In this issue of the Journal of Clinical Oncology, Rashidi et al [citation] report the results of a randomized trial of fecal microbiota transplantation (FMT) in recipients of induction chemotherapy for acute leukemia or allogeneic hematopoietic cell transplantation (allo-HCT). One hundred patients were randomized to receive FMT from healthy donors as oral capsules vs placebo upon recovery from neutrophil nadir. The primary endpoint was the all-cause infection rate over the following 4 months. The primary endpoint was not met, but the trial is still very instructive on several points.
Severe perturbations to microbiome composition have been described in both allo-HCT, and to a lesser but still significant extent in AML induction.1–4 Low fecal microbiota diversity is a reproducibly observed predictor of mortality after allo-HCT3, 5–7, primarily due to graft-vs-host disease (GVHD) and infections. Given the meticulous care with which indwelling catheters are now implanted and manipulated, bloodstream infections in allo-HCT patients are most often mucosal-barrier-injury associated. These infections of gut origin8 are very often preceded by a distortion of the usually diverse microbiome community such that ~30–100% of sequencing reads from fecal samples in the days prior to an infection are mapped to the single organism that caused the infection.9, 10 Under normal circumstances, such domination events are prevented by the normal microbiome community, which has evolved mechanisms to exert colonization resistance against such pathogens as Enterococci and Enterobacteriaceae (e.g. E. coli, Klebsiella).
The major mechanisms of colonization resistance by microbes are production of molecules that are toxic to neighboring organisms (most famously penicillin) and competition for key nutrients or other features of ecological niches. While restoring FMT is an investigational approach for systemic infection outcomes, restoring colonization resistance by these means is well-established in the case of C. difficile, for which fecal transplants (or products derived from fecal donors) have been recently authorized to prevent recurrence11, 12 With respect to other infections, the microbiome-derived metabolite desaminotyrosine can induce type-I interferon responses that protect experimental mice from influenza,13 and allo-HCT patients with upper-respiratory viral infections are more likely to progress to lower-respiratory tract infections if they harbor low diversity in their bacterial microbiomes.14 Invasive fungal infections have also been linked with intestinal fungal mycobiome domination events.15, 16 More broadly, an intact gut microbiome has been linked with more robust immune reconstitution after HCT in experimental animals, a prior randomized trial of FMT in allo-HCT recipients, and in a large observational cohort.17–20 In principle, the risk of any opportunistic infection could be lessened by augmenting immune reconstitution, or perhaps even by modulating responses to vaccines.21
Besides microbe-microbe interactions within the gut lumen, restoring a healthy microbiome is expected to improve gut-barrier function22–24, for example through provision of butyrate and indole metabolites that reduce permeability. Therefore, the risk of infection from any organism that gains entry by crossing the GI tract could be hypothesized to decrease upon restoring a normal microbiome by FMT; this might be the case in food-borne illnesses and in viral infections that may have a gastrointestinal reservoir.25, 26
Moreover, the intestinal microbiome produces a large output of small-molecule metabolites, many of which are bioactive and achieve micro- or millimilar concentrations in the lumen and in the systemic circulation. The best studied among these is butyrate, which modulates the metabolism of colonocytes, functions as a histone deacetylase inhibitor, and induces regulatory T cells in mouse intestines.27, 28 Primary bile acids are excreted into the intestine and subsequently processed by microbes into a diverse pool of secondary bile acids that are then reabsorbed and modulate T cell differentiation and organismal metabolism.29–32 A third example is the production of tryptophan-derived metabolites by microbiota, including inosines, kynurenanine, and serotonin, which have pleitotrophic effects on physiology.22, 33 Although many of these mechanisms have been well-studied only in animal models, their common theme is that a physiologic/homeostatic state is promoted by signals from a normal gut microbiome. The host, under normal circumstances, actively cultivates this homeostatic community of microbes by establishing a favorable gut-lumen niche that is temperature- and pH-regulated, anaerobic, and replete with nutrients from both alimentation and host-derived mucus, and into which antimicrobial peptides and IgA are secreted to further sculpt the composition of the community. Intensive cancer therapy, however, not only disrupts these host-derived inputs by causing mucositis but is also accompanied by poor nutrition and exposure to antibiotics and other drugs that directly affect bacteria as well.34
Since for most disease states, the key relevant strains or their bioactive metabolites35 that elicit the desired host response have yet to be defined, most FMT trials seek to restore this homeostatic feedback loop with a bulk transplant of feces from a healthy donor. A key assumption in these studies is that enough strains with relevant functions are likely to be in the stool of most healthy donors. A tacit assumption is that if the right strains can engraft, they might not only confer benefit direct but might jumpstart the feedback loop and nudge the host-microbiome interactions back toward a theoretically more homeostatic salutary state.
In order for a single (or perhaps a few) FMT treatments to plausibly affect clinical outcomes over the course of months, a transient presence of strains in the GI tract would need to durably modulate host biology, or the transplanted strains would need to durably engraft. Rashidi et al [citation] observed sustained engraftment of a considerable number of donor-origin taxa at 1 and even 9-months following the treatment. Notably, sustained engraftment was achieved by an encapsulated oral treatment (obviating enema or endoscopic instillation) and without the use of “conditioning” or “priming” regimen such as oral vancomycin, which has been employed in several recent and ongoing trials.36–38
Although trial did not meet its prespecified primary endpoint, the study intervention did improve two key microbiota endpoints that could be considered surrogates or risk factors as they themselves have been previously well-correlated with mortality outcomes. First, fecal microbiota α-diversity was increased in FMT recipients. This next-generation-sequencing-assessed biomarker has predicted overall survival following allo-HCT in multiple observational cohorts.3, 6, 7 Second, the study treatment was able to mitigate expansion of Enterococci, which is not only a risk factor for Enterococcal bloodstream infections9 and mortality in leukemia,4 but exacerbates GVHD in mice and predicts for higher rates of GVHD and of mortality in multiple observational cohorts.39
Of note, one design feature of the trial may have set the bar for success relatively high: The types of infections with the strongest evidence that they arise from microbial dysbiosis – C. difficle and mucosal-barrier-injury bloodstream infections – occur most frequently during neutropenic nadir which coincides with the period of greatest gut barrier damage. In this trial, the FMT was administered after neutrophil recovery/engraftment (also past the time of the initiation of alloreactivity which occurs in the first week post HCT). Thus the primary endpoint was more dependent types of infections that are less-clearly linked to the gut microbiome, such as viral (which comprised 33 of the 102 infection events in the whole study) and respiratory infections (also 33 of 102 events).
Another notable finding in the study was the overall safety of the FMT, even in these highly immunocompromised patients. Despite a prior report of a fatal case of transmission of multi-drug resistant pathogen via FMT to an HCT recipient40, this study expands considerably the otherwise good safety track record of FMT in immunocompromised patients treated for hematologic malignancy with chemotherapy and allo-HCT.38, 41–47 Although there was a higher rate of GVHD in the recipients of FMT, there only 16 GVHD events in the whole study. Combined with an imbalance in the GVHD-prophylaxis regimens administered on the two arms, this make it difficult to interpret any effect on GVHD in this study, especially as FMT has demonstrated treatment responses in several studies of steroid-refractory GVHD.42, 44, 45 and studies of this are ongoing.38
Future studies of FMT will need to study donor optimization, dose, route, timing, patient selection.36, 48 Antibiotic stewardship and evidence-based use of antibiotics and nutrition during treatment can help mitigate microbiome damage.49 Finally, another approach that might overcome some of the barriers to FMT (such as batch-to-batch heterogeneity, healthy-donor recruitment challenges) is combinations of strains that are individually cultivated under clinical-grade production conditions.50, 51
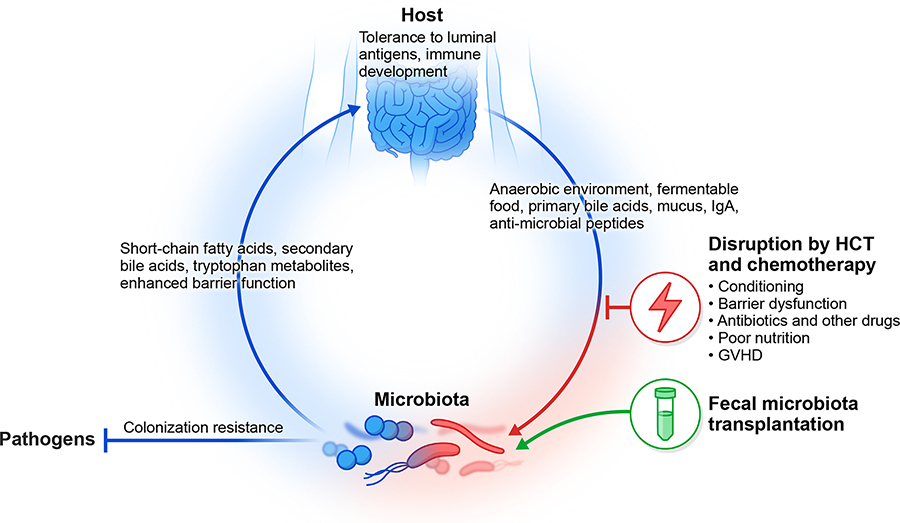
Figure: Fecal transplantation in the interaction between a patient with cancer and their microbiome.
The mammalian host cultivates a colonic microbiome through the provision of niche factors (e.g. temperature, pH, food, mucus) that favor the growth of certain microbial organisms. The microbiome, in turn, ferments foods the host cannot digest independently and also provides various inputs to the immune system and organismal metabolism, and also exerts colonization resistance against pathogens. This homeostatic feedback loop is disrupted by chemotherapy and hematopoietic transplantation. Fecal microbiota transplantation is hypothesized to restore colonization resistance and nudge this pathway back toward homeostasis.
Research Support:
JUP is supported by NHLBI NIH Award K08HL143189, MSKCC Cancer Center Core Grant P30 CA008748, the Society of Memorial Sloan Kettering Cancer Center. MVDB is supported by National Cancer Institute award numbers, R01-CA228358, R01-CA228308, P30 CA008748 MSK Cancer Center Support Grant/Core Grant and P01-CA023766; National Heart, Lung, and Blood Institute (NHLBI) award number R01-HL123340 and R01-HL147584; National Institute on Aging award number P01-AG052359, and Tri Institutional Stem Cell Initiative. Additional funding was received from The Lymphoma Foundation, The Susan and Peter Solomon Family Fund, The Solomon Microbiome Nutrition and Cancer Program, Cycle for Survival, Parker Institute for Cancer Immunotherapy, Paula and Rodger Riney Multiple Myeloma Research Initiative, Starr Cancer Consortium, and Seres Therapeutics.
References
- 1.Ubeda C, Taur Y, Jenq RR, et al. : Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest 120:4332–4341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galloway-Pena JR, Smith DP, Sahasrabhojane P, et al. : The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer 122:2186–96, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peled JU, Gomes ALC, Devlin SM, et al. : Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. The New England journal of medicine 382:822–834, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messina JA, Tan CY, Ren Y, et al. : Enterococcus Intestinal Domination is Associated with Increased Mortality in the Acute Leukemia Chemotherapy Population. Clin Infect Dis ciab 1043, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taur Y, Jenq RR, Perales MA, et al. : The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 124:1174–82, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golob JL, Pergam SA, Srinivasan S, et al. : Stool Microbiota at Neutrophil Recovery Is Predictive for Severe Acute Graft vs Host Disease After Hematopoietic Cell Transplantation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 65:1984–1991, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han L, Zhang H, Ma P, et al. : Intestinal microbiota score could predict survival following allogeneic hematopoietic stem cell transplantation. Ann Hematol 101:1283–1294, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamburini FB, Andermann TM, Tkachenko E, et al. : Precision identification of diverse bloodstream pathogens in the gut microbiome. Nat Med 24:1809–1814, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taur Y, Xavier JB, Lipuma L, et al. : Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 55:905–14, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoma I, Littmann ER, Peled JU, et al. : Compositional Flux Within the Intestinal Microbiota and Risk for Bloodstream Infection With Gram-negative Bacteria. Clin Infect Dis 73:e4627–e4635, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feuerstadt P, Louie TJ, Lashner B, et al. : SER-109, an Oral Microbiome Therapy for Recurrent Clostridioides difficile Infection. N Engl J Med 386:220–229, 2022 [DOI] [PubMed] [Google Scholar]
- 12.Khanna S, Assi M, Lee C, et al. : Efficacy and Safety of RBX2660 in PUNCH CD3, a Phase III, Randomized, Double-Blind, Placebo-Controlled Trial with a Bayesian Primary Analysis for the Prevention of Recurrent Clostridioides difficile Infection. Drugs 82:1527–1538, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steed AL, Christophi GP, Kaiko GE, et al. : The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science (New York, NY) 357:498–502, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haak BW, Littmann ER, Chaubard JL, et al. : Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood 131:2978–2986, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolling T, Zhai B, Gjonbalaj M, et al. : Haematopoietic cell transplantation outcomes are linked to intestinal mycobiota dynamics and an expansion of Candida parapsilosis complex species. Nat Microbiol 6:1505–1515, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhai B, Ola M, Rolling T, et al. : High-resolution mycobiota analysis reveals dynamic intestinal translocation preceding invasive candidiasis. Nat Med 26:59–64, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staffas A, Burgos da Silva M, Slingerland AE, et al. : Nutritional Support from the Intestinal Microbiota Improves Hematopoietic Reconstitution after Bone Marrow Transplantation in Mice. Cell Host Microbe, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schluter J, Peled JU, Taylor BP, et al. : The gut microbiota is associated with immune cell dynamics in humans. Nature 588:303–307, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miltiadous O, Waters NR, Andrlová H, et al. : Early intestinal microbial features are associated with CD4 T-cell recovery after allogeneic hematopoietic transplant. Blood 139:2758–2769, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josefsdottir KS, Baldridge MT, Kadmon CS, et al. : Antibiotics impair murine hematopoiesis by depleting intestinal microbiota. Blood 129:729–739, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagan T, Cortese M, Rouphael N, et al. : Antibiotics-Driven Gut Microbiome Perturbation Alters Immunity to Vaccines in Humans. Cell 178:1313–1328.e13, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatesh M, Mukherjee S, Wang H, et al. : Symbiotic Bacterial Metabolites Regulate Gastrointestinal Barrier Function via the Xenobiotic Sensor PXR and Toll-like Receptor 4. Immunity 41:296–310, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plöger S, Stumpff F, Penner GB, et al. : Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci 1258:52–59, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Mathewson ND, Jenq R, Mathew AV, et al. : Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nature immunology 17:505–13, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neurath MF, Überla K, Ng SC: Gut as viral reservoir: lessons from gut viromes, HIV and COVID-19. Gut 70:1605–1608, 2021 [DOI] [PubMed] [Google Scholar]
- 26.Campbell DE, Li Y, Ingle H, et al. : Impact of the Microbiota on Viral Infections. Annu Rev Virol, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atarashi K, Tanoue T, Oshima K, et al. : Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500:232–6, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Arpaia N, Campbell C, Fan X, et al. : Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–5, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell C, McKenney PT, Konstantinovsky D, et al. : Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581:475–479, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hang S, Paik D, Yao L, et al. : Bile acid metabolites control T(H)17 and T(reg) cell differentiation. Nature 576:143–148, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paik D, Yao L, Zhang Y, et al. : Human gut bacteria produce ΤΗ17-modulating bile acid metabolites. Nature 603:907–912, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wahlström A, Sayin SI, Marschall H-U, et al. : Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab 24:41–50, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Agus A, Planchais J, Sokol H: Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host & Microbe 23:716–724, 2018 [DOI] [PubMed] [Google Scholar]
- 34.Nguyen C, Markey KA, Miltiadous O, et al. : High-resolution analyses of associations between medications, microbiome and mortality in cancer patients. Cell, In Press, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ott SJ, Waetzig GH, Rehman A, et al. : Efficacy of Sterile Fecal Filtrate Transfer for Treating Patients With Clostridium difficile Infection. Gastroenterology 152:799–811 e7, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Porcari S, Benech N, Valles-Colomer M, et al. : Key determinants of success in fecal microbiota transplantation: From microbiome to clinic. Cell Host Microbe 31:712–733, 2023 [DOI] [PubMed] [Google Scholar]
- 37.Overman MJ: Pilot Trial of Fecal Microbiota Transplantation and Re-Introduction of Anti-PD-1 Therapy in dMMR Colorectal Adenocarcinoma Anti-PD-1 Non-Responders [Internet]. clinicaltrials.gov, 2023[cited 2023 May 25] Available from: https://clinicaltrials.gov/ct2/show/NCT04729322 [Google Scholar]
- 38.Malard, Florent: Evaluation of the Efficacy of MaaT013 as Salvage Therapy in Acute GVHD Patients With Gastrointestinal Involvement, Refractory to Ruxolitinib; a Multi-center Open-label Phase III Trial. [Internet] clinicaltrials.gov, 2022[cited 2023 May 25] Available from: https://clinicaltrials.gov/ct2/show/NCT04769895 [Google Scholar]
- 39.Stein-Thoeringer CK, Nichols KB, Lazrak A, et al. : Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science (New York, NY) 366:1143–1149, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeFilipp Z, Bloom PP, Torres Soto M, et al. : Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med 381:2043–2050, 2019 [DOI] [PubMed] [Google Scholar]
- 41.DeFilipp Z, Peled JU, Li S, et al. : Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv 2:745–753, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Lier YF, Davids M, Haverkate NJE, et al. : Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci Transl Med 12:eaaz8926, 2020 [DOI] [PubMed] [Google Scholar]
- 43.Taur Y, Coyte K, Schluter J, et al. : Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Science Translational Medicine, In Press; 10, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kakihana K, Fujioka Y, Suda W, et al. : Fecal microbiota transplantation for patients with steroid-resistant/dependent acute graft-versus-host disease of the gut. Blood 128:2083–2088, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spindelboeck W, Schulz E, Uhl B, et al. : Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft-versus-host-disease. Haematologica, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Zhao Y, Qi J, et al. : Fecal microbiota transplantation combined with ruxolitinib as a salvage treatment for intestinal steroid-refractory acute GVHD. Experimental Hematology & Oncology 11:96, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Y, Li X, Zhou Y, et al. : Safety and Efficacy of Fecal Microbiota Transplantation for Grade IV Steroid Refractory GI-GvHD Patients: Interim Results From FMT2017002 Trial [Internet]. Frontiers in Immunology 12, 2021[cited 2023 May 29] Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2021.678476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Markey KA, van den Brink MRM, Peled JU: Therapeutics Targeting the Gut Microbiome: Rigorous Pipelines for Drug Development. Cell Host Microbe 27:169–172, 2020 [DOI] [PubMed] [Google Scholar]
- 49.Shono Y, Docampo MD, Peled JU, et al. : Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med 8:339ra71, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ponce DM, Lombardo M-J, Ford CB, et al. : A phase 1b study to evaluate safety, tolerability, pharmacokinetics, and efficacy of SER-155 in adults undergoing hematopoietic stem cell transplantation to reduce the risk of infection and graft versus host disease (NCT04995653). JCO 40:TPS7074–TPS7074, 2022 [Google Scholar]
- 51.Tanoue T, Morita S, Plichta DR, et al. : A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 565:600–605, 2019 [DOI] [PubMed] [Google Scholar]
- 52.Lam KC, Araya RE, Huang A, et al. : Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 184:5338–5356.e21, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griffin ME, Espinosa J, Becker JL, et al. : Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science (New York, NY) 373:1040–1046, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mager LF, Burkhard R, Pett N, et al. : Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science (New York, NY) 369:1481–1489, 2020 [DOI] [PubMed] [Google Scholar]