Abstract
Background
Sickle cell disease (SCD) is a genetic blood disorder characterized by a painful vaso-occlusive crisis due to the sickling of red blood cells in capillaries. Complications often lead to liver and renal dysfunctions, contributing to morbidity and mortality, particularly for children under 5. This systematic review and meta-analysis aimed to evaluate the liver and renal functions of people with SCD (HbSS) compared to those without it (HbAA) in Africa.
Methods
The protocol was registered with PROSPERO (CRD42022346771). We searched PubMed, Embase, Web of Science, and Google Scholar using the keywords “liver function”, “renal function”, “sickle cell disease”, and “Africa” on 6th May 2023 for peer-reviewed articles with abstracts in English. We included case-control studies comparing SCD (HbSS) with controls without hemoglobinopathies (HbAA). We used the random-effect model to calculate the pooled average values for the blood tests of people with SCD in RStudio version 4.2.2.
Results
Overall, 17 articles were analyzed from five African countries involving 1312 people with SCD and 1558 controls. The pooled mean difference of liver enzymes aspartate transaminase (AST) was 8.62 (95% CI − 2.99–20.23, I2 = 97.0%, p < 0.01), alanine transaminase (ALT) 7.82 (95% CI − 0.16–15.80, I2 = 99%, p < 0.01) and alkaline phosphatase (ALP) − 2.54 (95% CI − 64.72 – 59.64, I2 = 99%, p < 0.01) compared to controls. The pooled mean difference for the renal biochemical profiles creatinine − 3.15 (95% CI − 15.02; 8.72, I2=99%, p < 0.01) with a funnel plot asymmetry of t = 1.09, df = 9, p = 0.3048 and sample estimates bias of 6.0409. The pooled mean difference for serum urea was − 0.57 (95% CI − 3.49; 2.36, I2 = 99%, p < 0.01), and the estimated glomerular filtration (eGFR) rate was 19.79 (95% CI 10.89–28.68 mL/min/1.73 m2, I2 = 87%, p < 0.01) compared to controls.
Conclusion
People with SCD have slightly elevated liver enzymes and estimated glomerular filtration rates compared to controls in Africa. With all the heterogeneity (I2) > 50%, there was substantial variation in the reported articles’ results.
Systematic review registration
PROSPERO CRD42022346771
Supplementary Information
The online version contains supplementary material available at 10.1186/s13643-024-02662-6.
Keywords: Sickle cell, Liver function, Renal function, Systematic review, Africa
Introduction
Sickle cell disease (SCD) is an autosomal recessive disease affecting normal β-globin function [1, 2]. It results from a point mutation in the short arm of chromosome 11, where the amino acid, glutamic acid, is replaced by valine at position 6 of the β-globin chain [1, 2]. That reduces the flexibility of the red blood cells under low oxygen tension in end organs, leading to the “sickling” of the red blood cells [1, 2]. Acute vaso-occlusive pain is caused by the entrapment of erythrocytes in the microcirculation, causing vascular obstruction and tissue ischemia [3]. Vaso-occlusion and ischemia of major organs may lead to life-threatening multi-organ failure [3–5]. Renal dysfunction may present as glomerular hyperfiltration and proteinuria [6–18], while liver dysfunction includes elevated liver enzymes, especially alanine transaminase (ALT) and aspartate transaminase (AST) [7, 11, 19–22].
SCD can cause progressive injury to the liver with significant fibrosis and decreased liver function by adulthood [5, 23]. The tissue ischemia experienced during vaso-occlusive crises also affects their major organs, explaining some SCD symptoms [3, 5]. Asymptomatic patients may have hepatomegaly and elevated liver enzyme levels [3, 5]. This is not affected by sex or gender [24]. Elevated liver enzymes may also signify viral hepatitis acquired from multiple transfusions [3, 5, 19, 23, 24]. However, most patients with SCD are given life-long treatment; therefore, drug toxicity-related liver injury cannot be excluded [25–27].
Hypoxia, acidosis, and hypertonicity resulting from multiple vaso-occlusive crises promote sickling, leading to endothelial injury and more vaso-occlusion [4]. The resultant renal medullary ischemia and infarction lead to a gradual loss of glomerular function [4]. The defective proximal tubular function may result in increased creatinine clearance due to increased creatinine secretion, therefore increasing the estimated glomerular filtration rate (eGFR) [4], especially in children [3, 4, 28]. This may also explain the reduced serum creatinine and normal urea levels in sickle cell disease [4]. Prolonged use of hydroxyurea was found to reduce glomerular hyperfiltration rate and protect against multi-organ damage in sickle cell patients [29, 30]. However, people with SCD are advised to stay hydrated always as part of their prophylaxis against vaso-occlusive crises. Their good hydration status may also result in good glomerular perfusion leading to hyperfiltration compared to controls [31]. Focal glomerular injury may progress to renal failure with age [4].
Approximately five percent of the world’s population carries the genes for hemoglobin disorders, with SCD being the most common among them [32, 33]. The prevalence of sickle cell genes is higher in sub-Saharan Africa, with 20–30% in western and eastern Africa [32, 33]. It is a major cause of mortality for every age group [34–36].
The Western and Eastern African people are known to consume foods with antisickling activities [37, 38]. Perhaps these foods modify the course of SCD and enable a higher chance of survival beyond 5 years of age. In Nigeria, niprisan (made from pepper seeds, clove flower buds, caprium stem, sorghum leaves, and “trona”) [38] and Cajanus cajan (pigeon peas) [37] are foods with antisickling properties commonly used to treat SCD [37, 38]. Therefore, this systematic review and meta-analysis evaluated the liver and renal function biochemical profiles of people with SCD in Africa.
Methods
Following registration in PROSPERO: CRD42022346771, we conducted this review according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines for systematic reviews and meta-analysis [39] and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guideline [40]. A review question generated using the Condition, Context, and Population (CoCoPop) guidelines by the Joanna Briggs Institute [41] was used. The condition was “liver function test” and “renal function test”; the context was “Africa,” and the population was “individuals living with SCD.” Therefore, the research question was, “What are the renal and liver biochemical profiles of people living with SCD (HbSS) compared to controls (HbAA) in Africa?” The following keywords were used to identify potential articles on 6th May 2023 from PubMed, Embase (OVID), Web of Science, and Google Scholar: “sickle cell”, “liver function test”, “renal function test”, and “Africa”. The search included papers from inception to 6th May 2023. The search strategy is presented in Supplementary file 1.
All identified articles were entered into Endnote for screening and removal of duplicates. The screen adhered to the following criteria: (i) observational studies reporting liver or renal function tests of people with SCD in Africa, (ii) in all languages (with abstracts in English), and (iii) peer-reviewed. Case reports, editorial, and qualitative studies were excluded. Two authors independently screened the title and abstract of each article, and in case of any discrepancies, the third author made the final decision by consensus. The same team followed the same procedure for full-text review, risk of bias, and data entry. All articles were assessed using the ROBINS-E, a tool for assessing the risk of bias in non-randomized studies of exposure [42]. The data extracted from each article included sample size, number of individuals with SCD tested for liver and renal function and their controls, study design, and study setting (country and city).
Statistical analysis
Data analysis was performed using RStudio version 4.2.2. Numerical continuous variables were summarized as mean and standard deviation. Heterogeneity across individual studies was assessed using Higgins’s inconsistency Q statistics and reported as I2 and p value. A random-effect model meta-analysis was performed for pooled outcomes with I2 of > 50%. The regression-based Egger test and visual evaluation of contour-enhanced funnel plots were assessed for small-study effects and risk of bias.
Results
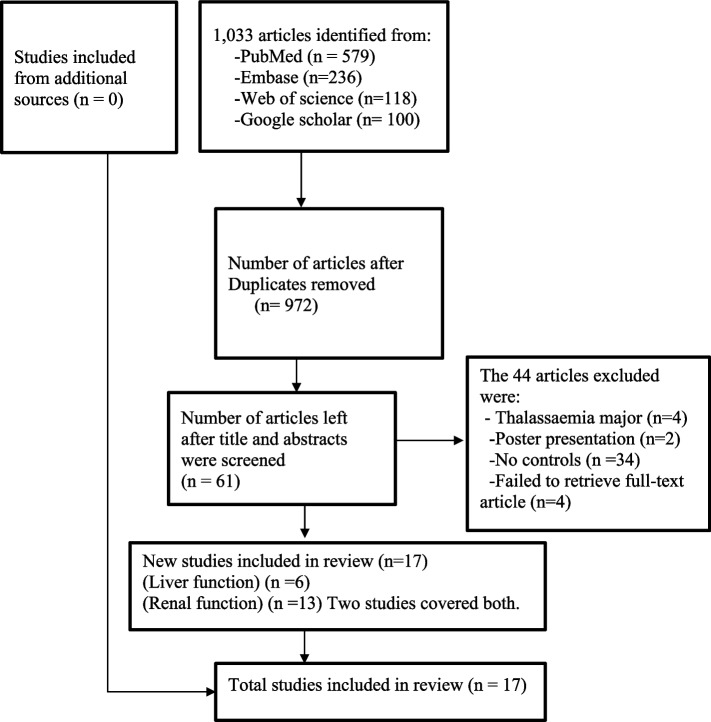
One thousand thirty-three articles were identified. Of this, 1016 were excluded, and only seventeen were included in the final synthesis. A summary of the data extraction processes is outlined in Fig. 1.
Fig. 1.
Flow diagram for data selection. Adapted from The PRISMA 2020 statement: an updated guideline for reporting systematic reviews [40]
Characteristics of excluded studies
We failed to retrieve the full-text article for two studies. The other excluded studies had no abstracts or full-text available, no results for controls, poster presentations, beta thalassemia studies, only sickle cell traits (HbAS) without HbSS or studies of HbSS without HbAA controls for comparison, as shown in the Supplementary file 2.
Characteristics of included studies
Most of the studies were conducted in Nigeria (n = 08). All the studies were observational studies with low sample sizes (ranging between 40 and 340). Liver and renal function tests are shown in Tables 1 and 2, respectively. Different authors used different units of measurement for the tests. For example, some authors used IU/L (international unit per liter of blood) [7, 19], µmol/L [20], and others used µkat/L (microunit per liter) [11] to measure liver enzymes. The final results reflected here were converted to most units used by other authors using an online tool at ScyMed® [43]. Details are found in Supplementary file 4. All liver enzyme units were converted to IU/L.
Table 1.
Liver function tests of people with sickle cell disease in Africa
| Authors | Setting | Study design | SCD/controls | Liver function |
|---|---|---|---|---|
| Asaolu et al. 2010 | Ekiti, Nigeria | Prospective case-control study | 42/42 | ALP mean = 133.23, sd = 61.69; ALT mean = 28.68, sd = 9.85; AST mean = 20.42, sd = 10.94 [7] |
| Obi et al. 2020 | Benin City, Nigeria | Prospective case-control study | 50/50 | ALT mean = 29.64, sd = 13.32; AST mean = 42.14, sd = 25.10 [21] |
| Mohamed et al. 1992 | Khartoum, Sudan | Prospective case-control study | 84/28 | ALT mean = 93.37, sd = 37.95; AST mean = 37.70, sd = 21.50 [11] |
| Akuyam et al. 2017 | Zaria, Nigeria | Prospective case-control study | 100/100 | ALP mean=108.8, sd = 5.40; ALT mean = 34.87, sd = 17.94; AST mean = 40.98, sd = 19.81 [19] |
| Saha 1982 | Khartoum, Sudan | Prospective case-control study | 5/320 | ALT mean = 33.00, sd = 2.51; AST mean = 35.00, sd = 2.32 [20] |
| Yahaya 2012 | Kano, Nigeria | Prospective case-control study | 150/100 | ALP mean = 82.67, sd = 10.87; ALT mean = 41.00, sd = 1.80; AST mean = 20.00, sd = 0.90 [22] |
ALP alkaline phosphatase, sd standard deviation, ALT alanine transaminase, AST aspartate transaminase
Table 2.
Renal function tests of people with sickle cell disease in Africa
| Authors | Setting | Study design | Sample size SCD/controls | Renal function |
|---|---|---|---|---|
| Kambale-Kombi et al 2022 | Kisangani, D R Congo | Prospective case-control study | 98/89 |
creatinine mean = 52.59, sd = 18.59; urea mean = 5.05, sd = 1.82; eGFR mean = 119.90, sd = 53.78 [10] |
| Ndour et al. 2022 | Dakar, Senegal | Prospective case-control study | 163/177 |
creatinine mean = 52.17, sd = 20.34; urea mean = 7.00, sd = 4.50 [12] |
| Olawale et al. 2021 | South-west, Nigeria | Prospective case-control study | 150/50 |
creatinine mean = 47.75, sd = 0.88; urea mean = 16.80, sd = 1.30; eGFR mean = 122.50, sd = 9.00 [16] |
| Suliman et al. 2020 | Khartoum, Sudan | Prospective case-control study | 32/23 |
creatinine mean = 57.47, sd = 15.92; urea mean = 21.60, sd = 8.40; eGFR mean = 149.70, sd = 29.60 [17] |
| Nnaji et al. 2020 | Owerri, Nigeria | Prospective case-control study | 60/60 |
creatinine mean = 44.24, sd = 17.68; eGFR mean = 146.50, sd = 31.10 [13] |
| Farouk et al. 2020 | Maiduguri, Nigeria | Prospective case-control study | 110/110 | eGFR mean = 125.90, sd = 31.90 [9] |
| Tantaway et al. 2017 | Cairo, Egypt | Prospective case-control study | 53/40 | Creatinine mean = 43.33, sd = 8.84 [18] |
| Obalide et al. 2017 | Lagos, Nigeria | Prospective case-control study | 20 / 20 |
creatinine mean = 54.90, sd = 21.73; eGFR mean = 130.24, sd = 42.57 [14] |
| Aloni et al. 2014 | Kinshasa, D R Congo | Prospective case-control study | 65 / 67 |
creatinine mean = 44.21, sd = 11.49; urea mean = 15.30, sd = 8.30; eGFR mean = 130.50, sd = 34.10 [6] |
| Asaolu et al. 2010 | Ekiti, Nigeria | Prospective case-control study | 42 / 42 |
creatinine mean = 10.55, sd = 8.13; urea mean = 10.50, sd = 6.28 [7] |
| Mohamed et al. 1992 | Khartoum, Sudan | Prospective case-control study | 84 / 28 |
creatinine mean = 22.20, sd = 4.00; urea mean = 22.70, sd = 5.70 [11] |
| El-Gamasy and El-Naghy 2019 | Tanta, Egypt | Prospective case-control study | 70 / 40 |
creatinine mean = 90.19, sd = 17.68; urea mean = 22.90, sd = 7.60 [8] |
| Okoro and Onwuameze 1991 | Enugu, Nigeria | Prospective case-control study | 60 / 305 | eGFR mean = 111, sd = 36.0 [15] |
eGFR estimated glomerular filtration rate
Risk of bias
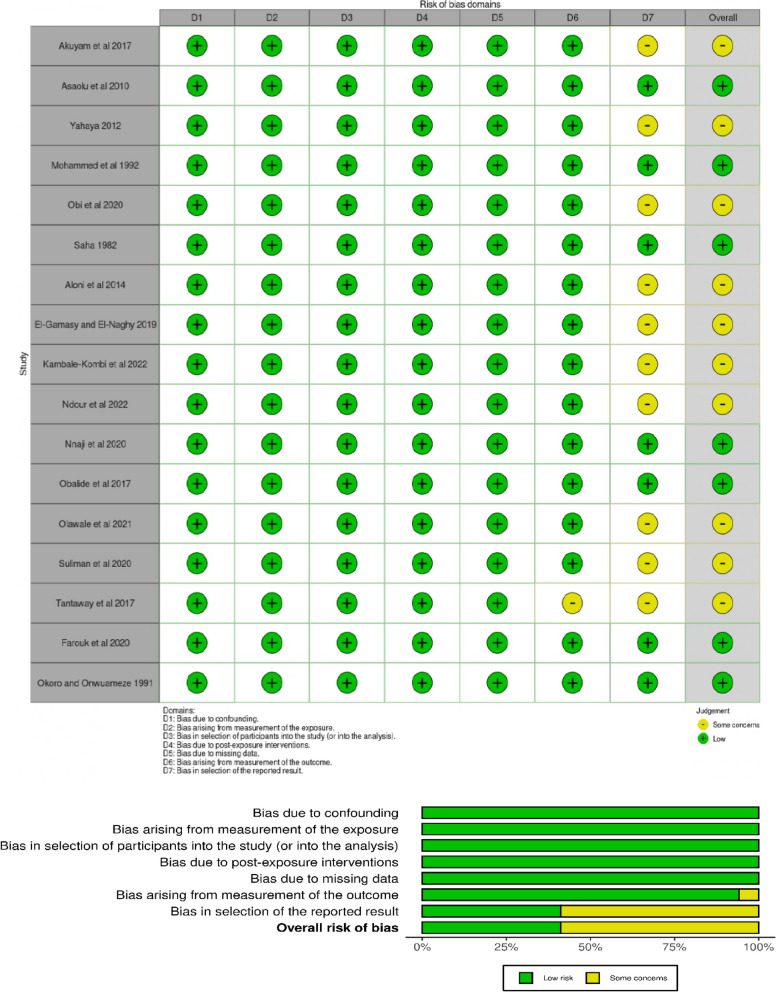
ROBINS-E, a tool for assessing the risk of bias in non-randomized studies of exposure effects [42], was used to assess the risk of bias. Seven risks of bias domains were identified and assessed online using ROBINS-E. There were overall some concerns about the risk of bias, as shown in Fig. 2, details in Supplementary file 3.
Fig. 2.
Assessment of risk of bias of non-randomized studies of exposure effects using ROBINS-E [42]
Most studies (10 out of 17) reported statistically significant differences between the people with SCD (HbSS) (exposed participants) and controls (HbAA). There were some concerns about the risk of bias, as summarized in Fig. 2 above.
Liver function in people with sickle cell disease in Africa
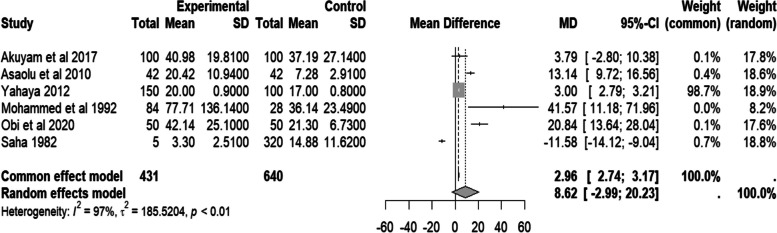
The studies [7, 11, 19–22] revealed that people with SCD in Africa were more likely to have elevated liver enzymes than controls. The studies considered serum aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) results from six and four studies, respectively [7, 11, 19–22], as shown in Table 1.
The finding showed that AST’s random effect pooled mean difference was not statistically significant (MD = 8.62 IU/L, 95% CI − 2.99–20.23, I2 = 97.0%, p < 0.01). However, most studies revealed that the people with SCD had a higher serum AST level than the control group. Notably, substantial heterogeneity (I2 > 50%) was found. The details are in Fig. 3.
Fig. 3.
Forest plot of serum AST of people with sickle cell disease compared to controls in Africa
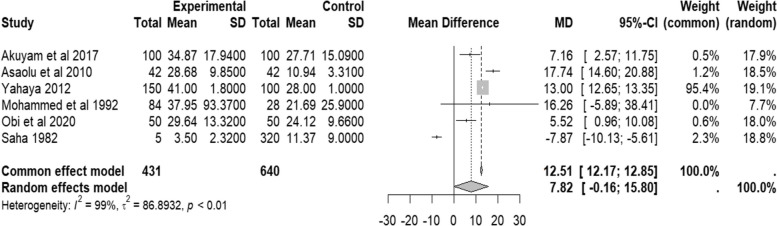
Similarly, the random effect of pooled mean difference (M.D.) of ALT was not statistically significant (MD = 7.82 IU/L, 95% CI − 0.16–15.58, I2 = 99.0%, p < 0.01). The forest plot (Fig. 4) shows a higher serum ALT level among people with SCD than controls.
Fig. 4.
Forest plot of serum ALT of people with sickle cell disease in Africa compared to controls
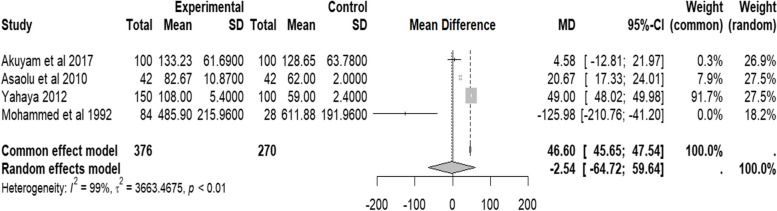
Findings from serum alkaline phosphatase (ALP) from four studies [7, 11, 19, 22] show a negative value of the random effect pooled mean difference (MD = − 2.54, 95% CI − 64.72–59.64, I2 = 99.0%, p < 0.01 IU/L). This could have been due to one study (Mohammed et al. 1992) [11] that had a relatively high mean value of ALP in the control group; the details are in Fig. 5. Due to the small sample size, inferential analysis of bias was not performed.
Fig. 5.
Forest plot of serum ALP of people with sickle cell disease compared to controls in Africa
Renal function in people with sickle cell disease in Africa
The studies [6–18] revealed that people with SCD in Africa may have a relatively compromised renal function. From the studies, three variables were used to evaluate the renal function in the study population, see Table 2.
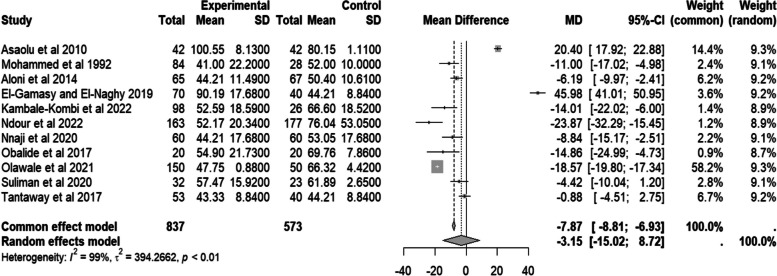
The random effect pooled mean difference for serum creatinine from the eleven studies [6–8, 10–14, 16–18] is negative (MD = − 3.15, 95% CI − 15.02–8.72, I2 = 99%, p < 0.01) units. The forest plot is skewed to the left of the experimental group, which may indicate increased creatinine clearance among people with SCD. Details are in Fig. 6.
Fig. 6.
Forest plot of serum creatinine for people with sickle cell disease compared to controls in Africa
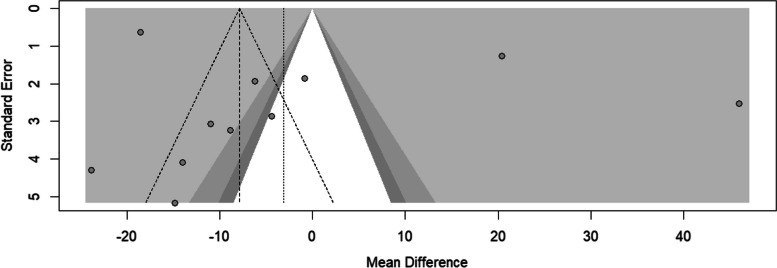
In Fig. 7, the funnel plot shows only two studies under the “white” area, and the asymmetry was tested using Egger’s regression. The finding suggested no statistically significant risk of bias (t = 1.09, df = 9, p = 0.305).
Fig. 7.
Funnel plot of serum creatinine for people with sickle cell disease compared to controls in Africa
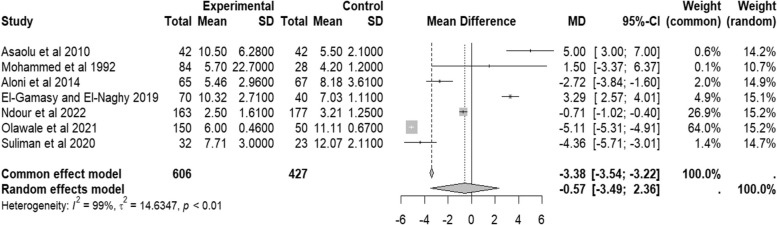
When the data of serum urea was considered, the random effect pooled mean difference from seven studies [6–8, 11, 12, 16, 17] was − 0.57 (95% CI − 3.49–2.36, I2 = 99%, p < 0.01) units as can be seen in Fig. 8. Though not statistically significant, it may still show that people with SCD have nearly similar serum urea levels to those of the control group.
Fig. 8.
Forest plot of serum urea of people with sickle cell disease compared to controls in Africa
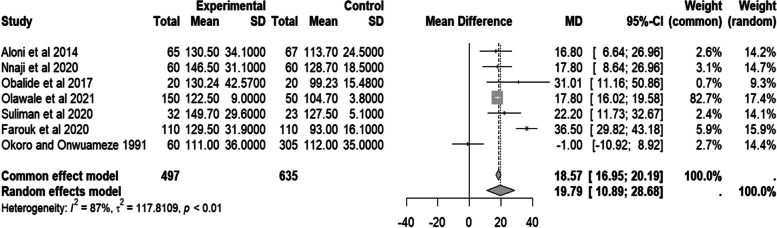
Meanwhile, the random effect pooled mean difference of estimated glomerular filtration rate (eGFR) from seven studies [6, 9, 14–17] was positive (MD = 19.79, 95% CI 10.89–28.68 mL/min/1.73 m2, I2 = 87.0%, p < 0.01). This demonstrates that people with sickle cell disease have higher eGFR (M.D. of estimated glomerular filtration > 0) than the control group. However, there was substantial heterogeneity (I2 > 50%). Details are in Fig. 9.
Fig. 9.
Forest plot of eGFR of people with sickle cell disease compared to controls in Africa
Discussion
This systematic review and meta-analysis found 17 articles from five African countries involving 1312 people with SCD (HbSS) and 1558 controls (HbAA). Compared to controls, there was no significant elevation of liver enzymes, urea, or creatinine among people with sickle cell disease.
The pooled mean difference of liver enzyme aspartate transaminase (AST) was 8.62 and alanine transaminase (ALT) was 7.82 above that of controls in this review. Normal levels of serum AST are 8–33 U/L, and ALT is less than 40 IU/L [44] and can rise to 2–3 times above normal levels in acute vaso-occlusive crises in sickle cell disease [45]. This was seen in a study in Pradesh, India [46], and the USA [45].
The pooled mean difference of serum alkaline phosphatase (ALP) in this review was -2.54 compared to controls. In acute hepatic sequestration [45] or bone disease [47], serum ALP may be elevated. However, the participants had a relatively lower serum ALP compared to controls. This may result from malnutrition and reduced dietary intake of zinc [48], and that cannot be excluded in these people with SCD in Africa.
The pooled mean difference for serum creatinine was − 3.15 while serum urea was − 0.57 compared to controls. The African population’s low serum urea and creatinine may be linked to glomerular hyperfiltration [4]. In the USA, where people with SCD are treated with hydroxyurea for a long time, increased creatinine and urea levels were seen in renal failure typically after 23 years of age [49], a drug that was only recently introduced to sub-Saharan Africa [50]. The pooled mean difference of estimated glomerular filtration rate (eGFR) was 19.79 compared to controls. Serum creatinine is used to estimate glomerular filtration rate [51]. The lower the serum creatinine level, the higher the eGFR [51].
Limitations and strengths of the study
Only 17 studies were found to have evaluated the liver and renal function tests in people with SCD in Africa and compared them to normal controls. Furthermore, most studies reported test results statistically significantly different from the controls. SCD vaso-occlusive crises insidiously affect the patients’ organs with repeated attacks as they age. However, different studies included participants of different age groups and perhaps lifestyles, which could have affected the test results. With all the heterogeneity (I2) > 50%, there was substantial variation in the reported articles’ results. Nonetheless, this systematic review forms a baseline for future research on SCD in Africa.
Conclusion
This review found non-significantly elevated liver enzymes. Glomerular filtration rates were higher in people with SCD than in controls. More extensive prospective studies are recommended to investigate further the impact of SCD on renal and liver function.
Supplementary Information
Supplementary Material 1. Literature search strategy.
Supplementary Material 2. Records of the excluded studies.
Supplementary Material 4. Extracted data.
Acknowledgements
Declared none.
Authors’ contributions
Silvia Awor drafted the research protocol, registered it in PROSPERO, did data screening processes, and drafted the manuscript. Benard Abola did the meta-analysis and reviewed the manuscript. Felix Bongomin provided expert advice on the subject, did data screening, and reviewed the manuscript. Mark Mohan Kaggwa did data extraction and reviewed the manuscript. Francis Pebolo Pebalo proofread and reviewed the manuscript. David Musoke, Proscovia Nnamuyomba, Jackline Epila, Maxwell Malinga, Acaye Ongwech, and Christine Oryema guided the protocol writing and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–31. [DOI] [PubMed] [Google Scholar]
- 2.Zittersteijn HA, Harteveld CL, Klaver-Flores S, Lankester AC, Hoeben RC, Staal FJT, Gonçalves MAFV. A small key for a heavy door: genetic therapies for the treatment of hemoglobinopathies. Front Genome Ed. 2021;2(34):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vichinsky EP. Overview of the clinical manifestations of sickle cell disease. UpToDate. 2023. https://www.uptodate.com/contents/overview-of-the-clinical-manifestations-of-sickle-cell-disease?search=renal%20manifestations%20in%20sickle%20cell%20disease&source=search_result&selectedTitle=4~150&usage_type=default&display_rank=4.
- 4.Lerma EV, Vichinsky EP. Sickle cell disease effects on the kidney. UpToDate. 2023. https://www.uptodate.com/contents/sicklecell-disease-effects-on-the-kidney?search=renal%20manifestations%20in%20sickle%20cell%20disease&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1.
- 5.Subhas B, DeBaun MR. Hepatic manifestations of sickle cell disease. UpToDate. 2023. https://www.uptodate.com/contents/hepatic-manifestations-of-sickle-cell-disease.
- 6.Aloni MN, Ngiyulu RM, Gini-Ehungu JL, Nsibu CN, Ekila MB, Lepira FB, Nseka NM. Renal function in children suffering from sickle cell disease: challenge of early detection in highly resource-scarce settings. PLoS One. 2014;9(5):e96561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asaolu MF, Oyeyemi AO, Fakunle JB, Ajose AO. Biochemical changes associated with sickle cell anaemia. Biosci Biotechnol Res Asia. 2010;7(1):21–4. [Google Scholar]
- 8.El-Gamasy MA, El-Naghy WS. Early predictors of renal dysfunction in pediatric patients with sickle cell disease. Indian J Nephrol. 2019;29(1):28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farouk AG, Ibrahim BA, Sulaiman MM, Asheikh MM, Yusuf H, Musa AH. Glomerular filtration rate in homozygous sickle cell disease children in steady state and healthy Nigerian children: a comparative study in north-eastern Nigeria. Borno Med J. 2020. (Online). https://pesquisa.bvsalud.org/gim/resource/en/afr-202067.
- 10.Kambale-Kombi P, Djang’eing’a RM, Alworong’a Opara JP, Mbo Mukonkole JP, Bours V, Tonen-Wolyec S, Mbumba Lupaka DM, Bome LB, Tshilumba CK, Batina-Agasa S. Renal abnormalities among sickle cell disease patients in a poor management setting: a survey in the Democratic Republic of the Congo. Mediterr J Hematol Infect Dis. 2022;14(1):e2022046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohamed AO, Bayoumi RA, Hofvander Y, Omer MIA, Ronquist G. Sickle cell anaemia in Sudan: clinical findings, haematological and serum variables. Ann Trop Paediatr. 1992;12(2):131–6. [DOI] [PubMed] [Google Scholar]
- 12.Ndour EHM, Mnika K, Tall FG, Seck M, Ly ID, Nembaware V, Mazandu GK, Bassene HATS, Dione R, Ndongo AA, et al. Biomarkers of sickle cell nephropathy in Senegal. PLoS ONE. 2022;17(11th November):e0273745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nnaji UM, Ogoke CC, Okafor HU, Achigbu KI. Sickle cell nephropathy and associated factors among asymptomatic children with sickle cell anaemia. Int J Pediatr. 2020;2020:1286432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obilade OA, Akanmu AS, Pipkin FB, Afolabi BB. Prostacyclin, thromboxane and glomerular filtration rate are abnormal in sickle cell pregnancy. PLOS ONE. 2017;12(9):e0184345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okoro BA, Onwuameze IC. Glomerular filtration rate in healthy Nigerian children and in children with sickle cell anaemia in a steady state. Ann Trop Paediatr. 1991;11(1):47–50. [DOI] [PubMed] [Google Scholar]
- 16.Olawale OO, Adekanmbi AF, Sonuga AA, Sonuga OO, Akodu SO, Ogundeyi MM. Assessment of renal function status in steady-state sickle cell anaemic children using urine human neutrophil gelatinase-associated lipocalin and albumin:creatinine ratio. Med Princ Pract. 2021;30(6):557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suliman MAO, Hassan AMA, Kaddam LA. Association between renal function parameters, clinical severity score and mortality risk among adult Sudanese sickle anemia patients. Am J Blood Res. 2020;10(6):434–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Tantawy AAG, Adly AAM, Ismail EAR, Abdelazeem M. Clinical predictive value of cystatin c in pediatric sickle cell disease: a marker of disease severity and subclinical cardiovascular dysfunction. Clin Appl Thromb Hemost. 2017;23(8):1010–7. [DOI] [PubMed] [Google Scholar]
- 19.Akuyam SA, Abubakar A, Lawal N, Yusuf R, Aminu SM, Hassan A, Musa A, Bello AK, Yahaya IA, Okafor PA. Assessment of biochemical liver function tests in relation to age among steady state sickle cell anemia patients. Niger J Clin Pract. 2017;20(11):1428–33. [DOI] [PubMed] [Google Scholar]
- 20.Saha N, Samuel AP. Sickle cell gene and liver functions in a Sudanese population. Acta Haematol. 1982;68(1):65–7. [DOI] [PubMed] [Google Scholar]
- 21.Obi CU, Stephen A, Nnamdi O, Arusiwon J, Agbiogwu I. Enzyme activities of liver function (bio-makers) in sickle cell anaemic patients attending Sickle Cell Anaemic Centre, Benin City, Edo State, Nigeria. Int J Blood Res Disord. 2020;7:1–5. [Google Scholar]
- 22.Yahaya IA. Biochemical features of hepatic dysfunction in Nigerians with sickle cell anaemia. Niger Postgrad Med J. 2012;19(4):204–7. [PubMed] [Google Scholar]
- 23.Schubert TT. Hepatobiliary system in sickle cell disease. Gastroenterology. 1986;90(6):2013–21. [DOI] [PubMed] [Google Scholar]
- 24.Kotila T, Adedapo K, Adedapo A, Oluwasola O, Fakunle E, Brown B. Liver dysfunction in steady state sickle cell disease. Ann Hepatol. 2005;4(4):261–3. [PubMed] [Google Scholar]
- 25.Ofakunrin AOD, Oguche S, Adekola K, Okpe ES, Afolaranmi TO, Diaku-Akinwumi IN, Zoakah AI, Sagay AS. Effectiveness and safety of hydroxyurea in the treatment of sickle cell anaemia children in Jos, North Central Nigeria. J Trop Pediatr. 2020;66(3):290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogwang P, Ralph T, Agwaya M, Gerosome M, Kyeyune GN, Badru G, Waako P. Repeat-dose effects of Zanthoxylum chalybeum root bark extract: a traditional medicinal plant used for various diseases in Uganda. Afr J Pharm Pharmacol. 2008;2:101–5. [Google Scholar]
- 27.Okoh MP, Alli LA, Tolvanen MEE, Nwegbu MM. Herbal Drug use in Sickle Cell Disease Management; Trends and Perspectives in Sub-Saharan Africa - A Systematic Review. Curr Drug Discov Technol. 2019;16(4):372–85. 10.2174/1570163815666181002101611. [DOI] [PubMed] [Google Scholar]
- 28.Afangbedji N, Jerebtsova M. Glomerular filtration rate abnormalities in sickle cell disease. Front Med (Lausanne). 2022;9:1029224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am J Hematol. 2013;88(2):116–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khargekar N, Banerjee A, Athalye S, Mahajan N, Kargutkar N, Tapase P, Madkaikar M. Role of hydroxyurea therapy in the prevention of organ damage in sickle cell disease: a systematic review and meta-analysis. Syst Rev. 2024;13(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirschberg R. Glomerular hyperfiltration in sickle cell disease. Clin J Am Soc Nephrol. 2010;5(5):748–9. [DOI] [PubMed] [Google Scholar]
- 32.Sickle cell disease. [https://www.afro.who.int/health-topics/sickle-cell-disease, https://www.afro.who.int/news/african-health-ministers-launch-drive-curb-sickle-cell-disease-toll].
- 33.Wastnedge E, Waters D, Patel S, Morrison K, Goh MY, Adeloye D, Rudan I. The global burden of sickle cell disease in children under five years of age: a systematic review and meta-analysis. J Glob Health. 2018;8(2):021103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ndeezi G, Kiyaga C, Hernandez AG, Munube D, Howard TA, Ssewanyana I, Nsungwa J, Kiguli S, Ndugwa CM, Ware RE, et al. Burden of sickle cell trait and disease in the Uganda Sickle Surveillance Study (US3): a cross-sectional study. Lancet Glob Health. 2016;4(3):e195–200. [DOI] [PubMed] [Google Scholar]
- 35.Nabongo P, Verver S, Nangobi E, Mutunzi R, Wajja A, Mayanja-Kizza H, Kadobera D, Galiwango E, Colebunders R, Musoke P. Two year mortality and associated factors in a cohort of children from rural Uganda. BMC Public Health. 2014;14(1):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elsevier-_Ltd. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000–2021: a systematic analysis from the Global Burden of Disease Study 2021. Lancet Haematol. 2023;10(8):e585–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akinsulie AO, Temiye EO, Akanmu AS, Lesi FE, Whyte CO. Clinical evaluation of extract of Cajanus cajan (Ciklavit) in sickle cell anaemia. J Trop Pediatr. 2005;51(4):200–5. [DOI] [PubMed] [Google Scholar]
- 38.Obodozie OO, Ameh SJ, Afolabi EK, Oyedele EO, Ache TA, Onanuga CE, Ibe MC, Inyang US. A normative study of the components of niprisan–an herbal medicine for sickle cell anemia. J Diet Suppl. 2010;7(1):21–30. [DOI] [PubMed] [Google Scholar]
- 39.Brooke BS, Schwartz TA, Pawlik TM. MOOSE reporting guidelines for meta-analyses of observational studies. JAMA Surg. 2021;156(8):787–8. [DOI] [PubMed] [Google Scholar]
- 40.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–53. [DOI] [PubMed] [Google Scholar]
- 42.Higgins JPT, Morgan RL, Rooney AA, Taylor KW, Thayer KA, Silva RA, Lemeris C, Akl EA, Bateson TF, Berkman ND, et al. A tool to assess risk of bias in non-randomised follow-up studies of exposure effects (ROBINS-E). Environ Int. 2024;186:108602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.UNIT CONVERSION PANEL. [http://www.scymed.com/en/smnxps/psxdf212_c.htm].
- 44.Murali AR, Carey WD. Liver test interpretation-approach to the patient with liver disease: a guide to commonly used liver tests. In: Cleveland Clinic-Center for Continuing Education. 2014. [Google Scholar]
- 45.Samuel SS, Jain N. Sickle Cell Hepatopathy. [Updated 2023 Jun 21]. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK574502/. [PubMed]
- 46.Tripathi P, Tripathi M. Biochemical assessment of liver in sickle cell disease patients at a tertiary care hospital of north India. Int J Res Med Sci. 2016;4:57–60. [Google Scholar]
- 47.Han J, Zhang X, Shah B, Saraf SL, Gordeuk VR. Alkaline phosphatase as a marker for acute complications in sickle cell disease. Blood. 2023;142:3683. [DOI] [PubMed] [Google Scholar]
- 48.Lum G. Significance of low serum alkaline phosphatase activity in a predominantly adult male population. Clin Chem. 1995;41(4):515–8. [PubMed] [Google Scholar]
- 49.Powars Darleen R, Elliott-Mills Donna D, Chan Linda, Niland Joyce, Hiti Alan L, Opas Lawrence M, Johnson Cage. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med. 1991;115(8):614–20. [DOI] [PubMed] [Google Scholar]
- 50.Opoka RO, Ndugwa CM, Latham TS, Lane A, Hume HA, Kasirye P, Hodges JS, Ware RE, John CC. Novel use Of Hydroxyurea in an African Region with Malaria (NOHARM): a trial for children with sickle cell anemia. Blood. 2017;130(24):2585–93. [DOI] [PubMed] [Google Scholar]
- 51.Florkowski CM, Chew-Harris JS. Methods of estimating GFR - different equations including CKD-EPI. Clin Biochem Rev. 2011;32(2):75–9. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Literature search strategy.
Supplementary Material 2. Records of the excluded studies.
Supplementary Material 4. Extracted data.
Data Availability Statement
Not applicable.