challenge of immunized mice were cultured in RPMI plus 3% FBS (mock control) or with addition of ConA (2.5 μg/ml) or anti-CD3 antibody (25 μg/well). Media collected from the lymphocyte cultures after days 2 and 3 were added to 96-well plates seeded with CTLL cells (2 × 103/well). The cells were cultured for 48 h and then labeled with 1 μCi of [methyl-3H]thymidine/well-(6.7 Ci/mmol; ICN Pharmaceuticals, Inc.) for 4 h, after which the cells were harvested and the incorporated radioactivity was counted.
Intracellular cytokine expression.
Lymphocytes from the brains of immunized, virus-challenged mice were prepared by gradient purification as described above. Cells were stimulated by incubation in vitro in the presence of phorbol ester (50 ng/ml; Sigma) or mock or viral antigen (1:120 to 1:240 final dilutions) for up to 48 h and then treated with GolgiPlug (PharMingen). The cells were collected and treated with Fc blocker for 15 min and stained with Cy-Chrome-conjugated anti-CD4 or anti-CD8 antibody (PharMingen), followed by fixation with 4% paraformaldehyde. The cells were then treated with Cytofix/Cytoperm solution (PharMingen) for 20 min at 4°C and washed with Perm/Wash. Intracellular IFN-γ was stained with phycoerythrin-conjugated anti-IFN-γ antibody (PharMingen) for 30 min at 4°C. IL-4 was stained with fluorescein isothiocyanate-conjugated anti-IL-4 antibody (PharMingen). Because only very low levels of IL-4 were detectable, positive-control reactions using IL-4-secreting cells (PharMingen) were used to establish a detection range of between 0.5 and 1.5% of the total cells in the assay. The stained cells were washed with Perm/Wash solution and resuspended in staining buffer for FACS analysis. Flow cytometry was used to profile the cytokine production of IFN-γ or IL-4 in the CD4+ or CD8+ populations.
Reverse transcription (RT)-PCR analysis of cytokine expression.
RNA was isolated from 20% brain suspensions in PBS plus 10% FBS using Trizol-LS (Gibco/BRL) according to the manufacturer's instructions. Final RNA preparations were dissolved in diethyl-pyrocarbonate-treated water and used for cDNA synthesis in the presence of cytokine-specific antisense primers: IFN-γ, 5′-GCTTCCTGAGGCTGGATTCC-3′; tumor necrosis factor alpha (TNF-α), 5′-TTCTCCAGCTGGAAGACTCC-3′; IL-1β, 5′-ACCACTGTTGTTTCCCAGGAAG-3′; or glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-ACCTTCTTGATGTCATCATACTTGGC-3′. cDNA synthesis was done with Superscript (Gibco/BRL). One-quarter of the reaction volumes were then used for PCR amplification in the presence of the same antisense primer and a 5′ primer: IFN-γ, 5′-GCTTTGCAGCTCTTCCTCATG-3′; TNF-α, 5′-AATTCGAGTGACAAGCCTGT-3′; IL-1β, 5′-ACCCATATGAGCTGAAAGCTC-3′; or GAPDH, 5′-ACCTCAACTACATGGTCTACATG-3′. The reactions were run with Taq DNA polymerase (Promega), using programs typically consisting of 1 min of denaturation at 95°C, 1 min of annealing at 50 to 55°C, and between 20 and 30 s of polymerization at 72°C for 30 to 35 cycles. The reaction products were visualized by agarose gel electrophoresis and ethidium bromide staining to confirm similar yields of DNA fragments over the range of cycles utilized. Plasmids containing the target sequences of the cytokine cDNAs were used as positive controls for PCR. The specificities of the PCR products were determined by Southern blotting after transfer of the DNA to a Nytran membrane (Midwest Scientific). Transfer was done in 20× SSC buffer (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate); DNA was then cross-linked to the membrane by UV irradiation and probed with 32P-labeled oligonucleotides corresponding to the following: IFN-γ, 5′-GATTTTCATGTCACCATCCTTTTGCCAGTT-3′; TNF-α, 5′-TAGTGGTGCCAGCCGATGGGTTGTACCTTG-3′; IL-1β, 5′-CAACGACAAAATACCTGTGGCCTTGGGCCT-3′; and GAPDH, 5′-GTGGAAGGGCTCATGACCACAGTCCATGCC-3′. Labeled probes were prepared by phosphorylation of the oligonucleotides with T4 polynucleotide kinase (Promega) in the presence of adenosine 5′-[γ-32P]triphosphate (5,000 Ci/mMol; Amersham). Approximately 1.5 × 107 cpm of each probe was added to portions of membranes containing the samples in hybridization buffer (0.13 M sodium phosphate [pH 7.0], 0.25 M NaCl, 7% [wt/vol] sodium dodecyl sulfate). The reaction mixtures were hybridized for 1 h at 55°C, and the membranes were washed twice with 6× SSC plus 0.1% sodium dodecyl sulfate and exposed to X-ray film.
Lymphocyte proliferation assay.
Brain-associated T cells were isolated as described above. For control experiments with splenocytes, spleens were harvested from anesthetized mice by a sterile technique and minced in RPMI 1640 medium (Biowhittaker). Splenocytes were isolated with lymphocyte separation medium (Pharmacia). Triplicate cultures of cells (105/well) were seeded into 96-well flat-bottom plates in RPMI 1640 containing 10% FBS (Hyclone), 50 μM β-mercaptoethanol (Sigma), 2 mM l-glutamine (Biowhittaker), 5 U of penicillin/ml, and 5 μg of streptomycin/ml. For proliferation assays, cells were treated at time zero with various stimuli, including ConA (2.5 μg/ml; Sigma) or either YF viral antigen or mock SW-13 cell antigen prepared as described above. In various experiments, the dose of antigen required to detect maximal proliferation of splenocytes varied from a 1:120 to 1:240 dilution of the extract. Cellular proliferation was determined by labeling the cells with 1 μCi of [methyl-3H]thymidine/well for 4 h prior to harvesting them. Depending on the experiment, the proliferative response was expressed by the equation net cpm = cpm (stimulated) − cpm (unstimulated [medium alone]) or as the stimulation index, cpm (stimulated)/cpm (unstimulated [medium alone]).
Statistical analysis.
Differences among average sample values were analyzed for significance using nonparametric methods (Mann-Whitney, Wilcoxon rank sum, or median tests where appropriate). Differences in survival ratios for mouse challenge experiments were assessed using Fisher's exact test.
RESULTS
Detection of T cells in virus-infected brains.
In initial experiments with i.c. inoculation of mice with PYF, signs of illness occurred by days 4 to 5, and the mice were moribund by day 6 or 7. Lymphocytes could be isolated from the brains of moribund mice, but greater numbers of viable cells were recovered when the harvesting was done on day 4 or 5. The yield of cells at these times ranged from 1.0 × 105 to 5.0 × 105 cells/brain and was consistent throughout the experiments. The mean levels of CD3+ CD4+ cells present in the brains of parental and GKO mice were 1.80 or 2.54%, respectively, and those of CD3+ CD8+ cells were 1.25 and 1.91% (Table 1). Only small numbers of cells could be isolated from the brains of parental or GKO mice which had not been inoculated with virus, and very few T cells were detected in these samples (data not shown). The low levels of T cells in the virus-infected brains did not result from insensitivity of the assay, as T cells isolated from the spleens of the mice used for these experiments were readily detectable. Among uninfected mice, virus-infected mice (i.c. challenge), and virus-immunized parental mice (i.p. inoculation), the levels of CD3+ CD4+ lymphocytes were approximately 17% and the levels of CD3+ CD8+ T cells were approximately 7%. Although these percentages are somewhat less than the expected values, similar results were repeatedly obtained over the course of the experiments.
TABLE 1.
T-cell FACS analysisa
| Sample | % CD3+ CD4+
|
% CD3+ CD8+
|
||
|---|---|---|---|---|
| Day 4 | Day 5 | Day 4 | Day 5 | |
| Acutely challenged | ||||
| C57 | 1.80 ± 0.34 | ND | 1.25 ± 0.16 | ND |
| GKO | 2.54 ± 0.36 | ND | 1.91 ± 0.24 | ND |
| Immunized and challenged | ||||
| C57 | 6.10 ± 2.291,5 | 8.34 ± 3.281,6 | 5.79 ± 3.362,7 | 11.12 ± 3.352,8 |
| GKO | 7.13 ± 1.943,5 | 13.22 ± 5.673,6 | 5.05 ± 2.304,7 | 17.97 ± 5.494,8 |
Percentages of CD3+ CD4+ and CD3+ CD8+ cells out of the total number of cells analyzed are shown. The values represent the mean ± the standard error of the mean. For acutely challenged mice, a total of 10 mice were individually analyzed for each strain. For immunized and challenged mice, a total of 15 normal mice and 13 GKO mice were individually analyzed in separate experiments. ND, samples not tested. Statistical significance is indicated by superior numbers as follows: 1, 5, 6, and 7, not significant; 2 and 4, P < 0.01; 3 and 8, P < 0.05.
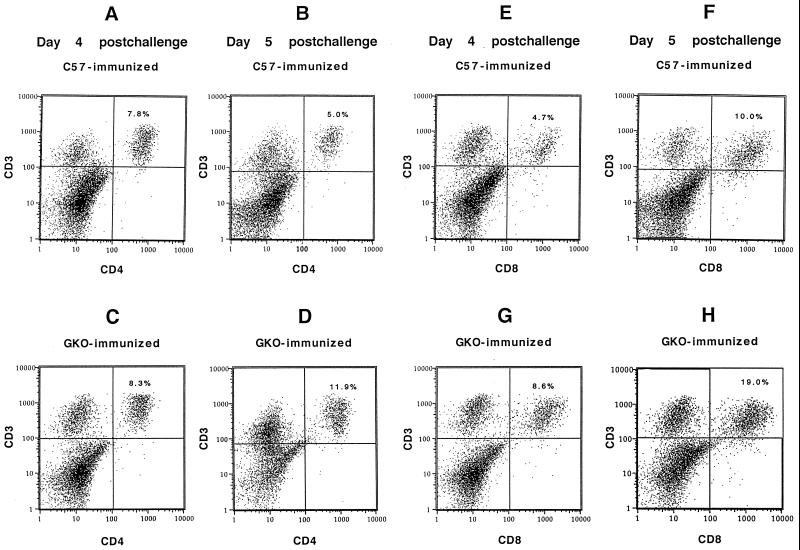
Because limited numbers of T cells were recovered from the brains after acute i.c. challenge, further characterization of their properties was difficult. Immunization with PYF was then done prior to i.c. challenge to determine whether augmentation of the brain-associated T-cell response would occur. Under these conditions, the vast majority of mice are protected from fatal disease and very few exhibit any signs of illness (see Table 3). On days 4 and 5 following challenge, the total numbers of cells in the brain-associated fraction from parental mice were increased 5 to 10-fold (approximately 1 × 106 to 2 × 106 cells/brain, relative to those of unimmunized mice). The percentages of both CD3+ CD4+ and CD3+ CD8+ cells were also increased (Table 1). In parental mice, the levels of CD3+ CD4+ T cells were 6.10% on day 4 and 8.34% on day 5. The levels of CD3+ CD8+ cells underwent a greater increase, from 5.79% on day 4 to 11.12% on day 5. Similar experiments were conducted with GKO mice. The total number of brain-associated cells recovered after gradient purification was less than for parental C57BL/6 mice (average, 4 × 105 cells/brain for GKO mice). These quantities of cells were significantly different from those recovered from parental mice (P < 0.05; median test). CD3+ CD4+ cells increased from 7.13 to 13.72% between days 4 and 5 after challenge. In contrast, CD3+ CD8+ cells increased from 5.05 to 17.97% between days 4 and day 5 (Table 1). FACS profiles of the CD3+ CD4+ and CD3+ CD8+ cells for typical samples from days 4 and 5 in these experiments are shown in Fig. 1. In additional experiments, the brains of immunized but nonchallenged parental mice had only low levels of T cells as detected by FACS analysis, similar to results with unimmunized, nonchallenged mice. In a typical experiment, the levels of CD3+ CD4+ and CD3+ CD8+ cells were 0.12 and 0.18%, respectively, in the absence of challenge (data not shown).
TABLE 3.
Immunization and challenge experimentsa
| Strain | Survival (%) | AST |
|---|---|---|
| C57 (parent) | 19/23 (82) | 7.5 |
| GKO | 24/30 (80)1 | 5.6 |
| CD8 KO | 24/28 (85)1 | 9.0 |
| CD4 KO | 1/15 (6.6)2 | 8.7 |
| Igh-6 | 0/9 (0)2 | 7.6 |
Survival ratios are given for normal and knockout (KO) mice after immunization and challenge with PYF virus. Average survival times (AST) (in days) are given for mice which did not survive. Statistical significance is indicated by superior numbers as follows: 1, compared with parental mice, not significant; 2, compared with parental mice, P < 0.005.
FIG. 1.
FACS analysis of CD3+ CD4+ T cells isolated from brains of immunized and virus-challenged mice. (A and B) CD3+ CD4+ cells from brains of parental mice on days 4 and 5 postchallenge. (E and F) CD3+ CD8+ cells on the same days. (C and D) CD3+ CD4+ cells on days 4 and 5 and (G and H) CD3+ CD8+ cells from GKO mice.
These experiments showed that both parental and GKO mice exhibited an absolute increase in the number of T cells during recruitment of the protective immune response into the CNS, which did not simply reflect nonspecific trafficking into the CNS as a result of prior immunization. Significant increases in numbers of CD8+ cells in parental mice, and of both CD4+ and CD8+ cells in GKO mice, occurred between days 4 and 5 postchallenge, although fewer T cells actually accumulated in the case of GKO mice. The relative percentages of the T-cell subsets in GKO mice were similar to those in parental mice, except for a higher percentage of CD8+ cells on day 5.
Cytokine production by brain-associated lymphocytes.
In order to assess some of the functional properties of the brain-infiltrating lymphocytes associated with protection of immunized mice, the production of the cytokines IL-4, IL-2, and IFN-γ by these cells in response to various stimuli was measured. The results of these experiments are shown in Table 2. IL-4 was detectable in cultures of unstimulated cells, and an increase in production significantly above the background level was generated by anti-CD3 antibody but not by viral antigen. IL-2 was detectable in unstimulated cultures but did not increase significantly in response to any stimuli. Background and stimulated levels of IL-4, but not IL-2, were higher from cells of GKO mice than from those of parental mice. IFN-γ was detectable in cultures of unstimulated cells and was maximally stimulated by anti-CD3 antibody. There was submaximal stimulation of IFN-γ significantly above background levels by treatment with viral antigen but not mock antigen. The differences between values of IFN-γ for unstimulated samples and those for anti-CD3- and viral antigen-stimulated samples were significant (P < 0.05). Thus, although the lymphocytes produced a background level of each of the cytokines, increases in production of both IL-4 and IFN-γ were detectable upon stimulation, but only the IFN-γ expression was antigen specific. The source of the antigen-presenting cells which elicit the IFN-γ production has not been identified but presumably includes peripherally recruited cells which are capable of processing exogenous viral proteins used for stimulation.
TABLE 2.
Detection of cytokines in T-cell culturesa
| Cytokine and sample | Amt with:
|
||||
|---|---|---|---|---|---|
| Control | ConA | Anti-CD3 | SW-13 | PYF | |
| IL-4 | |||||
| C57 | 101 ± 361,2 | 51 ± 151 | 286 ± 962 | 811 | 92 ± 81 |
| GKO | 194 | 252 | 702 | 226 | 187 |
| IL-2 | |||||
| C57 | 155 ± 573 | 124 ± 323 | 107 ± 703 | 175 ± 833 | 196 ± 173 |
| GKO | 139 | 108 | 163 | 125 | 100 |
| IFN-γ C57 | 486 ± 1794,5 | 407 ± 1024 | 2,279 ± 7385 | 3264 | 1,440 ± 4105 |
Brain-associated cells from several mice were pooled and cultured in triplicate at 105/well in the presence of medium only (control), ConA, anti-CD3 antibody, mock antigen (SW-13), or viral antigen (PYF). The supernatants of the media were pooled for ELISA (done in duplicate). The values are in picograms/milliliter (mean of several experiments ± the standard error of the mean) except for GKO (average of two experiments). Statistical significance is indicated by superior numbers as follows: 1, 3, 4, not significant; 2 (anti-CD3 versus control) and 5 (anti-CD3 or PYF versus control), P < 0.05.
IL2-IL4 bioassay.
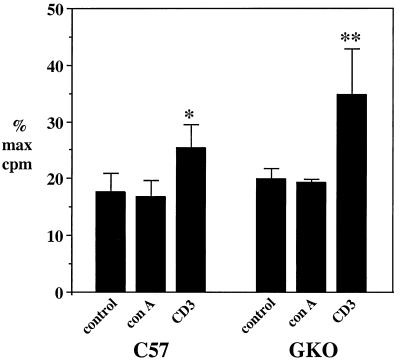
To further examine the in vitro production of IL-2 and IL-4, media from the cultures shown in Table 2 were tested using a bioassay for these cytokines. Figure 2 shows the results for cells isolated from parental and GKO mice after immunization and challenge as in previous experiments. Stimulation was done with medium only, ConA, anti-CD3 antibody, viral antigen, or mock antigen. Proliferation varied from approximately 10 to almost 50% of maximum among the various treatments. Anti-CD3 antibody was the most potent inducer, with levels of stimulation significantly different from those of controls in both groups of mice. The level of growth factor production by cells from GKO mice in response to anti-CD3 was substantially higher than that of parental mice, although this difference was not significant. Viral antigen did not elicit an increase in T-cell growth factor production in these experiments (data not shown). The specificity of the bioactivity in the assay was determined with anti-IL-2 and anti-IL-4 antibodies. In repeated experiments, antibody to IL-4 eliminated 85 to 90% of the CTLL cell proliferative response among the different stimulated groups (data not shown). In contrast, using spleen cells stimulated by ConA for IL-2 and IL-4 production, anti-IL-2 and anti-IL-4 antibodies exhibited roughly equivalent degrees of inhibition (between 40 and 60% of the proliferative response in various experiments) (data not shown). Thus, IL-4 accounted for the vast majority of the bioactivity in cultures of brain-associated lymphocytes. This capacity of the cells to produce IL-4 in a non-antigen-specific manner is consistent with the data in Table 2 and correlates with the time of accumulation of CD3+ CD4+ T cells in the brain on days 4 to 5 postchallenge.
FIG. 2.
Production of IL-4 as measured by CTLL cell assay for parental and GKO mice. Media were collected from cultures of brain-associated lymphocytes (harvested on days 4 and 5) that were stimulated for 2 to 3 days in the presence of control (no mitogen), ConA, or anti-CD3 antibody. The media were assayed for stimulation of CTLL cells as described in Materials and Methods. The results are expressed as the percentage of the maximal stimulation observed with recombinant IL-2. The values represent means + standard errors of the means. The asterisks indicate significant differences from control cultures: ∗, P < 0.005; ∗∗, P < 0.005 (Mann-Whitney test). The difference between anti-CD3-stimulated samples of GKO and parental mice was not significant (P > 0.05).
Intracellular staining for cytokines.
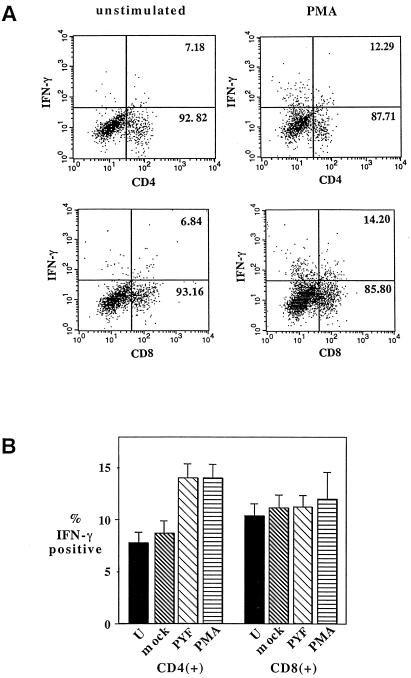
To determine if the antigen-specific production of IFN-γ observed in Table 2 could be localized to either the CD4+ or CD8+ compartment, intracellular staining was done on cells harvested from brains of immunized and challenged mice. In initial experiments, cells stained directly after isolation from the brain failed to yield detectable signals for IFN-γ (data not shown). After in vitro cultivation, IFN-γ was detectable in both the CD4+ and CD8+ compartments, and treatment with phorbol ester produced an increase in the fraction of both CD4+ and CD8+ cells which expressed it (Fig. 3). Permeabilization was required to detect positive signals, and staining with either isotype-matched control immunoglobulin G (IgG) or no IgG yielded no signal (data not shown). When the cells were stimulated with viral antigen, a significant increase above the background of unstimulated and mock-stimulated cells was detectable in the CD4+ population (Fig. 3B). The level of IFN-γ positive cells in the CD8+ population was slightly higher for PYF- versus mock-stimulated and unstimulated cultures, but the differences were not significant. It remains possible that some portion of the relatively high background level of IFN-γ production in both the CD4+ and CD8+ populations represents an antigen-specific response; however, further investigation is needed to determine this. In additional experiments, IL-4 production could be localized to the CD4+ compartments of phorbol ester-stimulated cells, but only at very low levels compared to the IFN-γ-positive signals, and antigen-specific stimulation was not tested by this method.
FIG. 3.
Analysis of IFN-γ expression in CD4+ and CD8+ compartments of brain-associated lymphocytes by intracellular staining. Cells were harvested from the brains of immunized, virus-challenged parental mice on day 4 or 5 following challenge, incubated for 2 days with various stimuli, and then processed as described in Materials and Methods. (A) Dot plots for typical experiments in which unstimulated and phorbol myristate acetate (PMA)-stimulated cells were used to establish the range of stimulation in the assay. (B) Percentages of CD4+ or CD8+ cells expressing IFN-γ in response to different stimuli (U, unstimulated; mock, SW-13 cell antigen; PYF, viral antigen; PMA, phorbol ester). The results represent composite means (± the standard errors of the means) for three separate experiments involving 16 samples for each stimulus. The differences in the levels of IFN-γ among CD4+ cells were significant for PYF and PMA versus unstimulated and mock-stimulated samples (P < 0.005 [Wilcoxon rank order test]).
In vivo expression of proinflammatory cytokines.
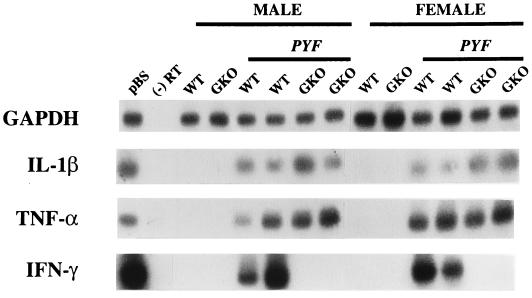
CNS infection of immunocompetent mice with YF 17D is associated with a profound acute inflammatory response, marked by diffuse infiltration of mononuclear cells (16). This suggested to us that the production of IFN-γ as seen in cultures of brain-associated lymphocytes is a principal factor driving the inflammatory response within the CNS. To determine if IFN-γ was in fact being produced during infection of the CNS in this model, expression of IFN-γ, as well as the proinflammatory cytokines TNF-α and IL-1β, was tested in acutely infected brains by PCR-based methods (Fig. 4). None of the mRNAs for these cytokines were detectable in the brains of uninfected mice. In contrast, expression of all three occurred during progressive infection of parental mice. In GKO mice, expression of TNF-α and IL-1β was observed in the absence of IFN-γ, indicating that IFN-γ is not required for activation of these endogenous CNS inflammatory mediators. The detection of GAPDH mRNA was used to verify the integrity of the RNA samples. These data provide evidence that the IFN-γ production observed in the cell culture experiments is not likely to be a simple artifact of in vitro stimulation of the brain-associated lymphocytes.
FIG. 4.
Inflammatory cytokine expression in parental and GKO mice during acute encephalitis with PYF. Brains were harvested on day 5 postinoculation, and RT-PCR assay for GAPDH and cytokine mRNAs (IL-1β, TNF-α, and IFN-γ) was performed as described in Materials and Methods. pBS, target PCR product generated from a plasmid containing cDNA of the respective mRNAs; (−) RT, reactions in which RT was not done prior to PCR amplification; WT, wild-type parental mice; GKO, GKO mice. The results for two male and two female mice of each strain with encephalitis (PYF) and for uninfected controls are shown.
Proliferative responses of brain-associated lymphocytes.
Because of the marked inflammatory responses occurring in the brains of mice infected with PYF virus, experiments were done to determine if any proliferation of brain-associated lymphocytes was involved. Splenocytes were used as controls in these experiments. The results for immunized parental mice whose splenocytes were tested after 2 to 4 days of cultivation showed peak stimulation indices (SI) for ConA and viral antigen of 67.6 ± 5.45 and 5.1 ± 3.5, respectively (mean and standard deviation for two groups of three mice tested [data not shown]). Splenocytes from GKO mice, tested on days 2 and 3 following stimulation, gave peak SI for ConA and viral antigen of 38 ± 6.9 and 1.7 ± 0.65, respectively (data not shown). Experiments with ICR mice indicated that the level of proliferation in C57BL/6 mice was typical of the murine response to the antigen preparation. The peak SI for ConA and viral antigen in ICR mice were 43 ± 15.2 and 4.1 ± 1.6, respectively (mean of three mice) (data not shown).
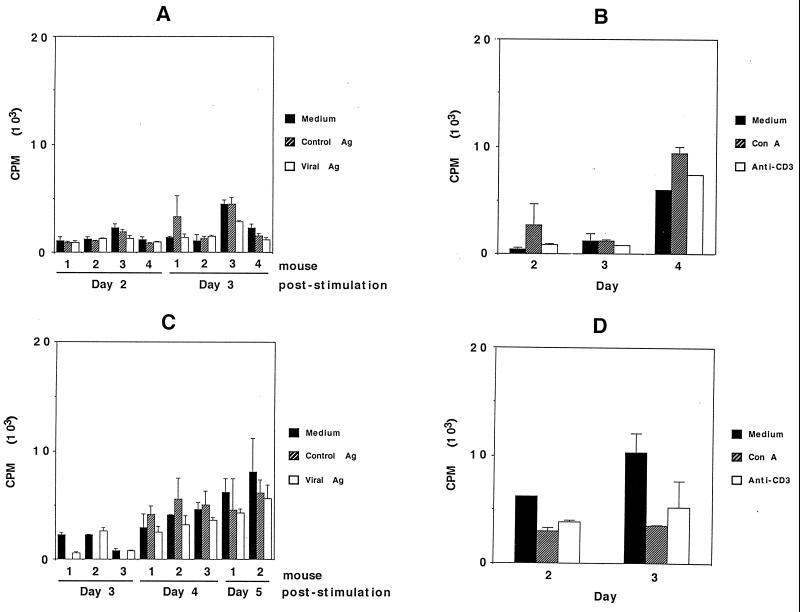
The proliferative responses of brain-associated lymphocytes from parental and GKO mice which had undergone immunization and virus challenge are shown in Fig. 5. Figure 5A shows that cells from parental mice harvested on day 5 following challenge did not proliferate significantly above the background level in response to viral antigen. In separate experiments, the cells failed to respond to either ConA or anti-CD3 antibody (Fig. 5B). Cells from GKO mice failed to proliferate above the background level in response to viral antigen (Fig. 5C). Responses to either ConA or anti-CD3 antibody also did not occur (Fig. 5D). Splenocytes from these same immunized, virus-challenged mice were tested in conjunction with these experiments. Peak antigen-specific responses in parental mice occurred on day 3 of stimulation (the SI were as follows: ConA, 71; anti-CD-3, 47; and viral antigen, 6.1 [mean of five mice tested]). For GKO mice, peak antigen-specific responses occurred on day 2 of stimulation (the SI were as follows: ConA, 32; anti-CD3, 15; viral antigen, 1.9 [mean of five mice tested]). Thus, the lack of proliferative responses in these experiments did not result from a general failure of lymphocytes from immunized mice to respond to stimulation in this assay but rather was related to the entry of these cells into the brain.
FIG. 5.
In vitro proliferation assay of brain-associated lymphocytes from parental (A and B) and GKO (C and D) mice which had been immunized and virus challenged. (A) Lymphocytes were isolated on day 5 postchallenge. The cells were stimulated with viral antigen (Viral Ag), SW-13 cell antigen (Control Ag), or medium alone for 2 and 3 days. (B) Cells harvested from three mice on day 5 postchallenge were pooled and tested for stimulation by ConA or anti-CD3 antibody after 2 to 4 days. (C) Cells were harvested on day 5 postchallenge and stimulated with viral antigen, control antigen, or medium. The results after 3 to 5 days of stimulation are shown. (D) Cells from three mice on day 5 postchallenge were pooled and stimulated with ConA or anti-CD3 antibody, and proliferation was measured on day 2 or 3 following stimulation. The error bars indicate standard deviations.
Viral replication in brains of unimmunized and immunized mice.
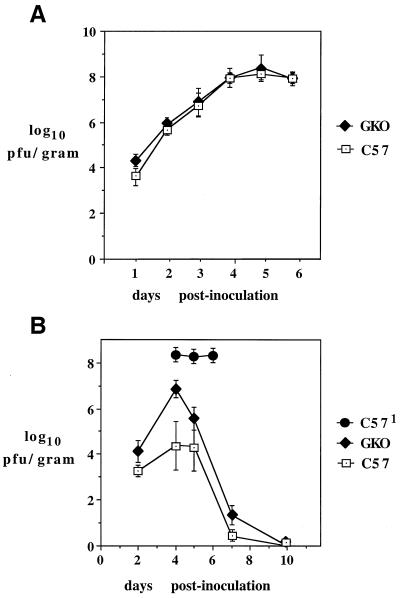
In previous studies, it was established that the CNS virus burden associated with infection of normal mice with PYF virus reaches very high levels (61). To determine if differences in the extent of this viral replication and, in particular, the pattern of virus clearance occurred in the brains of parental versus GKO mice, the time courses of virus production in both unimmunized and immunized mice which had been subjected to challenge were measured. Figure 6 shows the results of these experiments. In unimmunized mice of both strains, essentially identical levels of brain-associated virus were detectable between 1 and 6 days after infection (Fig. 6A). The virus burden increased from approximately 4 log units/g on day 1 to 8.0 log units/g on day 6, at which time the mice succumbed to disease. In contrast to this result, mice which had been immunized showed less accumulation of virus up to day 4, and the virus burden decreased thereafter and became undetectable by plaque assay 10 days after challenge. The brains of GKO mice contained higher levels of virus than those of parental mice on days 4, 5, and 7, with significant differences in the average levels on days 4 and 7 (see the legend to Fig. 6). These data indicate that a defect in virus clearance occurs in GKO mice, although this was not associated with a mortality different from that in normal mice (see below).
FIG. 6.
Viral replication in brains of parental and GKO mice. Brain-associated virus was measured as described in Materials and Methods. (A) Virus burdens (mean ± standard error of the mean [SEM]) in unimmunized mice after i.c. inoculation with 104 PFU of PYF. Three to five mice were tested for each time point. (B) Results for immunized parental and GKO mice that had been challenged with 104 PFU of PYF. Brain-associated virus (mean ± SEM) was measured for between 4 and 12 mice for each time point. The differences in the average values were significant for day 4 (P < 0.005) and day 7 (P < 0.05) (Wilcoxon rank order test). C571, unimmunized mice used as the control for peak virus burden.
Effects of IFN-γ, CD4+, CD8+, and B-cell deficiencies on susceptibility to fatal encephalitis.
To investigate whether IFN-γ, CD4+, CD8+, or B cells are required for mice to resist immunization and i.c. challenge with PYF, knockout strains were compared to parental mice for the proportion of survivors following the immunization-challenge protocol (Table 3). For all five of the mouse strains, only a small number of mice (<5.0%) sustained fatal encephalitis as a result of immunization with PYF, with no significant differences being observed in the rates of susceptibility (data not shown). These deaths occurred between 3 and 4 weeks postimmunization. Among immunized parental mice, 82% (19 of 23) were protected from virus challenge. Similar results were obtained for GKO mice (80% survival [24 of 30]) and CD8 knockout mice (85% survival [24 of 28]). The average survival times of mice which succumbed to challenge varied from 7.2 days for parental to 5.6 days for GKO and 9.0 days for CD8 knockout mice. In contrast to these results, CD4 knockout mice were highly susceptible to virus challenge (6.6% survival [1 of 15]). B-cell knockout mice were also highly susceptible to challenge (0% survival [0 of 9]). The average survival times for mice in these groups were 8.7 and 7.6 days, respectively.
DISCUSSION
Infiltration of CD4+ and CD8+ T cells into the CNS has been observed in various models of neurotropic virus infection in rodents (6, 11, 42, 46, 73). It has been stated that in general, CD4+ cells largely determine the protective immune response to viral infection of the brain (62), although a role for CD8+ cells has been documented in some cases (6, 21, 28, 65, 72). It is also known that T cells can generate an immunopathologic response, depending on the type of infecting virus, (57, 66, 71). To gain further understanding of the CNS immune response which occurs in association with flavivirus encephalitis, we studied the T cells which enter the brain during acute fatal encephalitis in unimmunized mice and sublethal encephalitis during challenge of immunized mice. The brains of mice that succumbed to acute encephalitis contained low numbers of CD4+ and CD8+ cells, whereas the levels of both of these cell types were significantly increased during challenge of immunized mice, suggesting that either individually or in combination they contribute to protection. The number of inflammatory cells present in the brains of GKO mice was less than for parental mice during challenge of both unimmunized and immunized animals. Since the virus burden in the brains of GKO mice was similar to or greater than that in parental mice, this suggests that the lesser inflammatory infiltrate in GKO mice is not explained by a reduced antigen load. Instead, it appears to depend on the lack of IFN-γ expression, which may limit the recruitment of inflammatory cells across the blood-brain barrier. IFN-γ deficiency did not substantially alter the percentages of CD4+ and CD8+ cells in the brain compared to parental mice, suggesting that its major effect is on the total accumulation of inflammatory cells rather than on T cells alone. However, further experiments are needed to determine if this is true for other specific cell types in the inflammatory infiltrate.
The ratio of CD8+ to CD4+ cells was greater in the brains of both parental and GKO mice. It is known that expansion of the CD8+ population can reach very high levels in response to viral infections (50, 76, 77). Some viral infections of the CNS are characterized by high levels of brain-associated CD8+ cells (11, 26) or increases in the relative proportions of CD8+ and CD4+ cells during control of disease (42, 73). Recruitment of CD8+ T cells with cytotoxic activity occurs in some cases where these cells contribute to protection (21, 26, 65). In previous studies of flavivirus encephalitis, infiltration of CD8+ cells into the brain has been observed (32, 43). In particular, virus-specific CD8+ cytotoxic T cells were isolated from West Nile virus-infected mice (43). Furthermore, protection against JE virus encephalitis in unimmunized mice was reported to require CD8+ cytotoxic T cells in conjunction with CD4+ cells (49). However, in our model, the absence of a deleterious effect on survival among CD8+ knockout mice undergoing immunization and challenge suggests that these cells do not provide a critical component of protection. Since the JE virus model used acutely challenged mice, the lack of immunization might explain the discrepancy with our data, if the role of CD8+ cells in protection is more important in the context of a primary versus a memory response. The extent of any differences in sublethal CNS disease or in the rate of virus clearance in the CD8-deficient mice compared with parental mice in our model remains to be determined. At least some defect in virus elimination may be expected if the brain-associated CD8+ cells produce IFN-γ, which is required for efficient virus clearance (see below).
In contrast to CD8+ cells, CD4+ T cells were required in order to resist challenge with PYF. This is consistent with other models in which these cells provide critical functions during control of CNS viral infections (19, 56; reviewed in reference 62). The mechanisms involved could include induction of antibody, production of cytokines, or even cytotoxic activity. In previous studies, the capacity of virus-specific antibody to mediate protection against YF encephalitis in mice has been demonstrated by passive transfer experiments (59, 60). It is not known, however, if activation of CD4+ lymphocytes occurs during clearance of virus in these cases or whether these cells are dispensable. Given the fact that both IgG-deficient and CD4-deficient mice are unable to resist virus challenge after immunization with PYF, we believe that an antibody response driven by CD4+ lymphocytes is the likely mechanism of protection in this model, although indirect effects of cytokines elaborated by these cells may also be a contributing factor.
Cytokine expression within the rodent CNS has been examined in response to infection with several types of RNA viruses (9, 53, 64, 70). Induction of the proinflammatory cytokines IL-1β and TNF-α may accompany activation of microglia and astrocytes soon after infection, but production of IFN-γ and IL-2 is believed to result from entry of the cellular immune response from the periphery in association with loss of integrity of the blood-brain barrier (5). The recruitment of either a Th1 or Th2 response has been observed in different models of infection and inflammation of the CNS, with a Th1 profile, involving production of IFN-γ, typical of diseases in which deleterious inflammation occurs (69, 75). IFN-γ causes many alterations in gene expression within the CNS that may affect virus-immune system interactions as well as neuronal survival (24, 54). Direct antiviral effects of this cytokine have also been proposed (37). In some models of CNS viral infection, it has been implicated in protection (10, 20, 40), although in others it is not required (39). IFN-γ also promotes IgG2A production, which is associated with antibody-mediated protection against YF encephalitis in mice (60). Thus, we suspected that IFN-γ deficiency might impair the survival of mice subjected to immunization and challenge with PYF virus. The lack of increased susceptibility of GKO mice to fatal encephalitis compared with parental mice indicates that this cytokine is not critical for protection in this model. However, the higher virus burden during virus clearance compared with that of parental mice suggests a partial role for IFN-γ in antiviral defense, as has been seen in other models (39). This effect of IFN-γ is consistent with the requirement for CD4+ cells for control of the infection but is not sufficient to fully explain the requirement.
The detection of antigen-specific expression of IFN-γ in the CD4+ compartment of brain-associated T cells suggests that activation of a Th1 pathway is a primary feature of the protective immune response in the CNS. Concordant with this, IL-2 production from these cells could also be detected. The lack of increased production of IL-2, even upon nonspecific stimulation, suggests that its expression is downregulated after entry of T cells into the CNS, a phenomenon described by others (30). However, we emphasize caution in interpreting the significance of the in vitro cytokine production, since the requirements for eliciting T-cell cytokines by antigen-presenting cells within the brain are not fully known (63). Nevertheless, detection of IFN-γ in whole-brain extracts from mice with acute encephalitis (Fig. 4) does suggest that the in vitro data generated here probably represent authentic T-cell activation events.
Detection of IL-4 during in vitro stimulation of the lymphocytes provides evidence that a mixed pattern of intracerebral cytokine production is generated in the context of protection. It has been shown that the relative levels and temporal profiles of Th1 and Th2 cytokine gene expression in the mouse CNS can vary, despite an overall bias of the T helper response (70). Expression of Th2 cytokines, such as IL-4 and IL-10, which induce neuroprotective effects (2, 12, 33), may be required for regulation of inflammatory responses driven by IFN-γ (17), thereby preventing immune-mediated injury caused by perpetuation of a proinflammatory cytokine environment (15, 74). Since IL-4 production was not antigen specific, we suggest that different mechanisms may exist for regulating its production from recruited T cells than for the antigen-specific stimulation of IFN-γ from T cells which are involved in virus clearance. It is known, for instance, that Th1 and Th2 lymphocytes can exhibit different requirements for activation by antigen-presenting cells (18, 23, 68). Finally, we observed that production of IL-4 by cells from GKO mice exceeded that seen in parental mice, raising the possibility that this elevation compensates in some way for the deleterious effects of IFN-γ deficiency on virus clearance from the CNS.
Infiltration of T cells into the CNS in the context of inflammatory disease may or may not be accompanied by their proliferation in this compartment (4, 26, 29, 52). It has been suggested that inhibition of T-cell proliferation occurs in situations where the immune response is primarily protective, whereas expansion of antigen-specific T cells is characteristic of immunopathologic processes (29). We observed that brain-associated T cells which provide protective immunity against PYF infection were inhibited for in vitro proliferation but not cytokine secretion. These results are similar to what has been reported for Sindbis virus encephalitis (29) and presumably reflect a general feature of immunoregulation of T cells which enter the brain parenchyma. It is possible, however, that a low level of T-cell proliferation occurs, as in the case where recovery from the acute encephalitis is followed by prolonged retention of the cells (26). This dissociation of T-cell proliferation from cytokine secretion after entry into the brain may reflect differences in the signaling pathways which regulate these activation events, as it is known, for instance, that secretion of IFN-γ by cytotoxic T cells can be elicited in the absence of cellular proliferation (38). Restriction of the antigen-specific stimulation of T cells to cytokine production, without accompanying proliferation, is presumably a mechanism for limiting a harmful accumulation of inflammatory cells within the brain (30). Additional studies with this model of PYF encephalitis may be useful for further study of this question and for determining what T-cell functions are critical for induction of an antiviral state during infection of the CNS by flaviviruses.
ACKNOWLEDGMENTS
This work was supported by grants from the NIAID (AI-37646) and the Edward Mallinckrodt, Jr., Foundation.
REFERENCES
- 1.Ahara H, Takasaki T, Matsutani T, Suzuki R, Kurane I. Establishment and characterization of Japanese encephalitis virus-specific human CD4+ T-cell clones: flavivirus cross-reactivity, protein recognition, and cytotoxic activity. J Virol. 1998;72:8032–8036. doi: 10.1128/jvi.72.10.8032-8036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awatsuji H, Furukawa Y, Hirota M, Murakami Y, Nii S, Furukawa S, Hayashi K. Interleukin-4 and -5 as modulators of nerve growth factor synthesis/secretion in astrocytes. J Neurosci Res. 1993;34:539–545. doi: 10.1002/jnr.490340506. [DOI] [PubMed] [Google Scholar]
- 3.Barrett A D T, Gould E A. Comparison of neurovirulence of different strains of yellow fever virus in mice. J Gen Virol. 1986;67:631–637. doi: 10.1099/0022-1317-67-4-631. [DOI] [PubMed] [Google Scholar]
- 4.Barten D M, Clark R B, Ruddle N H. Presence of T cells with activated and memory phenotypes in inflammatory spinal cord lesions. J Immunol. 1995;155:5409–5418. [PubMed] [Google Scholar]
- 5.Benveniste E N. Cytokine expression in the nervous system. In: Keane R W, Hickey W F, editors. Immunology of the nervous system. New York, N.Y: Oxford University Press; 1997. pp. 419–459. [Google Scholar]
- 6.Bi Z, Barna M, Komatsu T, Reiss C S. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol. 1995;69:6466–6472. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradish C J, Fitzgeorge R, Titmuss D. The responses of normal and athymic mice to infections by togaviruses: strain differentiation in active and adoptive immunization. J Gen Virol. 1980;46:255–265. doi: 10.1099/0022-1317-46-2-255. [DOI] [PubMed] [Google Scholar]
- 8.Camenga D L, Nathanson N, Cole G A. Cyclophosphamide-potentiated West Nile viral encephalitis: relative influence of cellular and humoral factors. J Infect Dis. 1974;130:634–641. doi: 10.1093/infdis/130.6.634. [DOI] [PubMed] [Google Scholar]
- 9.Campbell I L, Hobbs M V, Kemper P, Oldstone M B A. Cerebral expression of multiple cytokine genes in mice with lymphocytic choriomeningitis. J Immunol. 1994;152:716–723. [PubMed] [Google Scholar]
- 10.Cantin E M, Hinton D R, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceredig R, Allan J E, Tabi Z, Lynch F, Doherty P C. Phenotypic analysis of the inflammatory exudate in murine lymphocytic choriomeningitis. J Exp Med. 1987;165:1539–1551. doi: 10.1084/jem.165.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao C C, Molitor T W, Hu S. Neuroprotective role of IL-4 against activated microglia. J Immunol. 1993;151:1473–1481. [PubMed] [Google Scholar]
- 13.Cole G A, Nathanson N. Potentiation of experimental arbovirus encephalitis by immunosuppressive doses of cyclophosphamide. Nature. 1968;220:399–401. doi: 10.1038/220399a0. [DOI] [PubMed] [Google Scholar]
- 14.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1745. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 15.DiProspero N A, Meiners S, Geller H M. Inflammatory cytokines interact to modulate extracellular matrix and astrocytic support of neurite outgrowth. Exp Neurol. 1997;148:628–639. doi: 10.1006/exnr.1997.6700. [DOI] [PubMed] [Google Scholar]
- 16.Dominguez C, Baruch E. Histopathology of the central nervous system in swiss mice intracerebrally inoculated with 17-D strain of yellow fever virus. Am J Trop Med Hyg. 1963;12:815–819. doi: 10.4269/ajtmh.1963.12.815. [DOI] [PubMed] [Google Scholar]
- 17.Dore-Duffy P, Balabanov R, Rafols J, Swanborg R H. Recovery phase of acute experimental autoimmune encephalomyelitis in rats corresponds to development of endothelial cell unresponsiveness to interferon gamma activation. J Neuorsci Res. 1996;44:223–234. doi: 10.1002/(SICI)1097-4547(19960501)44:3<223::AID-JNR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 18.Fabry Z, Sandor M, Gajewski T F, Herlein J A, Waldschmidt M M, Lynch R G, Hart M N. Differential activation of Th1 and Th2 CD4+ cells by murine brain microvessel endothelial cells and smooth muscle/pericytes. J Immunol. 1993;151:38–47. [PubMed] [Google Scholar]
- 19.Finke D, Liebert U G. CD4+ T cells are essential in overcoming experimental murine measles encephalitis. Immunology. 1994;83:184–189. [PMC free article] [PubMed] [Google Scholar]
- 20.Finke D, Brinkmann U G, ter Meulen V, Liebert U G. Gamma interferon is a major mediator of antiviral defense in experimental measles virus-induced encephalitis. J Virol. 1995;69:5469–5474. doi: 10.1128/jvi.69.9.5469-5474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flory E, Pfleiderer M, Stuhler A, Wege H. Induction of protective immunity against coronavirus-induced encephalomyelitis: evidence for an important role of CD8+ T cells in vivo. Eur J Immunol. 1993;23:1757–1761. doi: 10.1002/eji.1830230804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fung-Leung W-P, Schilham M W, Rahemtulla A, Kundig T M, Vollenweider M, Potter J, van Ewijk W, Mak T W. CD8 is needed for development of cytotoxic T cells but not T helper cells. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 23.Gajewski T F, Schell S R, Fitch F W. Evidence implicating utilization of different T cell receptor-associated signaling pathways by TH1 and TH2 clones. J Immunol. 1990;144:4110–4120. [PubMed] [Google Scholar]
- 24.Geiger K D, Nash T C, Sawyer S, Krahl T, Patstone G, Reed J C, Krajewski S, Dalton D, Buchmeier M J, Sarvetnick N. Interferon-γ protects against herpes simplex virus type 1-mediated neuronal death. Virology. 1997;238:189–197. doi: 10.1006/viro.1997.8841. [DOI] [PubMed] [Google Scholar]
- 25.Hase T, Dubois D R, Summers P L. Comparative study of mouse brains infected with Japanese encephalitis virus by intracerebral or intraperitoneal inoculation. Int J Exp Pathol. 1990;71:857–869. [PMC free article] [PubMed] [Google Scholar]
- 26.Hawke S, Stevenson P G, Freeman S, Bangham C R M. Long-term persistence of activated cytotoxic T lymphocytes after viral infection of the central nervous system. J Exp Med. 1998;187:1575–1582. doi: 10.1084/jem.187.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch M S, Murphy F A. Effects of anti-thymocyte serum on 17-D yellow fever infection in adult mice. Nature. 1967;216:179–180. doi: 10.1038/216179a0. [DOI] [PubMed] [Google Scholar]
- 28.Hunneycutt B S, Bi Z, Aoki C J, Reiss C S. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. J Virol. 1993;67:6698–6706. doi: 10.1128/jvi.67.11.6698-6706.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irani D N, Lin K-I, Griffin D E. Brain-derived gangliosides regulate the cytokine production and proliferation of activated T cells. J Immunol. 1996;157:4333–4340. [PubMed] [Google Scholar]
- 30.Irani D N, Lin K-I, Griffin D E. Regulation of brain-derived T cells during acute central nervous system inflammation. J Immunol. 1997;158:2318–2326. [PubMed] [Google Scholar]
- 31.Jacoby R O, Bhatt P N, Schwartz A. Protection of mice from lethal flavivirus encephalitis by adoptive transfer of splenic cells from donors infected with live virus. J Infect Dis. 1980;141:617–624. doi: 10.1093/infdis/141.5.617. [DOI] [PubMed] [Google Scholar]
- 32.Johnson R T, Burke D S, Elwell M, Leake C J, Nisalak A, Hoke C H, Lorsomrudee W. Japanese encephalitis: immunocytochemical studies of viral antigen and inflammatory cells in fatal cases. Ann Neurol. 1985;18:567–573. doi: 10.1002/ana.410180510. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy M K, Torrance D S, Picha K S, Mohler K M. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- 34.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 35.Konishi E, Kurane I, Mason P W, Shope R E, Ennis F A. Poxvirus-based Japanese encephalitis vaccine candidates induce JE virus-specific CD8+ cytotoxic T lymphocytes in mice. Virology. 1997;227:353–360. doi: 10.1006/viro.1996.8331. [DOI] [PubMed] [Google Scholar]
- 36.Konishi E, Yamaoka M, Win K-S, Kurane I, Takada K, Mason P W. The anamnestic neutralizing antibody response is critical for protection of mice from challenge following vaccination with a plasmid encoding the Japanese encephalitis virus premembrane and envelope genes. J Virol. 1999;73:5527–5534. doi: 10.1128/jvi.73.7.5527-5534.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kundig T M, Hengartner H, Zinkernagel R M. T cell-dependent interferon-γ exerts an antiviral effect in the central nervous system but not in peripheral solid organs. J Immunol. 1993;150:2316–2321. [PubMed] [Google Scholar]
- 38.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V S, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lane T E, Paoletti A D, Buchmeier M J. Dissociation between the in vitro and in vivo effects of nitric oxide on a neurotropic murine coronavirus. J Virol. 1997;71:2202–2210. doi: 10.1128/jvi.71.3.2202-2210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leist T P, Eppler M, Zinkernagel R M. Enhanced virus replication and inhibition of lymphocytic choriomeningitis virus disease in anti-gamma interferon-treated mice. J Virol. 1989;63:2813–2819. doi: 10.1128/jvi.63.6.2813-2819.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levenbook I S, Pelleu L J, Elisberg B L. The monkey safety test for neurovirulence of yellow fever vaccines: the utility of quantitative clinical evaluation and histological examination. J Biol Stand. 1987;15:305–313. doi: 10.1016/s0092-1157(87)80003-3. [DOI] [PubMed] [Google Scholar]
- 42.Lindsley M D, Thiemann R, Rodriguez M. Cytotoxic T cells isolated from the central nervous system of mice infected with Theiler's virus. J Virol. 1991;65:6612–6620. doi: 10.1128/jvi.65.12.6612-6620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Blanden R V, Mullbacher A. Identification of cytolytic lymphocytes in West Nile virus-infected murine central nervous system. J Gen Virol. 1989;70:565–573. doi: 10.1099/0022-1317-70-3-565. [DOI] [PubMed] [Google Scholar]
- 44.Miura K, Onodera T, Nishida A, Goto N, Fujisaki Y. A single gene controls resistance to Japanese encephalitis virus in mice. Arch Virol. 1990;112:261–270. doi: 10.1007/BF01323170. [DOI] [PubMed] [Google Scholar]
- 45.Miyake M. The pathology of Japanese encephalitis. Bull W H O. 1964;30:153–160. [PMC free article] [PubMed] [Google Scholar]
- 46.Moench T R, Griffin D E. Immunocytochemical identification and quantitation of the mononuclear cells in cerebrospinal fluid, meninges and brain during acute viral meningoencephalitis. J Exp Med. 1984;159:77–88. doi: 10.1084/jem.159.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monath T P. Pathobiology of the flaviviruses. In: Schlesinger S, Schlesinger M J, editors. The Togaviridae and the Flaviviridae. New York, N.Y: Plenum Press; 1986. pp. 375–440. [Google Scholar]
- 48.Murali-Krishna K, Ravi V, Manjunath R. Cytotoxic T lymphocytes raised against Japanese encephalitis virus: effector cell phenotype, target specificity and in vitro virus clearance. J Gen Virol. 1994;75:799–807. doi: 10.1099/0022-1317-75-4-799. [DOI] [PubMed] [Google Scholar]
- 49.Murali-Krishna K, Ravi V, Manjunath R. Protection of adult but not newborn mice against lethal intracerebral challenge with Japanese encephalitis virus by adoptively transferred virus-specific cytotoxic T lymphocytes: requirement for L3T4+ T cells. J Gen Virol. 1996;77:705–714. doi: 10.1099/0022-1317-77-4-705. [DOI] [PubMed] [Google Scholar]
- 50.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 51.Ogata A, Nagashima K, Hall W W, Ichikawa M, Kimura-Kuroda J, Yasui K. Japanese encephalitis virus neurotropism is dependent on the degree of neuronal maturity. J Virol. 1991;65:880–886. doi: 10.1128/jvi.65.2.880-886.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohmori K, Hong Y, Fujiwara M, Matsumoto Y. In situ demonstration of proliferating cells in the rat central nervous system during experimental autoimmune encephalomyelitis. Lab Investig. 1992;66:54–62. [PubMed] [Google Scholar]
- 53.Pearce B D, Hobbs M V, McGraw T S, Buchmeier M J. Cytokine induction during T-cell-mediated clearance of mouse hepatitis virus from neurons in vivo. J Virol. 1994;68:5483–5495. doi: 10.1128/jvi.68.9.5483-5495.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Popko B, Corbin J G, Baerwald K D, Dupree J, Garcia A M. The effects of interferon-gamma on the central nervous system. Mol Neurobiol. 1997;14:19–35. doi: 10.1007/BF02740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahemtulla A, Fung-Leung W P, Schillham M W, Kundig T M, Sambhara S R, Narendran A, Arabian A, Wakeham A, Paige C J, Zinkernagel R M, Miller R G, Mak T W. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 56.Reich A, Erlwein O, Niewiesk S, ter Meulen V, Liebert U G. CD4+ T cells control measles virus infection of the central nervous system. Immunology. 1992;76:185–191. [PMC free article] [PubMed] [Google Scholar]
- 57.Richt J A, Stitz I, Wekerle H, Rott R. Borna disease, a progressive meningoencephalomyelitis as a model for CD4+ T cell-mediated immunopathology. J Exp Med. 1989;170:1045–1050. doi: 10.1084/jem.170.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sangster M Y, Urosevic N, Mansfield J P, Mackenzie J S, Shellam G R. Mapping the Flv locus controlling resistance to flaviviruses on mouse chromosome 5. J Virol. 1994;68:448–452. doi: 10.1128/jvi.68.1.448-452.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schlesinger J J, Brandriss M W, Walsh E E. Protection against 17D yellow fever encephalitis in mice by passive transfer of monoclonal antibodies to the nonstructural glycoprotein gp48 and by active immunization with gp48. J Immunol. 1985;135:2805–2809. [PubMed] [Google Scholar]
- 60.Schlesinger J J, Foltzer M, Chapman S. The Fc portion of antibody to yellow fever virus NS1 is a determinant of protection against YF encephalitis in mice. Virology. 1993;192:132–141. doi: 10.1006/viro.1993.1015. [DOI] [PubMed] [Google Scholar]
- 61.Schlesinger J J, Chapman S, Nestorowicz A, Rice C M, Ginocchio T E, Chambers T J. Replication of yellow fever virus in the mouse central nervous system: comparison of neuroadapted and non-neuroadapted virus and partial sequence analysis of the neuroadapted strain. J Gen Virol. 1996;77:1277–1285. doi: 10.1099/0022-1317-77-6-1277. [DOI] [PubMed] [Google Scholar]
- 62.Schneider-Schaulies J, Liebert U G, Dorries R, ter Meullen V. Establishment and control of viral infections of the central nervous system. In: Keane R W, Hickey W F, editors. Immunology of the nervous system. New York, N.Y: Oxford University Press; 1997. pp. 576–610. [Google Scholar]
- 63.Sedgwick J D, Hickey W F. Antigen presentation in the central nervous system. In: Keane R W, Hickey W F, editors. Immunology of the nervous system. New York, N.Y: Oxford University Press; 1997. pp. 364–418. [Google Scholar]
- 64.Shankar V, Kao M, Hamir A N, Sheng H, Koprowski H, Dietzschold B. Kinetics of virus spread and changes in levels of several cytokine mRNAs in the brain after intranasal infection of rats with Borna disease virus. J Virol. 1992;66:992–998. doi: 10.1128/jvi.66.2.992-998.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stevenson P G, Hawke S, Bangham C R M. Protection against lethal influenza-virus encephalitis by intranasally primed CD8+ memory T cells. J Immunol. 1996;157:3065–3073. [PubMed] [Google Scholar]
- 66.Sugamata M, Miyazawa M, Mori S, Spangrude G J, Ewalt L C, Lodmell D L. Paralysis of street rabies virus-infected mice is dependent on T lymphocytes. J Virol. 1992;66:1252–1260. doi: 10.1128/jvi.66.2.1252-1260.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vince V, Grcevic N. Development of morphological changes in experimental tick-borne meningoencephalitis induced in mice by different virus doses. J Neurol Sci. 1969;9:109–130. doi: 10.1016/0022-510x(69)90064-1. [DOI] [PubMed] [Google Scholar]
- 68.Weaver C T, Hawrylowicz C M, Unanue E R. T helper cell subsets require the expression of distinct costimulatory signals by antigen-presenting cells. Proc Natl Acad Sci. 1988;85:8181–8185. doi: 10.1073/pnas.85.21.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weinberg A D, Wallin J J, Jones R E, Sullivan T J, Bourdette D N, Vandenbark A A, Offner H. Target organ-specific up-regulation of the MRC OX-40 marker and selective production of Th1 lymphokine mRNA by encephalitogenic T helper cells isolated from the spinal cord of rats with experimental autoimmune encephalomyelitis. J Immunol. 1994;152:4712–4721. [PubMed] [Google Scholar]
- 70.Wesselingh S L, Levine B, Fox R J, Choi S, Griffin D E. Intracerebral cytokine mRNA expression during fatal and nonfatal alphavirus encephalitis suggests a predominant type 2 T cell response. J Immunol. 1994;152:1289–1297. [PubMed] [Google Scholar]
- 71.Whitton J L. Lymphocytic choriomeningitis virus CTL. Semin Virol. 1990;1:257–262. [Google Scholar]
- 72.Williamson J S P, Stohlman S A. Effective clearance of mouse hepatitis virus from the central nervous system requires both CD4+ and CD8+ T cells. J Virol. 1990;64:4589–4592. doi: 10.1128/jvi.64.9.4589-4592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williamson J S P, Sykes K C, Stohlman S A. Characterization of brain-infiltrating mononuclear cells during infection with mouse hepatitis virus strain JHM. J Neuroimmunol. 1991;32:199–207. doi: 10.1016/0165-5728(91)90189-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yong V W, Moumdjian R, Yong F P, Ruijs T C G, Freedman M S, Cashman N, Antel J P. γ-Interferon promotes proliferation of adult human astrocytes in vitro and reactive gliosis in the adult mouse brain in vivo. Proc Natl Acad Sci USA. 1991;88:7016–7020. doi: 10.1073/pnas.88.16.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu M, Johnson J M, Tuohy V K. Generation of autonomously pathogenic neo-autoreactive TH1 cells during the development of the determinant spreading cascade in murine autoimmune encephalomyelitis. J Neurosci Res. 1996;45:463–470. doi: 10.1002/(SICI)1097-4547(19960815)45:4<463::AID-JNR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 76.Zarozinski C C, Welsh R M. Minimal bystander activation of CD8+ T cells during the virus-induced polyclonal T cell response. J Exp Med. 1997;185:1629–1639. doi: 10.1084/jem.185.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zimmerman C, Brduscha-Riem K, Blaser C, Zinkernagel R M, Pircher H. Visualization, characterization and turnover of CD8+ memory T cells in virus-infected hosts. J Exp Med. 1996;183:1367–1375. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]