Abstract
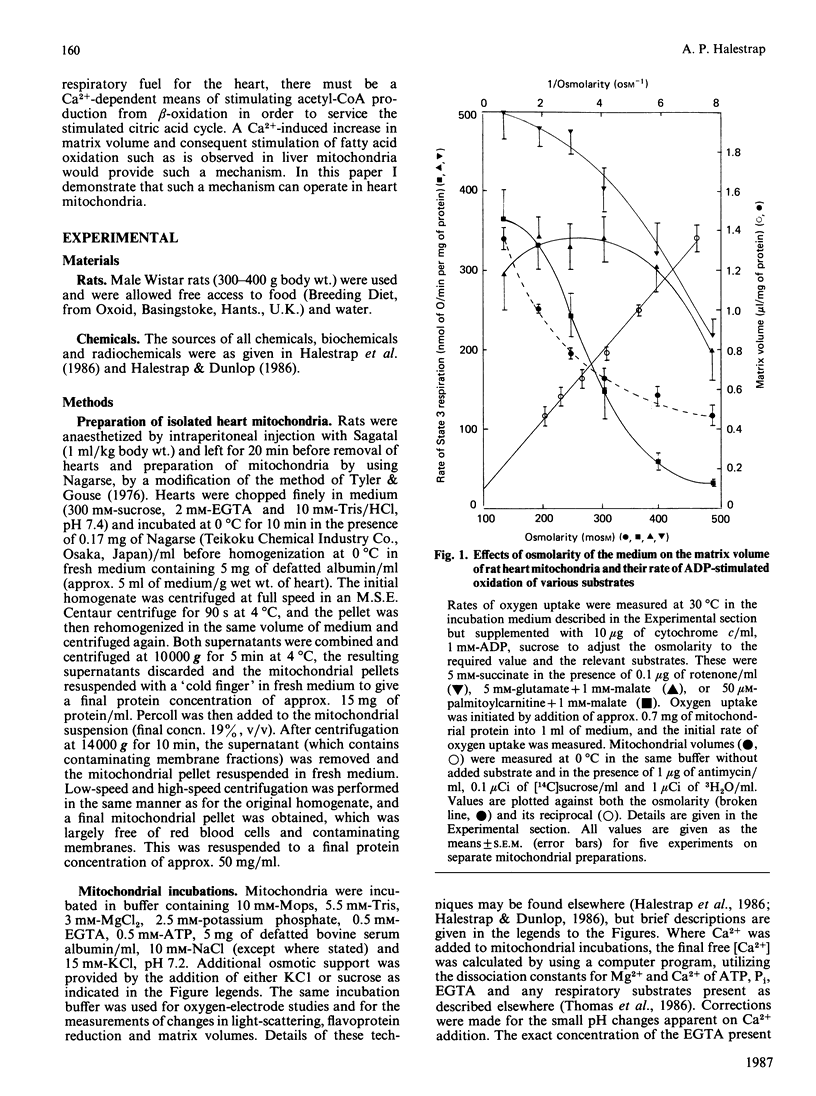
1. The rate of ADP-stimulated respiration with various substrates and the matrix volume of rat heart mitochondria were measured over a range of osmolarities of the medium. 2. The rate of oxidation of palmitoylcarnitine (in the presence of malate) was stimulated 7-fold by increasing the matrix volume from 0.6 to 1.0 microliter/mg of protein. Oxidation of octanoate showed a similar sensitivity to the matrix volume, whereas oxidation of other substrates showed little sensitivity until the volume fell below 0.7 microliter/mg of protein. 3. The matrix volume of heart mitochondria incubated under physiological conditions was about 0.8 microliter/mg of protein. 4. Low concentrations of valinomycin added to mitochondria incubated under such physiological conditions could activate the rate of ADP-stimulated palmitoylcarnitine oxidation by at least 100%. 5. Decreasing the matrix volume increased the reduction of the electron-transferring flavoprotein (ETF), suggesting an effect on electron flow between ETF and ubiquinone, as has been observed for liver mitochondria [Halestrap & Dunlop (1986) Biochem. J. 239, 559-565]. 6. A rapid decrease in light-scattering by heart mitochondria incubated in State 4 was induced by addition of Ca2+, reaching 50% of the maximal effect after about 30 s at 30 degrees C and with K0.5 for Ca2+ of 0.3 microM. This was not associated with a change in matrix volume, and is discussed in terms of a conformational change whose identity remains to be determined. 7. However, incubation of heart mitochondria at 37 degrees C in the presence of 0.65 microM-Ca2+ for 4 min did increase the matrix volume significantly, by 0.181 +/- 0.029 microliter/mg of protein (n = 7, P less than 0.001), similar to the Ca2+-induced changes observed with liver mitochondria [Halestrap, Quinlan, Whipps & Armston (1986) Biochem. J. 236, 779-787]. 8. The possible significance of these results in the co-ordinate regulation of fatty acid oxidation and the citric acid cycle in the heart responding to increased work load or hormonal stimulation is discussed.
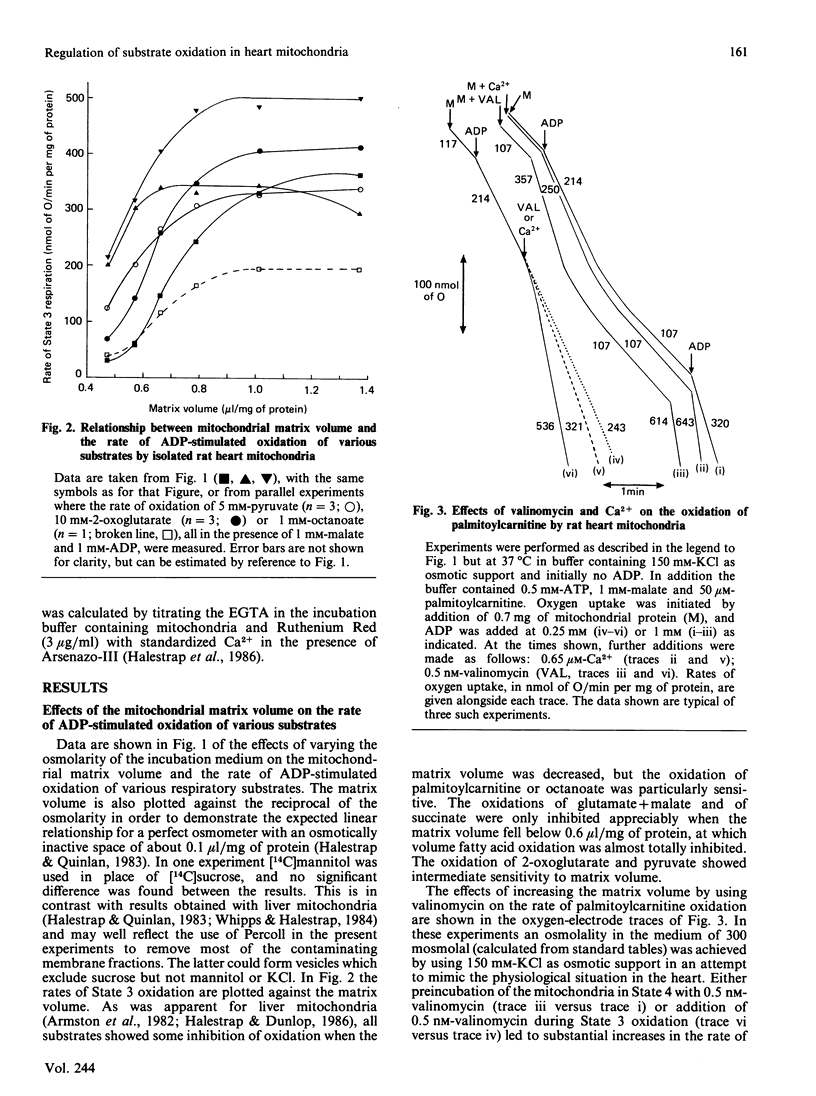
Full text
PDF
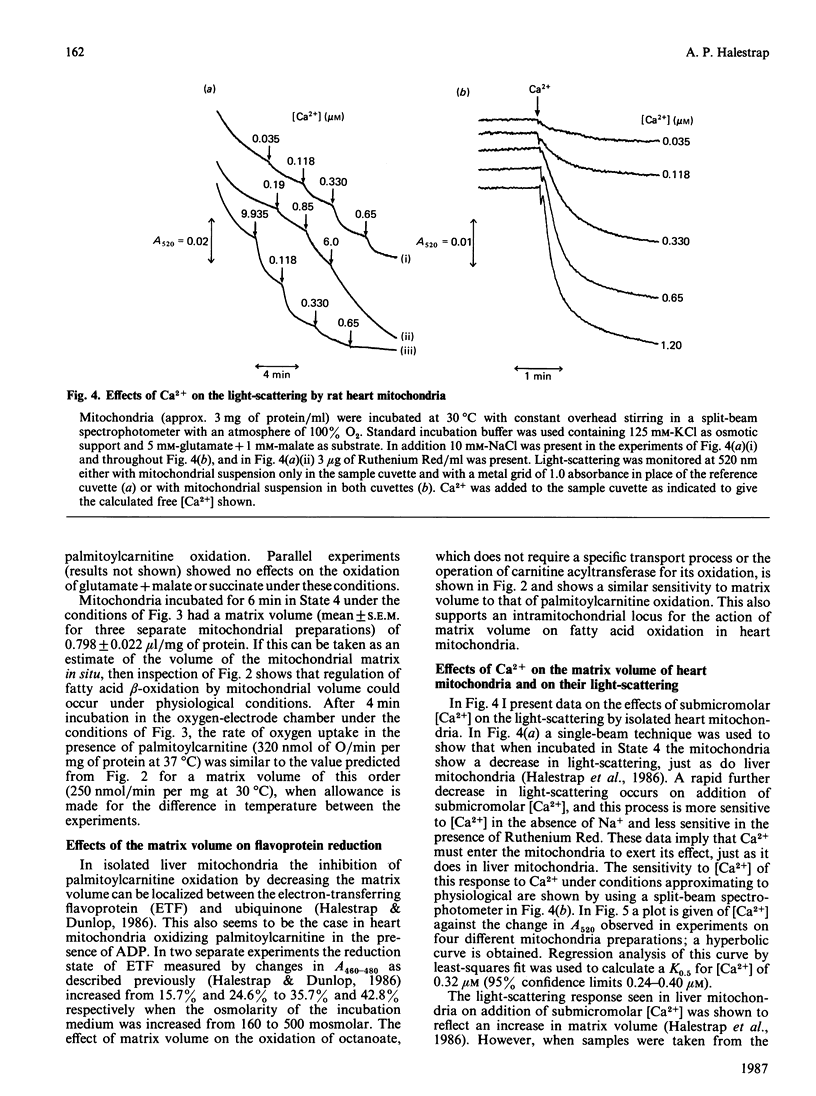
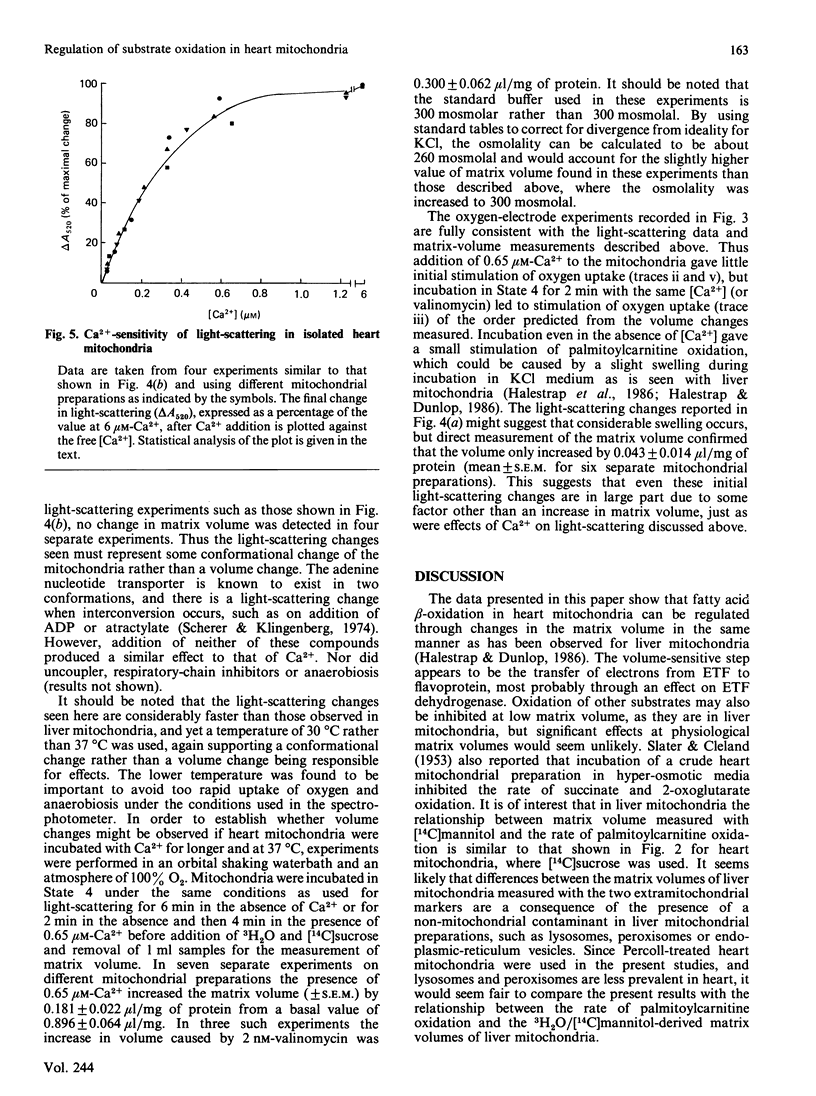

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armston A. E., Halestrap A. P., Scott R. D. The nature of the changes in liver mitochondrial function induced by glucagon treatment of rats. The effects of intramitochondrial volume, aging and benzyl alcohol. Biochim Biophys Acta. 1982 Sep 15;681(3):429–439. doi: 10.1016/0005-2728(82)90185-2. [DOI] [PubMed] [Google Scholar]
- Bünger R., Soboll S. Cytosolic adenylates and adenosine release in perfused working heart. Comparison of whole tissue with cytosolic non-aqueous fractionation analyses. Eur J Biochem. 1986 Aug 15;159(1):203–213. doi: 10.1111/j.1432-1033.1986.tb09854.x. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- From A. H., Petein M. A., Michurski S. P., Zimmer S. D., Uğurbil K. 31P-NMR studies of respiratory regulation in the intact myocardium. FEBS Lett. 1986 Oct 6;206(2):257–261. doi: 10.1016/0014-5793(86)80992-9. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P., Dunlop J. L. Intramitochondrial regulation of fatty acid beta-oxidation occurs between flavoprotein and ubiquinone. A role for changes in the matrix volume. Biochem J. 1986 Nov 1;239(3):559–565. doi: 10.1042/bj2390559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Quinlan P. T. The intramitochondrial volume measured using sucrose as an extramitochondrial marker overestimates the true matrix volume determined with mannitol. Biochem J. 1983 Aug 15;214(2):387–393. doi: 10.1042/bj2140387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Quinlan P. T., Whipps D. E., Armston A. E. Regulation of the mitochondrial matrix volume in vivo and in vitro. The role of calcium. Biochem J. 1986 Jun 15;236(3):779–787. doi: 10.1042/bj2360779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. The nature of the stimulation of the respiratory chain of rat liver mitochondria by glucagon pretreatment of animals. Biochem J. 1982 Apr 15;204(1):37–47. doi: 10.1042/bj2040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- Hess D. S., Bache R. J. Transmural distribution of myocardial blood flow during systole in the awake dog. Circ Res. 1976 Jan;38(1):5–15. doi: 10.1161/01.res.38.1.5. [DOI] [PubMed] [Google Scholar]
- Illingworth J. A., Ford W. C., Kobayashi K., Williamson J. R. Regulation of myocardial energy metabolism. Recent Adv Stud Cardiac Struct Metab. 1975;8:271–290. [PubMed] [Google Scholar]
- Latipä P. M., Kärki T. T., Hiltunen J. K., Hassinen I. E. Regulation of palmitoylcarnitine oxidation in isolated rat liver mitochondria. Role of the redox state of NAD(H). Biochim Biophys Acta. 1986 Feb 12;875(2):293–300. doi: 10.1016/0005-2760(86)90179-7. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., England P. J. Ruthenium Red inhibits the activation of pyruvate dehydrogenase caused by positive inotropic agents in the perfused rat heart. Biochem J. 1983 Aug 15;214(2):581–585. doi: 10.1042/bj2140581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moravec J., Corsin A., Owen P., Opie L. H. Effect of increased aortic perfusion pressure on fluorescent emission of the isolated rat heart. J Mol Cell Cardiol. 1974 Apr;6(2):187–200. doi: 10.1016/0022-2828(74)90021-2. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Denton R. M., England P. J., Randle P. J. The effects of increased heart work on the tricarboxylate cycle and its interactions with glycolysis in the perfused rat heart. Biochem J. 1972 Jun;128(1):147–159. doi: 10.1042/bj1280147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie L. H., Mansford K. R., Owen P. Effects of increased heart work on glycolysis and adenine nucleotides in the perfused heart of normal and diabetic rats. Biochem J. 1971 Sep;124(3):475–490. doi: 10.1042/bj1240475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan P. T., Halestrap A. P. The mechanism of the hormonal activation of respiration in isolated hepatocytes and its importance in the regulation of gluconeogenesis. Biochem J. 1986 Jun 15;236(3):789–800. doi: 10.1042/bj2360789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan P. T., Thomas A. P., Armston A. E., Halestrap A. P. Measurement of the intramitochondrial volume in hepatocytes without cell disruption and its elevation by hormones and valinomycin. Biochem J. 1983 Aug 15;214(2):395–404. doi: 10.1042/bj2140395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLATER E. C., CLELAND K. W. The effect of tonicity of the medium on the respiratory and phosphorylative activity of heart-muscle sarcosomes. Biochem J. 1953 Mar;53(4):557–567. doi: 10.1042/bj0530557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson D. Carnitine palmitoyltransferase in extrahepatic tissues. Biochem Soc Trans. 1986 Aug;14(4):679–681. doi: 10.1042/bst0140679. [DOI] [PubMed] [Google Scholar]
- Scherer B., Klingenberg M. Demonstration of the relationship between the adenine nucleotide carrier and the structural changes of mitochondria as induced by adenosine 5'-diphosphate. Biochemistry. 1974 Jan 1;13(1):161–170. doi: 10.1021/bi00698a025. [DOI] [PubMed] [Google Scholar]
- Scholte H. R., Luyt-Houwen I. E., Dubelaar M. L., Hulsmann W. C. The source of malonyl-CoA in rat heart. The calcium paradox releases acetyl-CoA carboxylase and not propionyl-CoA carboxylase. FEBS Lett. 1986 Mar 17;198(1):47–50. doi: 10.1016/0014-5793(86)81182-6. [DOI] [PubMed] [Google Scholar]
- Soboll S., Bünger R. Compartmentation of adenine nucleotides in the isolated working guinea pig heart stimulated by noradrenaline. Hoppe Seylers Z Physiol Chem. 1981 Feb;362(2):125–132. doi: 10.1515/bchm2.1981.362.1.125. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Diggle T. A., Denton R. M. Sensitivity of pyruvate dehydrogenase phosphate phosphatase to magnesium ions. Similar effects of spermine and insulin. Biochem J. 1986 Aug 15;238(1):83–91. doi: 10.1042/bj2380083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipps D. E., Halestrap A. P. Rat liver mitochondria prepared in mannitol media demonstrate increased mitochondrial volumes compared with mitochondria prepared in sucrose media. Relationship to the effect of glucagon on mitochondrial function. Biochem J. 1984 Jul 1;221(1):147–152. doi: 10.1042/bj2210147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Jamieson D. Metabolic effects of epinephrine in the perfused rat heart. I. Comparison of intracellular redox states, tissue pO2 and force of contraction. Mol Pharmacol. 1966 May;2(3):191–205. [PubMed] [Google Scholar]


