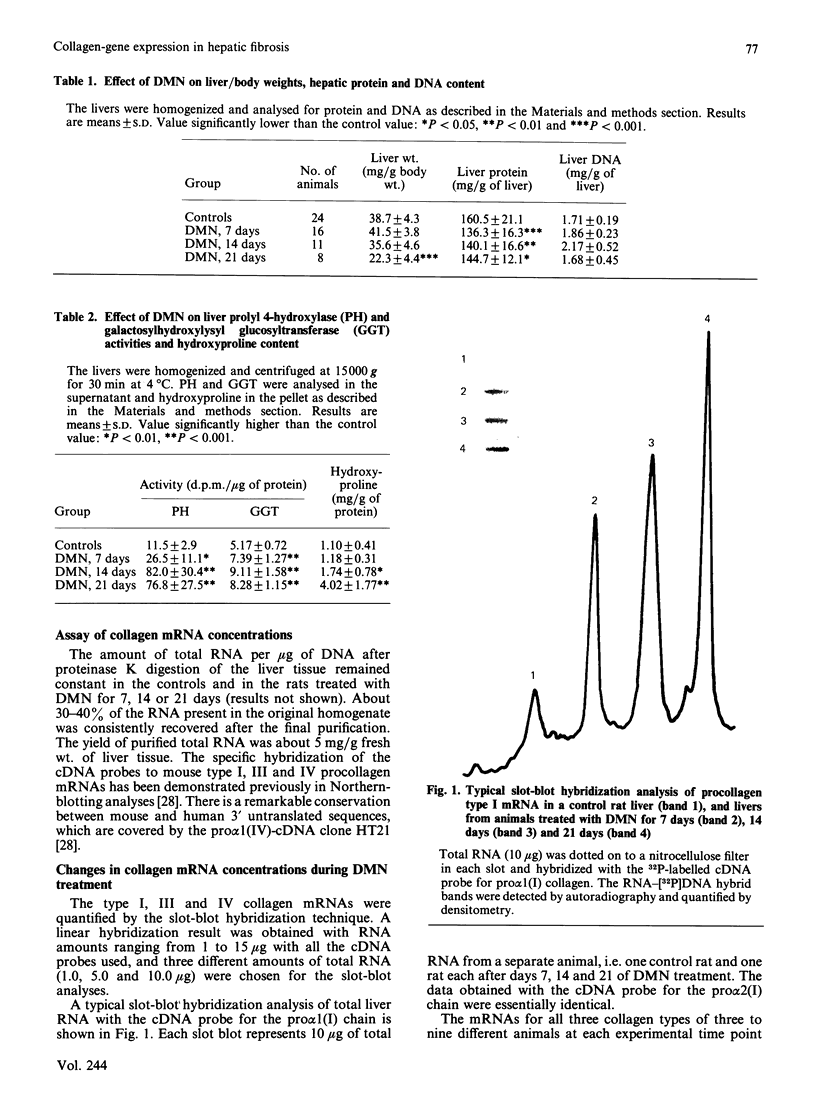
Abstract
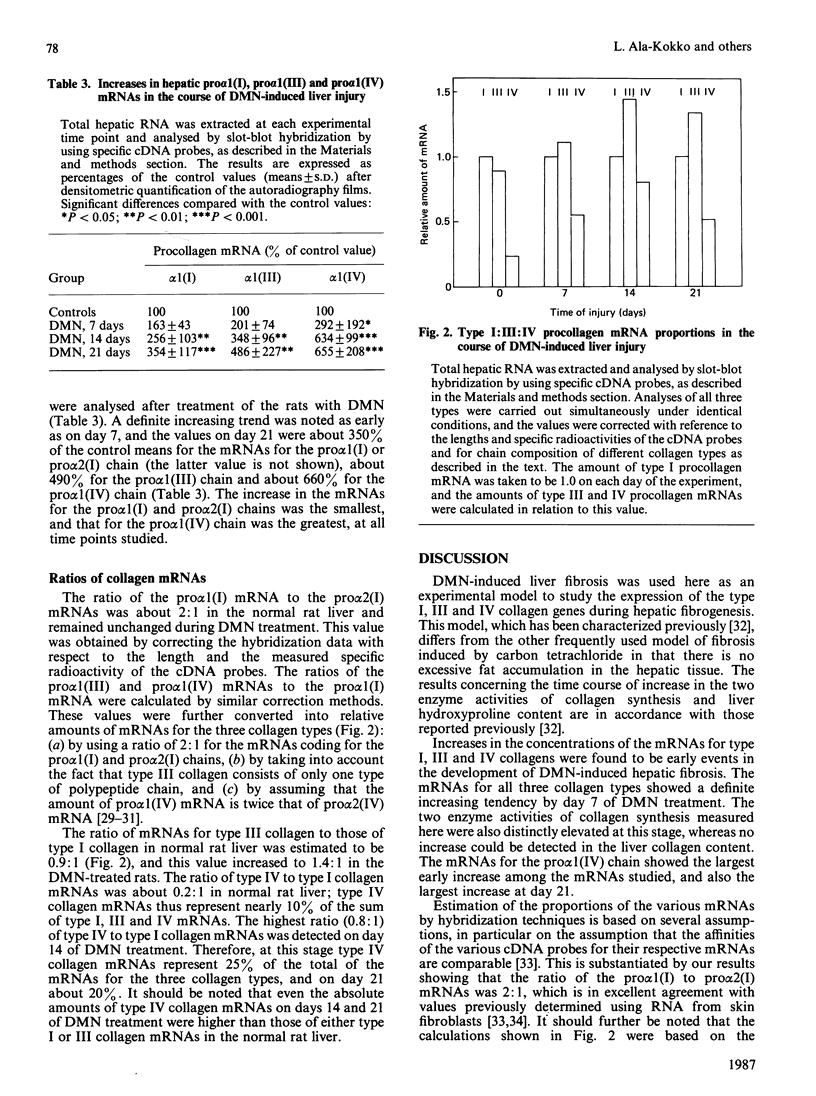
Dimethylnitrosamine (DMN)-induced hepatic fibrosis was used as an experimental model to study collagen-gene expression during liver fibrogenesis. Increase in the concentrations of the mRNAs for type I, III, and IV collagens was found to be an early event in the development of hepatic fibrosis, as the mRNAs for all three collagen types showed a definite increasing tendency by day 7 of DMN treatment. Prolyl 4-hydroxylase (EC 1.14.11.2) and galactosylhydroxylysyl glucosyltransferase (EC 2.4.1.66) activities were also distinctly elevated at this stage, whereas no increase could be detected in the liver collagen content. The increase in the mRNAs for type I collagen was the smallest and that for type IV collagen the greatest at all the time points studied. The relative concentrations of the mRNAs for the three collagen types on day 21 of DMN treatment were 350% of the control mean for type I collagen, 490% for type III and 660% for type IV. The data further indicate that the proportions of the mRNAs for the three collagen types are 1.0:0.9:0.2 in normal rat liver, 1.0:1.4:0.8 on day 14 of DMN treatment, and 1.0:1.3:0.5 on day 21. The early marked increase in the mRNA for type IV collagen suggests that enhanced production of basement-membrane collagen may be an early event in the development of hepatic fibrosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter E. A., McCarron M. J., Alpert E., Isselbacher K. J. Lysyl oxidase and collagenase in experimental acute and chronic liver injury. Gastroenterology. 1982 Mar;82(3):526–534. [PubMed] [Google Scholar]
- Galambos J. T., Shapira R. Natural history of alcoholic hepatitis. IV. Glycosaminoglycuronans and collagen in the hepatic connective tissue. J Clin Invest. 1973 Nov;52(11):2952–2962. doi: 10.1172/JCI107492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R. W., Matthew W. T., Dubowik D. A. Factors influencing the determination of DNA with indole. Anal Biochem. 1970 Nov;38(1):190–201. doi: 10.1016/0003-2697(70)90169-7. [DOI] [PubMed] [Google Scholar]
- Hämäläinen L., Oikarinen J., Kivirikko K. I. Synthesis and degradation of type I procollagen mRNAs in cultured human skin fibroblasts and the effect of cortisol. J Biol Chem. 1985 Jan 25;260(2):720–725. [PubMed] [Google Scholar]
- Kivirikko K. I., Laitinen O., Prockop D. J. Modifications of a specific assay for hydroxyproline in urine. Anal Biochem. 1967 May;19(2):249–255. doi: 10.1016/0003-2697(67)90160-1. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Collagen glycosyltransferases. Int Rev Connect Tissue Res. 1979;8:23–72. doi: 10.1016/b978-0-12-363708-6.50008-4. [DOI] [PubMed] [Google Scholar]
- Kresina T. F., Miller E. J. Isolation and characterization of basement membrane collagen from human placental tissue. Evidence for the presence of two genetically distinct collagen chains. Biochemistry. 1979 Jul 10;18(14):3089–3097. doi: 10.1021/bi00581a028. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindblad W. J., Fuller G. C. Hepatic collagenase activity during carbon tetrachloride induced fibrosis. Fundam Appl Toxicol. 1983 Jan-Feb;3(1):34–40. doi: 10.1016/s0272-0590(83)80170-5. [DOI] [PubMed] [Google Scholar]
- Loidl H. R., Brinker J. M., May M., Pihlajaniemi T., Morrow S., Rosenbloom J., Myers J. C. Molecular cloning and carboxyl-propeptide analysis of human type III procollagen. Nucleic Acids Res. 1984 Dec 21;12(24):9383–9394. doi: 10.1093/nar/12.24.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Feinman L., Fainsilber Z., Nakano M., Okazaki I., Lieber C. S. Mammalian collagenase increases in early alcoholic liver disease and decreases with cirrhosis. Life Sci. 1982 Apr 19;30(16):1379–1384. doi: 10.1016/0024-3205(82)90023-6. [DOI] [PubMed] [Google Scholar]
- Mayne R., Zettergren J. G. Type IV collagen from chicken muscular tissues. Isolation and characterization of the pepsin-resistant fragments. Biochemistry. 1980 Aug 19;19(17):4065–4072. doi: 10.1021/bi00558a025. [DOI] [PubMed] [Google Scholar]
- Montfort I., Pérez-Tamayo R. Collagenase in experimental carbon tetrachloride cirrhosis of the liver. Am J Pathol. 1978 Aug;92(2):411–420. [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Brinker J. M., Kefalides N. A., Rosenbloom J., Wang S. Y., Gudas L. J. Discrimination among multiple AATAAA sequences correlates with interspecies conservation of select 3' untranslated nucleotides. Nucleic Acids Res. 1986 Jun 11;14(11):4499–4517. doi: 10.1093/nar/14.11.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Chu M. L., Faro S. H., Clark W. J., Prockop D. J., Ramirez F. Cloning a cDNA for the pro-alpha 2 chain of human type I collagen. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3516–3520. doi: 10.1073/pnas.78.6.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllylä R., Risteli L., Kivirikko K. I. Assay of collagen-galactosyltransferase and collagen-glucosyltransferase activities and preliminary characterization of enzymic reactions with transferases from chick-embryo cartilage. Eur J Biochem. 1975 Apr 1;52(3):401–410. doi: 10.1111/j.1432-1033.1975.tb04008.x. [DOI] [PubMed] [Google Scholar]
- Oikarinen J., Ryhänen L. Cortisol decreases the concentration of translatable type-I procollagen mRNA species in the developing chick-embryo calvaria. Biochem J. 1981 Sep 15;198(3):519–524. doi: 10.1042/bj1980519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajaniemi T., Tryggvason K., Myers J. C., Kurkinen M., Lebo R., Cheung M. C., Prockop D. J., Boyd C. D. cDNA clones coding for the pro-alpha1(IV) chain of human type IV procollagen reveal an unusual homology of amino acid sequences in two halves of the carboxyl-terminal domain. J Biol Chem. 1985 Jun 25;260(12):7681–7687. [PubMed] [Google Scholar]
- Risteli J., Kivirikko K. I. Intracellular enzymes of collagen biosynthesis in rat liver as a function of age and in hepatic injury induced by dimethylnitrosamine. Changes in prolyl hydroxylase, lysyl hydroxylase, collagen galactosyltransferase and collagen glucosyltransferase activities. Biochem J. 1976 Aug 15;158(2):361–367. doi: 10.1042/bj1580361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risteli J., Tuderman L., Kivirikko K. I. Intracellular enzymes of collagen biosynthesis in rat liver as a function of age and in hepatic injury induced by dimethylnitrosamine. Purification of rat prolyl hydroxylase and comparison of changes in prolyl hydroxylase activity with changes in immunoreactive prolyl hydroxylase. Biochem J. 1976 Aug 15;158(2):369–376. doi: 10.1042/bj1580369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojkind M., Pérez-Tamayo R. Liver fibrosis. Int Rev Connect Tissue Res. 1983;10:333–393. doi: 10.1016/b978-0-12-363710-9.50012-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstoshev P., Haber R., Trapnell B. C., Crystal R. G. Procollagen messenger RNA levels and activity and collagen synthesis during the fetal development of sheep lung, tendon, and skin. J Biol Chem. 1981 Sep 25;256(18):9672–9679. [PubMed] [Google Scholar]
- Trüeb B., Gröbli B., Spiess M., Odermatt B. F., Winterhalter K. H. Basement membrane (type IV) collagen is a heteropolymer. J Biol Chem. 1982 May 10;257(9):5239–5245. [PubMed] [Google Scholar]
- Zern M. A., Leo M. A., Giambrone M. A., Lieber C. S. Increased type I procollagen mRNA levels and in vitro protein synthesis in the baboon model of chronic alcoholic liver disease. Gastroenterology. 1985 Nov;89(5):1123–1131. doi: 10.1016/0016-5085(85)90219-7. [DOI] [PubMed] [Google Scholar]
- Zern M. A., Saber M. A., Shafritz D. A. Molecular mechanisms for changes in hepatic protein synthesis induced by schistosomiasis infection in mice. Biochemistry. 1983 Dec 20;22(26):6072–6077. doi: 10.1021/bi00295a005. [DOI] [PubMed] [Google Scholar]
- de Wet W. J., Chu M. L., Prockop D. J. The mRNAs for the pro-alpha 1(I) and pro-alpha 2(I) chains of type I procollagen are translated at the same rate in normal human fibroblasts and in fibroblasts from two variants of osteogenesis imperfecta with altered steady state ratios of the two mRNAs. J Biol Chem. 1983 Dec 10;258(23):14385–14389. [PubMed] [Google Scholar]