Abstract
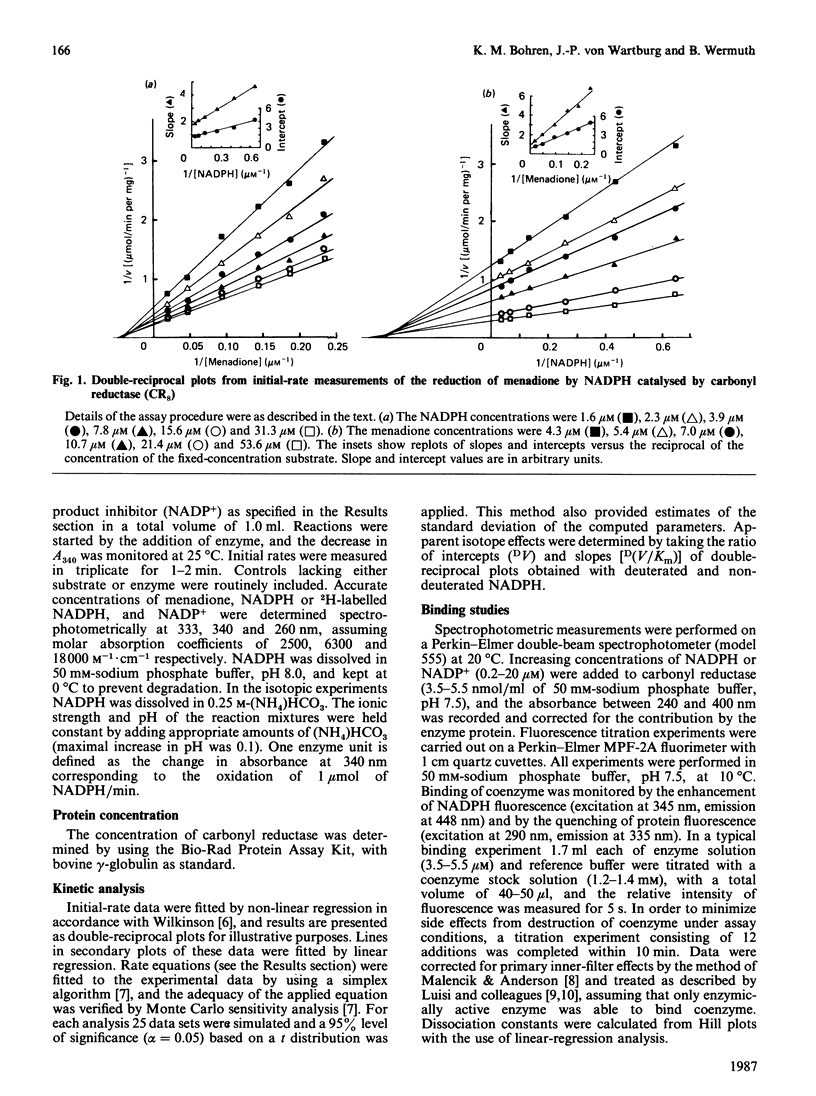
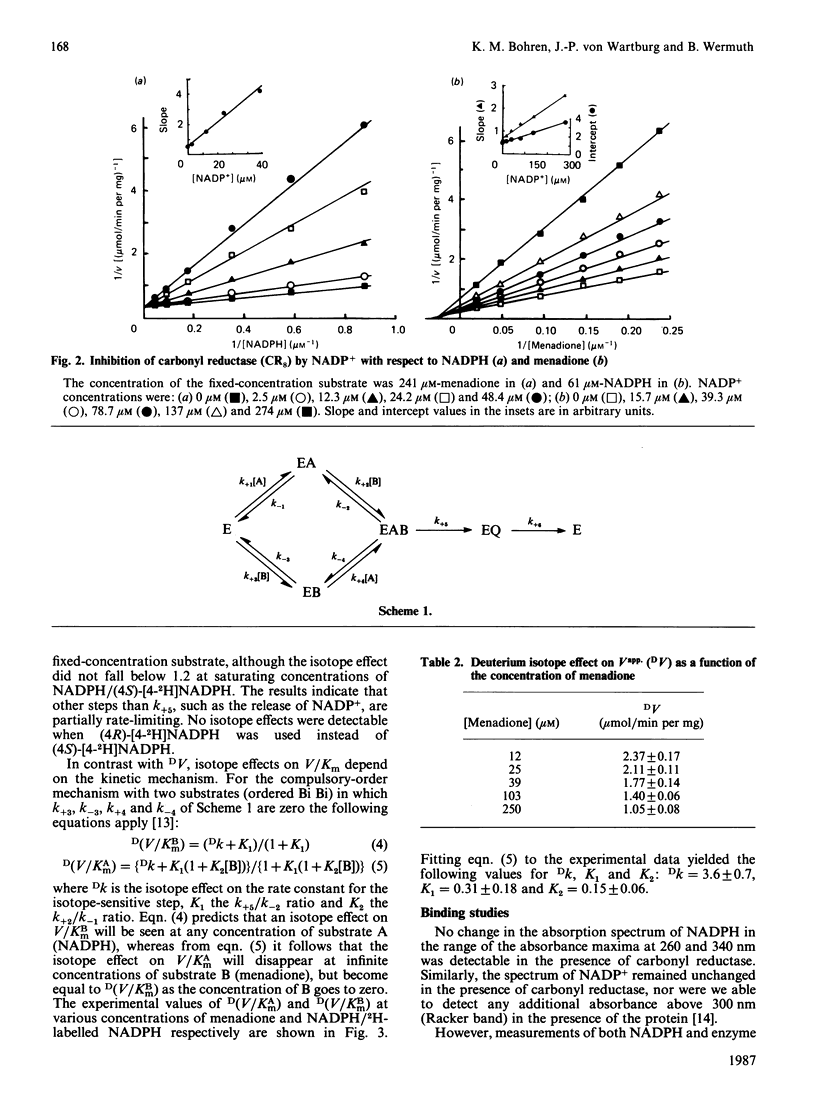
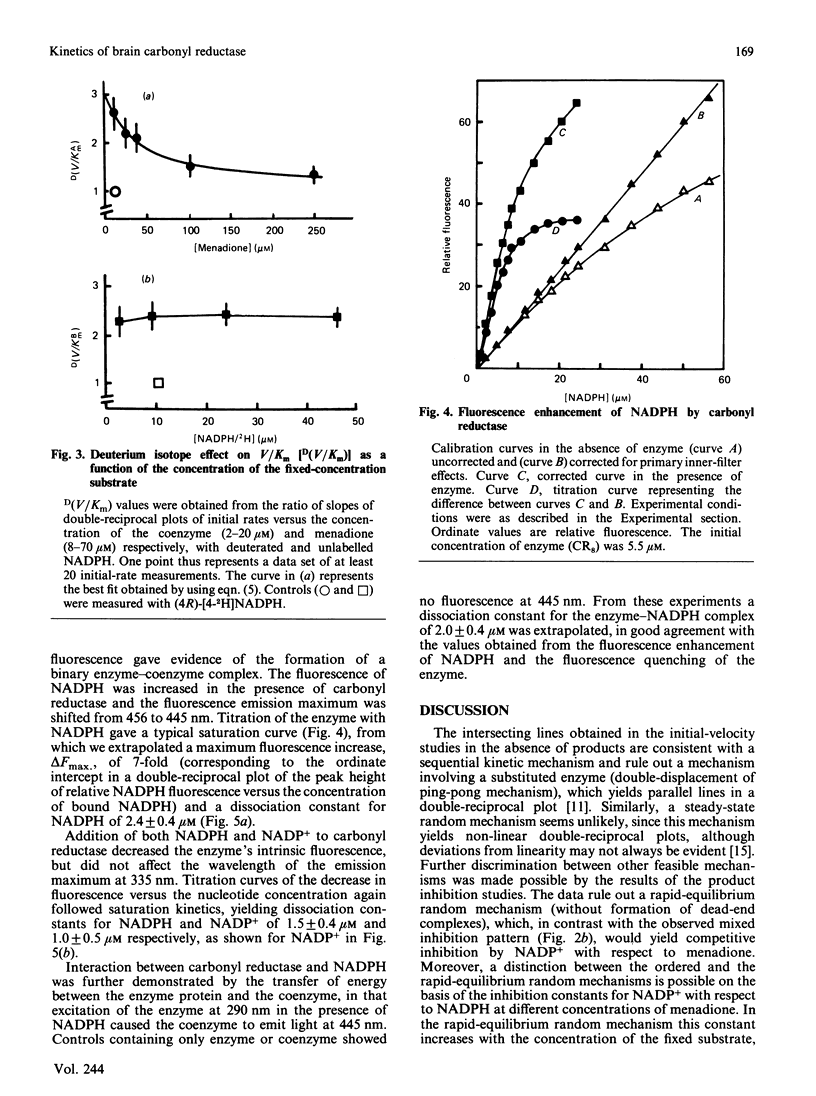
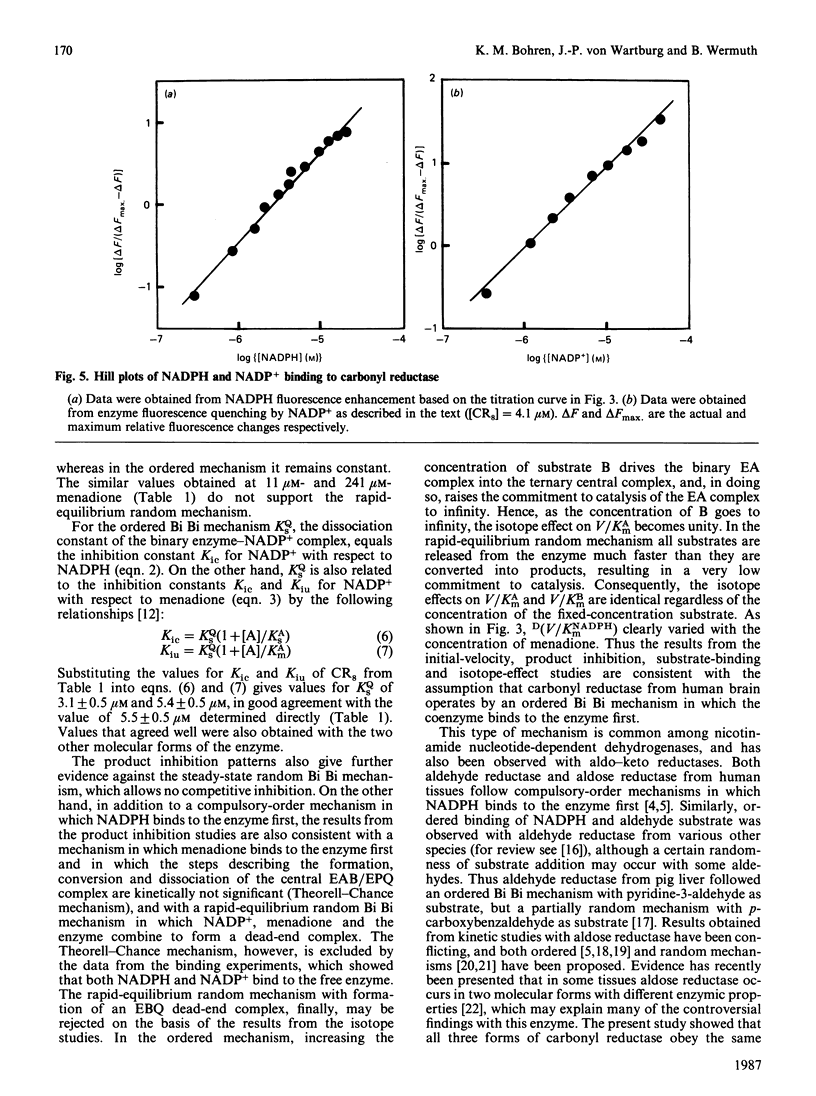
Initial-rate analysis of the carbonyl reductase-catalysed reduction of menadione by NADPH gave families of straight lines in double-reciprocal plots consistent with a sequential mechanism being obeyed. The fluorescence of NADPH was increased up to 7-fold with a concomitant shift of the emission maximum towards lower wavelength in the presence of carbonyl reductase, and both NADPH and NADP+ caused quenching of the enzyme fluorescence, indicating formation of a binary enzyme-coenzyme complex. Deuterium isotope effects on the apparent V/Km values decreased with increasing concentrations of menadione but were independent of the NADPH concentration. The results, together with data from product inhibition studies, are consistent with carbonyl reductase obeying a compulsory-order mechanism, NADPH binding first and NADP+ leaving last. No significant differences in the kinetic properties of three molecular forms of carbonyl reductase were detectable.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boghosian R. A., McGuinness E. T. Pig brain aldose reductase: a kinetic study using the centrifugal fast analyzer. Int J Biochem. 1981;13(8):909–914. doi: 10.1016/0020-711x(81)90017-3. [DOI] [PubMed] [Google Scholar]
- Branlant G., Biellmann J. F. Purification and some properties of aldehyde reductases from pig liver. Eur J Biochem. 1980 Apr;105(3):611–621. doi: 10.1111/j.1432-1033.1980.tb04539.x. [DOI] [PubMed] [Google Scholar]
- Branlant G. Properties of an aldose reductase from pig lens. Comparative studies of an aldehyde reductase from pig lens. Eur J Biochem. 1982 Dec;129(1):99–104. doi: 10.1111/j.1432-1033.1982.tb07026.x. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Cook P. F., Cleland W. W. Mechanistic deductions from isotope effects in multireactant enzyme mechanisms. Biochemistry. 1981 Mar 31;20(7):1790–1796. doi: 10.1021/bi00510a013. [DOI] [PubMed] [Google Scholar]
- Cromlish J. A., Flynn T. G. Purification and characterization of two aldose reductase isoenzymes from rabbit muscle. J Biol Chem. 1983 Mar 10;258(5):3416–3424. [PubMed] [Google Scholar]
- Das B., Srivastava S. K. Activation of aldose reductase from human tissues. Diabetes. 1985 Nov;34(11):1145–1151. doi: 10.2337/diab.34.11.1145. [DOI] [PubMed] [Google Scholar]
- Doughty C. C., Conrad S. M. A reaction mechanism for aldose reductase from lens. Biochim Biophys Acta. 1982 Nov 19;708(3):358–364. doi: 10.1016/0167-4838(82)90449-6. [DOI] [PubMed] [Google Scholar]
- Flynn T. G., Shires J., Walton D. J. Properties of the nicotinamide adenine dinucleotide phosphate-dependent aldehyde reductase from pig kidney. Amino acid composition, reactivity of cysteinyl residues, and stereochemistry of D-glyceraldehyde reduction. J Biol Chem. 1975 Apr 25;250(8):2933–2940. [PubMed] [Google Scholar]
- Halder A. B., Crabbe M. J. Bovine lens aldehyde reductase (aldose reductase). Purification, kinetics and mechanism. Biochem J. 1984 Apr 1;219(1):33–39. doi: 10.1042/bj2190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi P. L., Favilla R. Tryptophan fluorescence quenching in horse liver alcohol dehydrogenase. Eur J Biochem. 1970 Nov;17(1):91–94. doi: 10.1111/j.1432-1033.1970.tb01139.x. [DOI] [PubMed] [Google Scholar]
- Luisi P. L., Olomucki A., Baici A., Karlovic D. Fluorescence properties of octopine dehydrogenase. Biochemistry. 1973 Oct 9;12(21):4100–4106. doi: 10.1021/bi00745a012. [DOI] [PubMed] [Google Scholar]
- Magnien A., Branlant G. The kinetics and mechanism of pig-liver aldehyde reductase. Comparative studies with pyridine-3-aldehyde and p-carboxybenzaldehyde. Eur J Biochem. 1983 Mar 15;131(2):375–381. doi: 10.1111/j.1432-1033.1983.tb07273.x. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Reduced pyridine nucleotide binding to beef liver and dogfish liver glutamate dehydrogenases. Biochemistry. 1972 Jul 18;11(15):2766–2771. doi: 10.1021/bi00765a005. [DOI] [PubMed] [Google Scholar]
- Morpeth F. F., Dickinson F. M. Some properties of pig kidney-cortex aldehyde reductase. Biochem J. 1980 Nov 1;191(2):619–626. doi: 10.1042/bj1910619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson G. Asymptotic properties of enzymatic rate equations of the Wong-Hanes type. Acta Chem Scand. 1970;24(4):1271–1274. doi: 10.3891/acta.chem.scand.24-1271. [DOI] [PubMed] [Google Scholar]
- RACKER E., KRIMSKY I. The mechanism of oxidation of aldehydes by glyceralde-hyde-3-phosphate dehydrogenase. J Biol Chem. 1952 Oct;198(2):731–743. [PubMed] [Google Scholar]
- Ryle C. M., Tipton K. F. Kinetic studies with the low-Km aldehyde reductase from ox brain. Biochem J. 1985 Apr 15;227(2):621–627. doi: 10.1042/bj2270621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermuth B., Bürgisser H., Bohren K., von Wartburg J. P. Purification and characterization of human-brain aldose reductase. Eur J Biochem. 1982 Oct;127(2):279–284. doi: 10.1111/j.1432-1033.1982.tb06867.x. [DOI] [PubMed] [Google Scholar]
- Wermuth B. Human carbonyl reductases. Prog Clin Biol Res. 1982;114:261–274. [PubMed] [Google Scholar]
- Wermuth B., Münch J. D., von Wartburg J. P. Purification and properties of NADPH-dependent aldehyde reductase from human liver. J Biol Chem. 1977 Jun 10;252(11):3821–3828. [PubMed] [Google Scholar]
- Wermuth B. Purification and properties of an NADPH-dependent carbonyl reductase from human brain. Relationship to prostaglandin 9-ketoreductase and xenobiotic ketone reductase. J Biol Chem. 1981 Feb 10;256(3):1206–1213. [PubMed] [Google Scholar]


