Abstract
The small envelope protein of hepatitis B virus (HBsAg-S) can self-assemble into highly organized virus like particles (VLPs) and induce an effective immune response. In this study, a restriction enzyme site was engineered into the cDNA of HBsAg-S at a position corresponding to the exposed site within the hydrophilic a determinant region (amino acid [aa] 127–128) to create a novel HBsAg vaccine vector allowing surface orientation of the inserted sequence. We inserted sequences of various lengths from hypervariable region 1 (HVR1) of the hepatitis C virus (HCV) E2 protein containing immunodominant epitopes and demonstrated secretion of the recombinant HBsAg VLPs from transfected mammalian cells. A number of different recombinant proteins were synthesized, and HBsAg VLPs containing inserts up to 36 aa were secreted with an efficiency similar to that of wild-type HBsAg. The HVR1 region exposed on the particles retained an antigenic structure similar to that recognized immunologically during natural infection. VLPs containing epitopes from either HCV-1a or -1b strains were produced that induced strain-specific antibody responses in immunized mice. Injection of a combination of these VLPs induced antibodies against both HVR1 epitopes that resulted in higher titers than were achieved by vaccination with the individual VLPs, suggesting a synergistic effect. This may lead to the development of recombinant particles which are able to induce a broad anti-HCV immune response against the HCV quasispecies or other quasispecies-like infectious agents.
Hepatitis C virus (HCV) is now recognized as the major cause of non-A, non-B hepatitis. It has been estimated that about 170 million people worldwide are infected with HCV, of whom 70 to 80% will develop chronic liver disease, leading to cirrhosis in 10 to 20% and liver cancer (hepatocellular carcinoma) in 1 to 5% of chronically infected individuals (6). The linear, single-stranded, positive-sense HCV RNA genome of ca. 9.5 kb contains a single open reading frame (ORF) encoding a polyprotein which is cleaved into the individual mature viral proteins by host- and virus-specific proteinases. Three structural proteins have been identified, the core protein and two envelope proteins, E1 and E2.
It has been reported that cellular and humoral immune responses play a pivotal role in the host defense mechanism against HCV (8, 36). Most HCV carriers have circulating antibodies to the virus envelope proteins and to a region located in the extreme amino terminus of E2, hypervariable region 1 (HVR1), which has been reported to contain neutralizing B-cell epitopes and a T-cell epitope (16, 17, 44). HVR1 probably represents the major site of HCV genetic drift, with amino acid substitutions leading to escape from recognition by existing anti-HVR1 antibodies. Due to the variability within the HVR1 region, it has been proposed that these mutations are responsible for the persistence of HCV infection through neutralizing antibody escape mutants (25, 46, 52). Qualitative antibody changes accompany HVR1 epitope shifts during the clinical course of hepatitis (25). Antibodies to HVR1 can be protective against infection and contribute to the selective replication of HCV in chimpanzees (26).
Despite its hypervariability, some amino acid positions in HVR1 are highly conserved, and even variable positions are occupied by a limited number of amino acids. Mimotopes of HVR1 which react with antibodies from a range of patients have been identified (14, 37, 54). An understanding of the cross-reactivity of these antibodies or the induction of a spectrum of anti-HVR1 antibodies which react against different HVR1 sequences may be vital for the future development of a vaccine against HCV.
The particulate nature of virus-like particles (VLPs) generally induces a more effective immune response than denatured or soluble proteins. VLPs have a number of advantages over conventional immunogens as vaccines (20). Antigens from various infectious agents can be synthesized as VLPs in heterologous expression systems (20, 48). In addition to the ability of certain capsid or envelope proteins to self-assemble, these particles can be produced in large quantities and are easily enriched and purified. Vaccination with chimeric VLPs can induce both insert-specific B- and T-cell responses even in the absence of adjuvant (40); furthermore, VLPs cannot replicate and are noninfectious.
The hepatitis B virus (HBV) small envelope protein (HBsAg-S) has the capacity to self-assemble with host-derived lipids into empty envelope particles without the participation of nucleocapsids (reviewed in references 18, 29, and 32). These distinct subviral particles, produced as 22-nm-diameter spherical or filamentous forms, bud into the lumen of a pre-Golgi compartment and are subsequently secreted (24, 29, 32). During synthesis of these particles, HBsAg-S is cotranslationally inserted into the membrane of the endoplasmic reticulum to result in a short, luminally exposed N-terminal sequence, two transmembrane regions separated by a 50-amino-acid (50 aa) cytosolic loop, a luminal (external) 60 aa domain containing the major B-cell epitopes (the a determinant), and a glycosylation site. The a determinant consists of a limited number of epitopes and is located within a double-looped structure with the antigenically important residues between aa 122 and 150 of HBsAg-S (19, 23, 45). It is estimated that one 22-nm particle contains about 100 HBsAg-S molecules (2). These subviral HBsAg particles are used successfully worldwide for hepatitis B vaccination.
The aim of this study was to develop recombinant HBsAg particles containing foreign B-cell epitopes of the HVR1 region of the E2 protein of HCV inserted within the surface-oriented loop of HBsAg and to determine if these particles could induce humoral immune responses in mice. By using a range of foreign epitopes to induce a broad but specific immune response, this approach may have applications to a vaccine for HCV and other quasispecies-like viruses.
MATERIALS AND METHODS
Plasmids.
Plasmid pSVHBs (22) was used as the template to amplify the gene for HBsAg-S. Two oligonucleotides (On#23 and On#24, Table 1) were designed to create an AgeI restriction site in the HBsAg-S cDNA which encodes the a determinant region. The HBsAg-S-specific oligonucleotides On#24 and On#17 (Table 1), which anneal in the pSVL vector region upstream of the multiple cloning site, were used to amplify the 5′ half of the HBsAg-S-specific cDNA. The HBsAg-S-specific oligonucleotides On#23 and On#9 (Table 1), which anneal to the pSVL vector region downstream of the multiple cloning site, were used to amplify the 3′ half of the HBsAg-S-specific cDNA. Both PCR products contain an EcoRI restriction site outside the HBsAg-S ORF and an AgeI restriction site within the HBsAg-S ORF. To obtain the construct pD3-HBs/AgeI, both PCR fragments encoding the 5′ and 3′ half of the HBsAg-S ORF were digested with EcoRI and AgeI, and the vector pcDNA3 (Invitrogen) was digested with EcoRI followed by ligation of the DNA molecules via the EcoRI and AgeI restriction sites. Ligation of the two PCR fragments via the AgeI site resulted in restoration of the complete HBsAg-S ORF with an AgeI restriction site at a position that corresponds to aa 127–128 within the HBsAg-S. Due to the introduction of this AgeI restriction site, the codon for aa 128-alanine of the wild-type HBsAg-S protein was mutated to glycine. To clone the construct pD3-HBsAg/AgeI-7, oligonucleotides On#33 and On#34 (Table 1) were annealed and ligated into pD3-HBs/AgeI via the AgeI restriction site. The construct pD3-HBsAg/AgeI-22 was generated in a similar manner by using On#35 and On#36 (Table 1). For the other constructs, an HCV cDNA template, genotype 1b (47), was used to amplify E2-specific products. The PCR product was digested with AgeI and then inserted into D3-HBs/AgeI. For the construction of plasmid pD3-HBsAg/AgeI-35-1a, a genotype 1a HCV cDNA template was used (27). The following sets of oligonucleotides were used; for pD3-HBsAg/AgeI-35-1a, On#63 and On#64 (Table 1); for pD3-HBsAg/AgeI-36-1b, On#44 and On#62 (Table 1); for pD3-HBsAg/AgeI-60, On#62 and On#69; and for pD3-HBsAg/AgeI-82, On#62 and On#70 (Table 1). Plasmid pSV27, which expresses large hepatitis delta virus antigen (L-HDAg) under the control of the simian virus 40 promoter, has been described (7).
TABLE 1.
Sequences of oligonucleotides
| Oligonucleotide | Sequence |
|---|---|
| On#9 | 5′-GATGAATTCTCACTGCATTCTAGTTGTGG-3′ |
| On#17 | 5′-GATGAATTCCTTCTGCTCTAAACCGGATCG-3′ |
| On#23 | 5′-GACTACCGGTCAAGGAACCTCTATGTATCC-3′ |
| On#24 | 5′-CTTGACCGGTAGTCATGCAGGTCCGGCATGG-3′ |
| On#33 | 5′-CCGGTGGGGACACCCACACGA-3′ |
| On#34 | 5′-CCGGTCGTGTGGGTGTCCCCA-3′ |
| On#35 | 5′-CCGGTGGGGACACCCACACGACGGGGGGGGTGGCGGGCCGCGACACGCTGCGCTTCACGGGGTTCA-3′ |
| On#36 | 5′-CCGGTGAACCCCGTGAAGCGCAGCGTGTCGCGGCCCGCCACCCCCCCCGTCGTGTGGGTGTCCCCA-3′ |
| On#44 | 5′-TGACTACCGGTGGTGTTTACAAGCTGGATC-3′ |
| On#62 | 5′-TGACTACCGGTGGGGACACCCACACGAC-3′ |
| On#63 | 5′-TGACTACCGGTGGGGAAACCCACGTCACCGGG-3′ |
| On#64 | 5′-TGACTACCGGTGTTGATCAGTTGGATG-3′ |
| On#70 | 5′-GACTACCGGTGATGGGGTGGCAGCTGGC-3′ |
Cell line and transfection.
The human hepatoma cell line HuH-7 (35) was grown in Dulbecco's modified Eagle's medium (Gibco-BRL) supplemented with GlutaMax-1 (Gibco-BRL), 10% fetal calf serum, penicillin, and streptomycin (Gibco-BRL).
Transfection.
HuH-7 cells were transfected by the Ca3(PO4)2 method as described (21). The supernatant was harvested 5 days later, and HBV surface antigen (HBsAg) was measured by the Abbott Prism HBsAg assay (Abbott Diagnostics). The level of HBsAg-S in the cell culture fluid was quantitated by comparison with a commercially available vaccine (Engerix-B; 20 μg/ml; SmithKline Beecham). The presence of L-HDAg was identified by immunoblot using a human anti-delta virus antibody and assayed by the ECL-Plus detection system (Amersham). The transfection efficiency of different plasmids was normalized to the activity of secreted alkaline phosphatase (SEAP) as described previously (1, 30). The variation in the range of SEAP activity was less than twofold.
Peptides.
Peptides representing the corresponding HVR1 regions of the HCV E2 protein, HVR1-1a and HVR1-1b, were synthesized at the Queensland Institute for Medical Research, Brisbane, Australia, and had a purity of at least 50%: HCV HVR1-1a genotype, ETHVTGGSAGRTTAGLVGLLTPGAKQN; and HCV HVR1-1b genotype; DTHTTGGVAGRDTLRFTGFFSFGPKQK. An unrelated peptide derived from the E7 protein of human papilloma virus type 16 (DSTLRLCVQSTHVDIRTL) was synthesized by Chiron Technologies.
ELISA.
Peptides (0.5 μg/well) in phosphate-buffered saline (PBS) were bound to microtiter plates (Maxisorb; Nunc) at 4°C overnight, and then each well was blocked in PBS containing gelatin (0.25%) and Tween 20 (0.1%) at room temperature for 2 h. Serum samples were incubated at an appropriate dilution in PBS with gelatin (0.125%) and Tween 20 (0.05%) for 1 h at 37°C. Bound antibody was detected with an anti-mouse or anti-human immunoglobulin (Ig) antibody conjugated to horseradish peroxidase (Dako). After several washing steps, antibody binding was detected by the addition of ABTS [2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid); Sigma] and H2O2 in a citrate phosphate buffer. Cell culture-derived VLPs expressing the HVR1-1b peptide were purified over a sucrose cushion and by a CsCl gradient (see below). Cell culture medium derived from untransfected HuH-7 cells was treated in the same way, and mock-treated fractions of the appropriate density were collected. The fractions were concentrated, and the particles were purified from the CsCl solution using a Microcon YM-100 filter device (Amicon). Each well was coated with about 500 ng of VLPs in PBS, as estimated by using the commercially available vaccine as a standard. The human serum sample was incubated in cell culture medium to decrease the background signal and then analyzed by enzyme-linked immunosorbent assay (ELISA). Results were deemed positive if the optical density of the medium value was above the OD of the negative control plus 2 standard deviations.
Human serum.
Human serum was derived from the patient from whom the Australian HCV isolate was cloned (47).
Animals.
C57BL/6 and BALB/c mice were used at 6 to 15 weeks of age. Within a given experiment, the mice were littermates or were closely age and sex matched. The mice were housed under specific-pathogen-free conditions. Groups of two to four mice were immunized subcutaneously at the base of the tail with 250 to 500 ng of recombinant HBsAg VLPs in the presence of Alhydrogel adjuvant (a kind gift from Peter Cooper, Australian National University, Canberra). Mice used as negative controls were immunized with adjuvant alone. Mice were bled from the retro orbital plexus at intervals, and antibody levels were measured by ELISA.
Centrifugation.
Cell culture supernatant containing VLPs was overlaid on a 20% sucrose cushion (20% sucrose in STE buffer (100 mM NaCl, 10 mM Tris [pH 8], and 1 mM EDTA), and centrifuged for 16 h at 23,000 rpm (AH-629 rotor, Sorvall). The partially purified VLPs were resuspended in HEPES buffer and used for vaccination procedures. To determine the buoyant density of the VLPs and for purification purposes, the resuspended VLPs were loaded onto a 10 to 40% (wt/wt) CsCl step gradient (in STE buffer) and centrifuged for 22 h at 36,000 rpm (SW41 Ti; Beckman), and 200- to 300-μl fractions were taken from the bottom of the tube. The fractions containing VLPs were identified by the Prism HBsAg assay (Abbott). The positive fractions were desalted, concentrated, washed with PBS by using Microcon YM-100 (Millipore) filter devices, and used for electron microscopy studies.
Electron microscopy.
Particles in PBS were visualized by negative staining with 1% ammonium molybdate.
RESULTS
HBsAg-S/HCV HVR1 chimeric proteins.
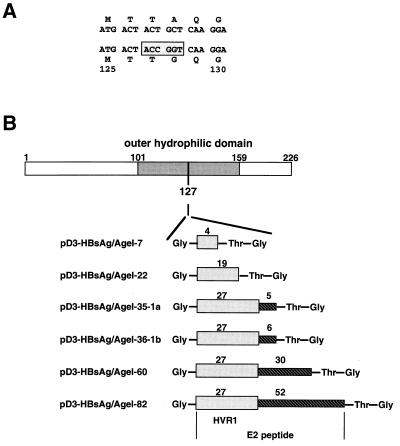
Anti-HVR1 antibodies have been shown to be neutralizing, and some human sera with anti-HVR1 activity showed a degree of cross-reactivity to different HVR1 variants (37, 38). Consequently, we used the immunodominant HVR1 region as the foreign sequence to be inserted into the HBsAg-S subviral particles. To synthesize these VLPs, we modified the HBsAg-S gene to create a new AgeI restriction site that permitted insertion of the HVR1 into an exposed region of the major external hydrophilic loop of the a determinant. The construct was designed to ensure a surface orientation of the inserted HCV-specific B-cell epitope(s). The new AgeI site within the HBsAg-S ORF led to an alanine-to-glycine change at position 128 (Fig. 1A). A series of cDNA sequences encoding HCV-specific peptides of different lengths were inserted into the AgeI site. Each insert begins with a glycine, followed by the HVR1 sequence of the E2 protein derived from the Australian HCV-1b isolate (47), then by threonine and glycine at the C-terminal end of the insert. The different plasmids encoded 4, 19, or the complete 27 aa of the HVR1 region (Fig. 1B). Plasmids encoding the complete HVR1 region also contained the downstream 6, 30, or 52 aa of the E2 protein, as indicated (Fig. 1B). In addition, one construct (pD3-HBsAg/AgeI-35-1a) which expressed the complete HVR1 polypeptide and the downstream 5 aa derived from an HCV-1a isolate was created (27).
FIG. 1.
Illustration of the strategy used to insert the HCV HVR1 peptide into the hydrophilic loop of HBsAg-S. (A) Part of the HBsAg-S nucleotide and corresponding amino acid sequence before and after introduction of the AgeI cloning site. The numbers indicate the amino acid position within HBsAg-S. The nucleotide sequence within the rectangle represents the AgeI site. The introduction of this restriction enzyme site leads to an alanine-to-glycine change at position 128. (B) Constructs derived by inclusion of HCV sequences into the AgeI cloning site of the modified HBsAg-S DNA sequence. The first amino acid is glycine, which is not part of the sequence of the HCV E2 protein; the last two amino acids (threonine and glycine) are encoded by the AgeI nucleotide sequence downstream of the HCV E2 insert. The shaded rectangle indicates the HVR1 region of E2, and the hatched rectangle represents the E2 sequence downstream of the HVR1 region. The numbers above the shaded and hatched rectangles indicate the number of encoded amino acids of the corresponding HVR1 region and the downstream E2 region. The HBsAg-S sequence between aa 101 and 159 represents the outer hydrophilic domain.
Recombinant particles are secretion competent.
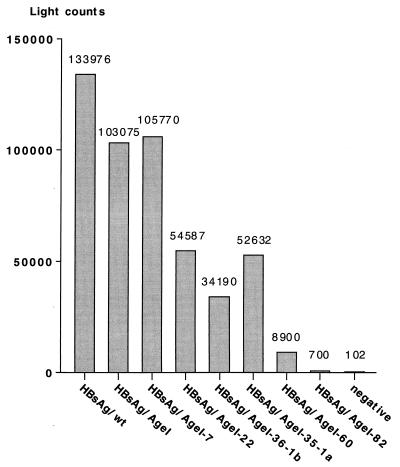
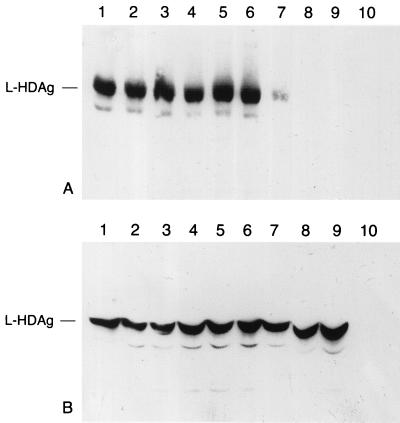
Individual plasmids containing the above constructs were transfected into HuH-7 cells, and the cell culture fluid was harvested 5 days later and tested for secreted HBsAg by an in vitro chemiluminescent immunoassay based on an anti-HBsAg IgM antibody (Abbott Prism). The transfections were standardized by a SEAP assay; a representative result with the corrected light counts is shown in Fig. 2. The A128G mutation resulted in an apparent reduction in the level of secretion from pD3-HBsAg/AgeI to approximately 77% compared with the wild-type HBsAg. Insertion of 7 aa also decreased the number of light counts to about 79%, while increasing the length of the insert beyond this resulted in an even greater apparent reduction in light counts. As a result, the HBsAg which contained an insert of 82 aa showed a level of HBsAg activity in the cell culture fluid similar to that of the negative control (Fig. 2). However, the decrease in HBsAg levels may reflect either decreased secretion efficiency of the recombinant HBsAg proteins or decreased affinity of the anti-HBsAg IgM antibody resulting from the insertions. To address this question, cotransfections with a plasmid expressing the large hepatitis delta virus antigen (L-HDAg) were performed. L-HDAg can only be packaged and secreted in the presence of functional HBV envelope proteins, with HBsAg-S being sufficient for packaging (3, 51). In this case, secretion was quantified by measurement of L-HDAg in the supernatant by immunoblot analysis (Fig. 3A); the presence of the expressed L-HDAg in the corresponding cell pellets is shown in Fig. 3B. The results of this experiment showed that recombinant HBsAg-S proteins containing up to 35 or 36 additional aa in the external loop (Fig. 3A, lanes 5 and 6) were as efficient as wild-type HBsAg-S (Fig. 3A, lane 1) in supporting L-HDAg secretion. Furthermore, secretion of L-HDAg also indicated that these recombinant HBsAg-S proteins retained the structural features necessary for the L-HDAg/ HBsAg-S interaction. Thus, the combined data from this and the previous experiment suggest that HBsAg/Agel-35-1a and HBsAg/AgeI -36-1b were secreted with an efficiency similar to that of wild-type HBsAg-S. On the other hand, coexpression with recombinant envelope proteins containing an insert of 60 aa resulted in a decreased potential to support L-HDAg secretion (Fig. 3A, lane 7), and the recombinant protein with 82/aa inserted into HBsAg-S was totally unable to support L-HDAg secretion (Fig. 3A, lane 8). These larger insertions may interfere with the stability or the secretion ability of the recombinant HBsAg-S and/or may lead to conformational changes which preclude L-HDAg secretion.
FIG. 2.
Detection of recombinant HBsAg in cell culture fluid. HuH-7 cells were cotransfected with plasmids encoding one of the HBsAg proteins, a plasmid encoding L-HDAg (see Fig. 3), and pSEAP. Supernatants were harvested, and HBsAg was measured by a chemiluminescence assay. The light counts were normalized by an SEAP assay.
FIG. 3.
Detection of L-HDAg in the presence of the different recombinant HBsAg proteins. (A) Cell culture supernatant (10 ml) (identical samples as used in Fig. 2) was pelleted through a sucrose cushion, resuspended in sample buffer, and analyzed by an immunoblot specific for HDAg. Expression of L-HDAg in the presence of (lane 1) HBsAg wild type, (lane 2) HBsAg/AgeI, (lane 3) HBsAg/AgeI-7, (lane 4) HBsAg/AgeI-22, (lane 5) HBsAg/AgeI-36-1b, (lane 6) HBsAg/AgeI-35-la, (lane 7) HBsAg/AgeI-60, (lane 8) HBsAg/AgeI-82, (lane 9) no HBsAg, and (lane 10) neither HDAg-L nor HBsAg. (B) Analysis of the corresponding cell pellets for the presence of L-HDAg.
Characterization of recombinant particles.
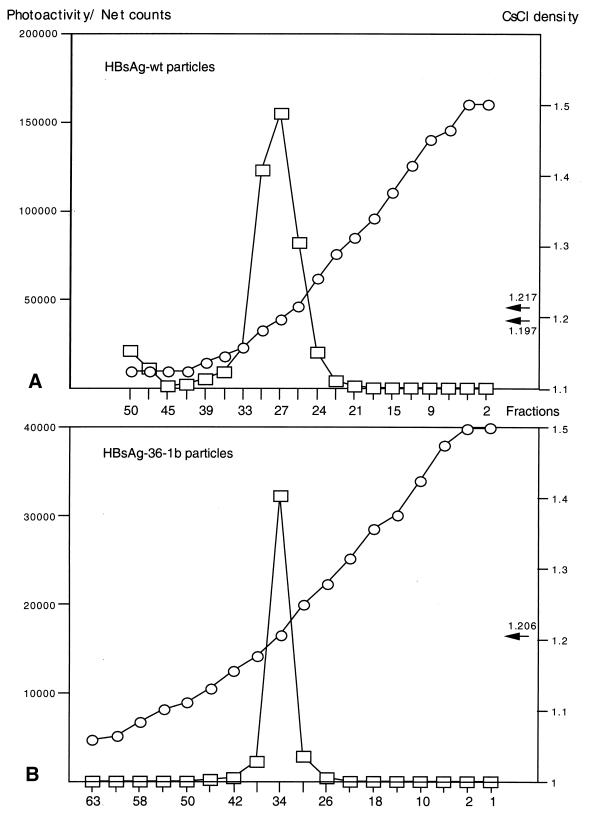
Although the above HBsAg preparations containing insertions of 36 aa appeared to be secreted and recognized by the Prism HBsAg assay, it was important to determine if particle formation occurred. To this end, plasmids expressing wild-type HBsAg-S or the recombinant HBsAg/AgeI-36-1b protein were transfected independently into HuH-7 cells, the cell culture medium was collected, and the particles were concentrated and purified by centrifugation through a 20% sucrose cushion followed by a CsCl density gradient. The HBsAg content of individual gradient fractions was measured by the Prism HBsAg assay. Wild-type and recombinant HBsAg were detected in fractions with a density of 1.2 g/ml (Fig. 4). As this represents the density of wild-type HBsAg particles (13, 34), this provides strong evidence that the recombinant HBsAg formed particles in a similar manner to wild-type HBsAg.
FIG. 4.
Equilibrium density gradient analysis of HBsAg VLPs isolated from cell culture fluid. The VLPs were centrifuged through a 20% sucrose cushion, resuspended in PBS, and then centrifuged to equilibrium on preformed step gradients of CsCl (10 to 40% [wt/wt]). (A) Wild-type (wt) HBsAg particles. (B) Recombinant particles expressing HVR1-1b.
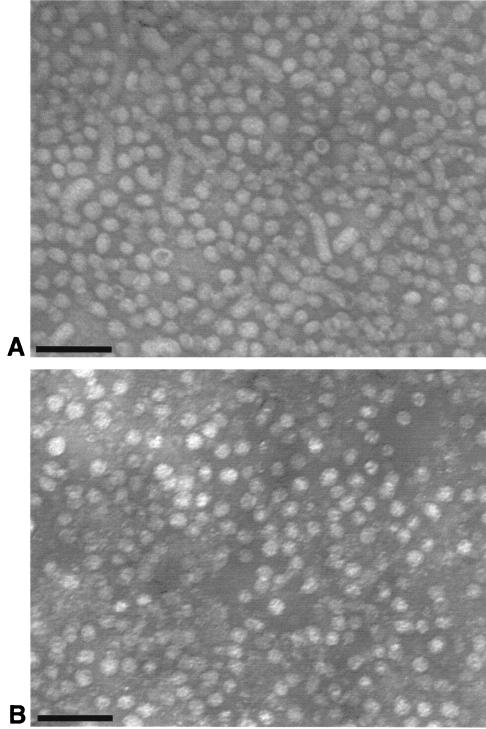
To confirm this, the putative particles were examined by electron microscopy. Particles were derived from an HBV chronic carrier and from the supernatant of HuH-7 cells transfected with the plasmid expressing HBsAg/AgeI-36-1b and then purified as described above. Both samples contained particles of approximately 22 nm (Fig. 5), and the particles derived from the recombinant HBsAg were virtually indistinguishable from wild-type particles. Filaments and Dane particles were not present in the sample derived from the recombinant protein.
FIG. 5.
Identification of particles by electron microscopy. (A) Particles derived from the serum of a chronic HBV carrier. (B) Recombinant particles derived from the construct pD3-HBsAg/AgeI-36-1b. Bars, 100 nm.
Reactivity of recombinant particles with human serum.
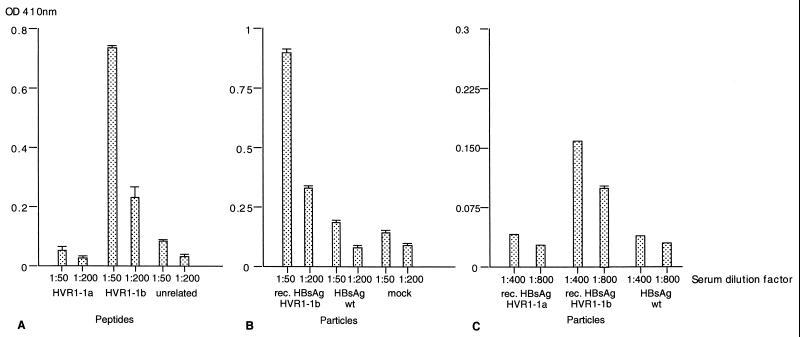
The recombinant particles were then examined to determine if the HCV HVR1 region was displayed on the surface of the recombinant 22-nm particles and if the associated antigenicity was retained. As the Australian isolate of HCV (genotype 1b) was cloned from a single individual who acquired acute hepatitis C after receiving an allogeneic bone marrow transplant (47), the serum from this patient was examined by ELISA for antibodies directed against the HVR1 region, initially using HVR1-specific peptides and later using the recombinant particles. HVR1-specific peptides were used that represented the sequence of the authentic Australian HCV1-1b isolate and an HVR1-1a isolate (27). The human serum showed a specific reaction with the HVR1-1b peptide but did not react with the HVR1-1a peptide or an unrelated peptide (Fig. 6A). We then investigated if the HVR1-1b epitope present in the VLPs had retained its antigenicity. The serum reacted exclusively with the VLPs with the HVR1-1b epitope but not with VLPs containing the HVR1-1a epitope, wild-type VLPs, or the mock-treated fraction (Fig. 6B and C). This indicated that the HVR1-1b epitope present within the a determinant of HBsAg-S had retained its antigenicity and was most likely displayed on the surface of the VLP in a conformation identical or similar to the conformation of HVR1 during natural infection.
FIG. 6.
Reactivity of recombinant particles with human serum as determined by ELISA. (A) Serum from a patient infected with HCV was tested against peptides representing HVR1-1a and HVR1-1b sequences and an unrelated peptide. (B and C) Two independently performed assays testing the human serum against recombinant (rec.) HBsAg particles containing the HVR1-1a epitope or HVR1-1b epitope, wild-type (wt) HBsAg particles, and a control fraction derived from the cell culture fluid of untransfected HuH-7 cells (mock).
Recombinant VLPs are immunogenic in mice.
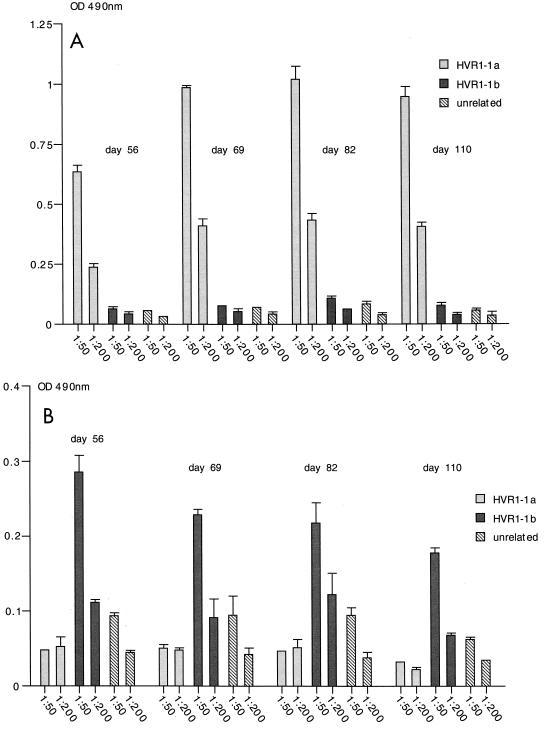
We then wished to determine if the recombinant HBsAg particles containing the complete HVR1 region were immunogenic in mice. The particles expressed from plasmids HBsAg/AgeI-35-1a and HBsAg/AgeI-36-1b (Fig. 1B) were partially purified and injected into mice. In two experiments, four mice were immunized with the HVR1-1a VLPs and four mice were immunized with the HVR1-1b VLPs. As determined by ELISA, the sera of all four mice injected with the HBsAg-S/HVR1-1a particles were reactive with the HVR1-1a peptide. A representative result for one mouse immunized with HBsAg/Agel-35-1a VLPs is shown for the 1:50 and 1:200 dilutions (Fig. 7A). The serum showed a highly specific immune response against the HVR1-1a peptide and was not reactive with the HVR1-1b peptide or an unrelated peptide. Similarly, in two experiments, four mice were immunized with the HVR1-1b particles, and two of four mice injected with the HBsAg-S/HVR1-1b particles were reactive with the HVR1-1b peptide. A representative result for one mouse immunized with HBsAg/AgeI-35-1b VLPs is shown for the 1:50 and 1:200 serum dilutions (Fig. 7B). The serum showed a highly specific immune response against the HVR1-1b peptide and was not reactive with the HVR1-1a peptide or an unrelated peptide. Mice immunized with HBsAg/AgeI-35-1a VLPs did not develop antibodies which were cross-reactive with the HVR1-1b peptide and vice versa. This is consistent with the above data from ELISA examination of the patient's serum, which, although reactive with the HVR1-1b peptide, showed no cross-reactivity with the HVR1-1a peptide (Fig. 6A and C).
FIG. 7.
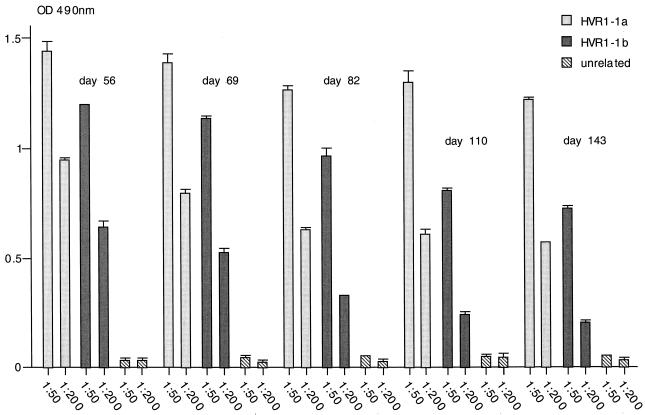
Immunogenicity of recombinant VLPs in mice as determined by ELISA. Serum samples were from a mouse immunized with HBsAg/AgeI-35-1a recombinant particles (A) or HBsAg/AgeI-35-1b recombinant particles (B). The mice were immunized three times on days 0, 33, and 47, and serum samples were taken on days 56, 69, 82, and 110 and tested (1:50 and 1:200 dilutions) against the HVR1-1a peptide, the HVR1-1b-specific peptide, and an unrelated peptide. The results show the mean OD of multiple tests and the standard deviation.
Immunization with a combination of VLPs.
To investigate whether antibodies could be raised simultaneously against the HVR1-1a and HVR1-1b epitopes, four mice were immunized with an equimolar mix of HBsAg/AgeI-35-1a and HBsAg/AgeI-36-1b VLPs, and the antibody response against the individual peptides was tested by ELISA. Serum samples from three of four mice reacted strongly with both epitopes, and the sample from the fourth mouse reacted weakly against the HVR1-1a epitope (titer between 1:50 and 1:200) but not against the HVR1-1b epitope (data not shown). A representative result for one mouse immunized with HBsAg/AgeI-35-1a and HBsAg/AgeI-36-1b VLPs is shown for the 1:50 and 1:200 serum dilutions (Fig. 8). The serum samples responded strongly to both HVR1-1a and HVR1-1b epitopes.
FIG. 8.
Induction of antibodies in mice immunized with a combination of VLPs as determined by ELISA. The mice were immunized three times on days 0, 33, and 47, and serum samples were taken on days 56, 69, 82, 110, and 143. The results shown are from a representative mouse immunized with a combination of HBsAg/AgeI-35-1a and HBsAg/AgeI-35-1b recombinant particles. Serum samples (diluted 1:50 and 1:200) were tested against the HVR1-1a peptide, the HVR1-1b-specific peptide, and an unrelated peptide. The results show the mean OD of multiple tests and the standard deviation.
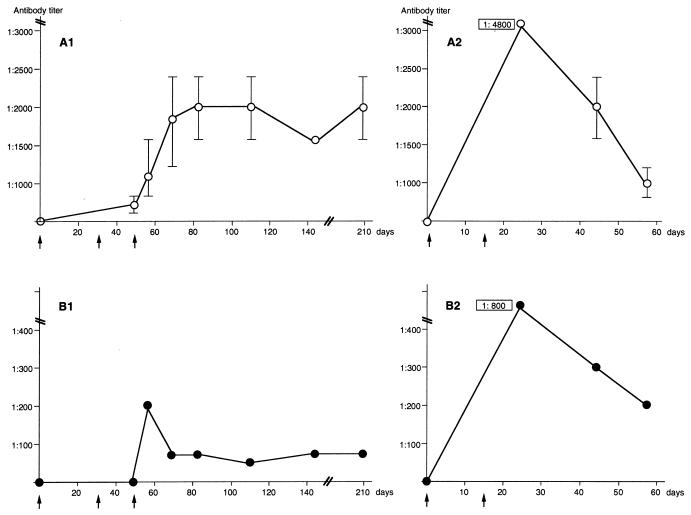
Antibody titer plotted as a function of time.
The OD values (Fig. 7A and B and Fig. 8) suggested that the VLPs containing the HVR1 epitopes were immunogenic, with the generation of a higher antibody titer after immunization with a combination of different VLPs. Hence, the sera harvested at different time points from each mouse were titrated. The titer of sera derived from four animals immunized with VLPs containing the HVR1-1a epitope in two independently performed experiments was determined. These data were plotted as a function of time (Fig. 9A1 and A2). The highest titer against the HVR1-1a epitope measured was 1:4,800 (Fig. 9A2). Similarly, four animals were immunized with VLPs containing the HVR1-1b epitope. One animal responded positively against the HVR1-1b epitope in each experiment (Fig. 9B1 and B2), with the highest titer measured being 1:800 (Fig. 9B2). The animals immunized with the VLPs carrying the HVR1-1a epitope did not develop antibodies reactive with the HVR1-1b and vice versa at any time point. For each set of experiments (Fig. 9A1 and B1 and Fig. 9A2 and B2) the antibody titer obtained after injection of VLPs containing the HVR1-1b epitope was lower than the titer obtained after immunization with VLPs containing the HVR1-1a epitope, suggesting that the HVR1-1b epitope was less immunogenic in mice. The anti-HVR1-1a antibody titer persisted at least to day 209 (162 days after the last booster injection), with titers of 1:1,600 and 1:2,400 for each of the two animals immunized with HVR1-1a-specific VLPs (Fig. 9A1). The anti-HVR1-1b antibody titer in the mouse immunized with HVR1-1b-specific VLPs was 1:75 at day 209 (Fig. 9B1).
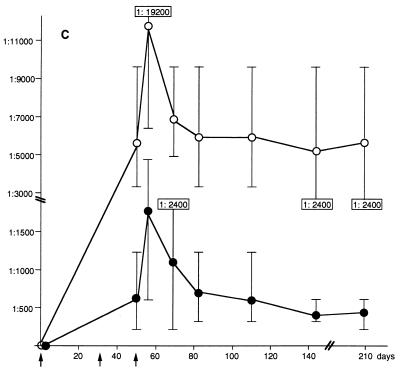
FIG. 9.
Antibody titer against HVR1 epitopes in mouse sera taken at different time points, shown as a function of time. Mice were immunized on days 0, 33, and 47 (indicated by arrows), and the serum was taken on days 0 (prebleed), 47, 56, 69, 82, 110, 143, and 209 (A1, B1 and C), or mice were immunized on days 0 and 15 and serum was taken on days 0 (prebleed), 24, 44, and 57 (A2 and B2). Antibody titers against the HVR1-1a epitope in two mice immunized with HBsAg/Age-35-1a (A1 and A2), against HVR1-1b in one mouse immunized with HBsAg/Age-36-1b (B1 and B2), and in three mice immunized with a combination of particles (C). ○, antibody titer against the HVR1-1a epitope; ●, antibody titer against the HVR1-1b epitope. The titer is given as the arimethic mean, and the bars indicate the range of antibody titers obtained. The values shown in panels A2, B2, and C indicate titers outside the range of the y axis.
In parallel to the experiments shown in Fig. 9A1 and B1, the antibody titers from three mice immunized with a combination of HVR1-1a and HVR1-1b-specific VLPs were plotted against time. The highest antibody titer obtained was 1:19,200 against the HVR1-1a epitope and 1:4,800 against the HVR1-1b epitope (Fig. 9C). The antibody titer obtained persisted at least to day 209, with an arimethic mean of 1:5,600 against the HVR1-1a epitope (range, 1:9,600 to 1:2,400) and with a mean of 1:470 against the HVR1-1b epitope (range, 1:600 and 1:200) (Fig. 9C). In both instances, these titers were higher than those generated by immunization with the individual recombinant particles. The results suggest that a synergistic effect may account for the higher titers resulting from immunization with the mixed recombinant particles.
DISCUSSION
For optimal immunogenicity, epitopes should ideally be presented as several copies on a defined particulate structure (20). Subviral HBsAg particles are used for hepatitis B vaccination, and because of their particulate structure, they are useful as a carrier matrix for foreign epitopes. We have created particles with an insertion site that is localized within the major antigenic site (a determinant) of the HBsAg protein. HCV-specific epitopes were inserted, chimeric particles were synthesized, and the insertion of up to 36 aa did not interfere with the secretion efficiency compared to wild-type particles. The antigenicity of the HCV-specific epitope was retained, and the particles were able to induce an immune response against the foreign epitope.
Because of its intrinsic immunogenic potential, particulate HBsAg has been investigated thoroughly in terms of antigen preparation and mode of delivery (39–41). It has also been used as a carrier for the presentation of foreign epitopes like tetanus toxoid (4), the glycoprotein D of herpes simplex virus (49), capsid protein of poliovirus (9, 11, 12), antigens derived from the malaria parasite (29, 50), HCV (31), and human immunodeficiency virus (33). The external hydrophilic loop is a preferred site of insertion, and the BamHI restriction site (corresponding to aa 112 and 113) within the HBsAg gene has been used to insert DNA fragments of various lengths (10). This resulted in expression of the foreign epitope close to the transmembrane region that may prevent an optimal surface orientation of the foreign insert. Moreover, insertions of short peptides into this site tended to reduce the level of secretion of the corresponding particles, while chimeric proteins with insertions of 24 and 33 aa failed to be secreted (10). In contrast, Lee et al. (31) reported the secretion of chimeric HBsAg particles with an insertion of the HCV E2/HVR1 domain at aa position 112–113, suggesting that the level of secretion may depend on the inserted sequence itself. This is in agreement with the observation by Delpeyroux et al. (10, 12). Particles with foreign sequences expressed within the external loop but close to the transmembrane region were shown to induce a B-cell immune response against the foreign epitope in the presence of Freund's adjuvant (11, 12).
To avoid any deleterious effects on secretion that may indicate a misfolding of the recombinant protein resulting from insertion of foreign sequences close to the transmembrane region and to ensure a surface orientation of the inserted foreign peptide on the VLP, we engineered an insertion site for foreign sequences into the cDNA encoding the a determinant. We have chosen sequences derived from the HVR1 region of the HCV envelope protein E2. It has been shown that antibodies to HVR1 interfered with virus attachment (42, 53, 54), provided an effective prophylaxis in chimpanzees (15, 17), and prevented HCV infection in cell culture (43, 55) and in chimpanzees (5, 16).
Due to the importance of anti-HVR1 antibodies, we created VLPs with the HVR1 peptide expressed within an exposed region of the major antigenic site of HBsAg and investigated the immunogenicity of these recombinant particles in the presence of aluminum hydroxide (Alhydrogel). These particles contained the HCV epitopes within the first loop of the exposed a determinant. Recombinant HBsAg-S proteins with insertions of up to 36 aa could support the secretion of L-HDAg as efficiently as wild-type HBsAg. This indicates that the recombinant and wild-type envelope proteins were secreted with the same efficiency. Moreover, we showed that these recombinant proteins were able to assemble into particles that were recognized by an anti-HBsAg IgM monoclonal antibody (Abbott Prism). Thus, the overall structure of the recombinant proteins was not changed dramatically, and consequently the foreign epitope within the a determinant was expected to be exposed in a surface-oriented way. A human anti-HCV-positive serum which contains anti-HVR1-1b antibodies was reactive with the HVR1-1b epitope contained within the a determinant. This confirmed that the HVR1 epitope was not only exposed but retained a conformation which mimics or is close to the natural conformation. The recombinant HBsAg particles were injected into mice in the presence of aluminum hydroxide. It was previously shown that the adsorption of HBsAg to aluminum hydroxide induced a strong B-cell immune response but inhibited the induction of a specific CD8+ cytotoxic T lymphocyte response in vivo (39–41). Since the HBV vaccine used for humans contains aluminum hydroxide and its use is most successful, the insertion of the HVR1 sequence into the major antigenic site of HBsAg was expected to elicit a prominent antibody response against the foreign peptide. The recombinant particles comprised of the proteins HBsAg/Agel-35-1a and HBsAg/AgeI-36-1b were able to induce a corresponding antibody response in mice. Mouse antibodies raised against the HVR-1b epitope expressed on VLPs could immunoprecipitate the corresponding E2 protein synthesized in BHK cells (S. Greive, personal communication). This indicates that these antibodies recognize native epitopes presented on the E2 protein, in agreement with the observation that the natural conformation of the HVR1 epitope was retained within HBsAg-S. The immune response against HBsAg/AgeI-35-1a was stronger than that against HBsAg/AgeI-36-1b, perhaps due to the presence of a more potent T-helper epitope in proximity to the B-cell epitope (44). Immunization with a combination of both particles raised an immune response against both the HVR1-1a and -1b epitopes. Hence, the antigen contained in one population of particles is not immunodominant over the epitopes presented by the second population. After immunization with a combination of two types of VLPs, we observed a higher antibody titer compared with the titers obtained after immunization with either VLP type alone. The combination of two particles doubled the total amount of carrier protein injected, although the total amount of each foreign epitope inserted into the carrier protein was the same. Therefore, the number of T-helper epitopes present within the carrier protein was also doubled, and this probably contributed to the higher antibody responses.
We have combined different particles and injected these into mice, but it has also been shown that mixed particles can be synthesized in which both HBsAg and a recombinant HBsAg with a poliovirus-specific epitope are presented. These mixed particles induced antibodies to both the HBsAg and the poliovirus-specific epitope (11). Therefore, it may be possible to use a combination of different recombinant particles to induce an appropriate immune response against a range of different foreign epitopes.
In this article, we have reported the development of a novel HBsAg vector with generic significance for the delivery of foreign epitopes. We have demonstrated the applicability of this approach to the generation of antibody responses to the HVR1 of HCV. The use of a combination of different particles which may themselves contain different epitopes may induce anti-HCV antibodies that are able to recognize a spectrum of HCV-specific sequences. Hence, these particles may have the potential to inhibit the development of sequentially emerging variants. A suitable combination of different hybrid particles may represent a “potpourri” vaccine required for successful vaccination against a quasispecies. Moreover, although it is likely that many potential recipients of such a vaccine will have existing anti-hepatitis B surface antigen, this can be expected to target the recombinant particles to antigen-presenting cells, and this may result in an enhanced immune response against the HVR1 epitope.
ACKNOWLEDGMENTS
We thank Charles Rice, who provided HCV-1a cDNA, and staff in the Australian Red Cross Blood Transfusion Service for performing the HBsAg assays. We are also grateful to Jason Mackenzie for assistance with the electron microscopy.
This work was supported by an NHMRC research grant and by the Royal Children's Hospital Foundation.
Footnotes
Manuscript number 126 from SASVRC.
REFERENCES
- 1.Berger J, Hauber J, Hauber R, Geiger R, Cullen B R. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eucaryotic cells. Gene. 1988;66:1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- 2.Bruss V, Gerhardt E, Vieluf K, Wunderlich G. Functions of the large hepatitis B virus surface protein in viral particle morphogenesis. Intervirology. 1996;39:23–31. doi: 10.1159/000150471. [DOI] [PubMed] [Google Scholar]
- 3.Chang F-L, Chen P-J, Tu S-J, Wang C-J, Chen D-S. The large form of hepatitis δ antigen is crucial for assembly of hepatitis δ virus. Proc Natl Acad Sci USA. 1991;88:8490–8494. doi: 10.1073/pnas.88.19.8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chengalvala M V, Bhat R A, Bhat B M, Vernon S K, Lubeck M D. Enhanced immunogenicity of hepatitis B surface antigen by insertion of a helper T cell epitope from tetanus toxoid. Vaccine. 1999;17:1035–1041. doi: 10.1016/s0264-410x(98)00318-1. [DOI] [PubMed] [Google Scholar]
- 5.Choo Q L, Kuo G, Ralston A, Weiner A, Chien D, Van Nest G, Han J, Berger K, Thudium K, Kuo C, Kansopon J, McFarland J, Tabrizi A, Ching K, Moss B, Cummins L B, Houghton M, Muchmore E. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA. 1994;91:1294–1298. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen J. The scientific challenge of hepatitis C. Science. 1999;285:26–30. doi: 10.1126/science.285.5424.26. [DOI] [PubMed] [Google Scholar]
- 7.Cole S. Ph. D. thesis. Adelaide, Australia: University of Adelaide; 1994. [Google Scholar]
- 8.Cooper S, Erickson A L, Adams E J, Kansopon J, Weiner A J, Chien D Y, Houghton M, Parham P, Walker C M. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439–449. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 9.Delpeyroux F, Chenciner N, Lim A, Malpièce Y, Blondel B, Crainic R, van der Werf S, Streeck R E. A poliovirus neutralization epitope expressed on hybrid hepatitis B surface antigen particles. Science. 1986;233:472–475. doi: 10.1126/science.2425433. [DOI] [PubMed] [Google Scholar]
- 10.Delpeyroux F, Chenciner N, Lim A, Lambert M, Malpièce Y, Streeck R E. Insertions in the hepatitis B surface antigen: effect on assembly and secretion of 22-nm particles from mammalian cells. J Mol Biol. 1987;195:343–350. doi: 10.1016/0022-2836(87)90655-3. [DOI] [PubMed] [Google Scholar]
- 11.Delpeyroux F, Peillon N, Blondel B, Crainic R, Streeck R E. Presentation and immunogenicity of the hepatitis B surface antigen and a poliovirus neutralizing antigen on mixed empty envelope particles. J Virol. 1988;62:1836–1839. doi: 10.1128/jvi.62.5.1836-1839.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delpeyroux F, van Wezel E, Blondel B, Crainic R. Structural factors modulate the activity of antigenic poliovirus sequences expressed on hybrid hepatitis B surface antigen particles. J Virol. 1990;64:6090–6100. doi: 10.1128/jvi.64.12.6090-6100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubois M F, Pourcel C, Rousset S, Chany C, Tiollais P. Excretion of hepatitis B surface antigen particles from mouse cells transformed with cloned viral DNA. Proc Natl Acad Sci USA. 1980;77:4549–4553. doi: 10.1073/pnas.77.8.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esumi M, Ahmed M, Zhou Y, Takahashi H, Shikata T. Murine antibodies against E2 and hypervariable region 1 cross-reactively capture hepatitis C virus. Virology. 1998;251:158–164. doi: 10.1006/viro.1998.9393. [DOI] [PubMed] [Google Scholar]
- 15.Esumi M, Rikihisa T, Nishimura S, Goto J, Mizuno K, Zhou Y H, Shikata T. Experimental vaccine activities of recombinant E1 and E2 glycoproteins and hypervariable region 1 peptides of hepatitis C virus in chimpanzees. Arch Virol. 1999;144:973–980. doi: 10.1007/s007050050559. [DOI] [PubMed] [Google Scholar]
- 16.Farci P, Alter H J, Wong D C, Miller R H, Govindarajan S, Engle R, Shapiro M, Purcell R H. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci USA. 1994;91:7792–7796. doi: 10.1073/pnas.91.16.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farci P, Shimoda A, Wong D, Cabezon D, De Gioannis D, Strazzera A, Shimizu Y, Shapiro M, Alter H J, Purcell R H. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc Natl Acad Sci USA. 1996;93:15394–15399. doi: 10.1073/pnas.93.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganem D. Hepadnaviridae and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2703–2737. [Google Scholar]
- 19.Gavilanes F, Gonzalez-Ros J M, Peterson D L. Structure of hepatitis B surface antigen. J Biol Chem. 1982;257:7770–7777. [PubMed] [Google Scholar]
- 20.Gerlich W H, Krüger D H, Ulrich R, editors. Chimeric virus-like particles as vaccines. Intervirology. 1996;39:126–132. [Google Scholar]
- 21.Graham F L, van der Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 22.Harvey T J, Macnaughton T B, Gowans E J. The development and characterisation of a SV40 T -antigen positive cell line of human hepatic origin. J Virol Methods. 1997;65:67–74. doi: 10.1016/s0166-0934(96)02170-2. [DOI] [PubMed] [Google Scholar]
- 23.Howard C R, Stirk H J, Brown S E, Steward M W. Towards the development of synthetic hepatitis B vaccines. In: Zuckerman A J, editor. Viral hepatitis and liver disease. New York, N.Y: Alan R. Liss, Inc.; 1988. pp. 1094–1101. [Google Scholar]
- 24.Huovila A P, Eder A M, Fuller S D. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J Cell Biol. 1992;118:1305–1320. doi: 10.1083/jcb.118.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato N, Ootsuyama Y, Sekiya H, Ohkoshi S, Nakazawa T, Hijikata M, Shimotohno K. Genetic drift in hypervariable region 1 of the viral genome in persistent hepatitis C virus infection. J Virol. 1994;68:4776–4784. doi: 10.1128/jvi.68.8.4776-4784.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima M, Osuga T, Tsuda F, Tanaka T, Okamoto H. Influence of antibodies to the hypervariable region of E2/NS1 glycoprotein on the selective replication of hepatitis C virus in chimpanzees. Virology. 1994;204:665–672. doi: 10.1006/viro.1994.1582. [DOI] [PubMed] [Google Scholar]
- 27.Kolykhalov A A, Agapov E V, Blight K J, Mihalik K, Feinstone S M, Rice C M. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science. 1997;277:570–574. doi: 10.1126/science.277.5325.570. [DOI] [PubMed] [Google Scholar]
- 28.Lalvani A, Moris P, Voss G, Pathan A A, Kester K E, Brookes R, Lee E, Koutsoukos M, Plebanski M, Delchambre M, Flanagan K L, Carton C, Slaoui M, van Hoecke C, Ballou W R, Hill A V S, Cohen J. Potent induction of focused Th1-type cellular and humoral immune responses by RTS,S/SBAS2, a recombinant Plasmodium falciparum malaria vaccine. J Infect Dis. 1999;180:1656–1664. doi: 10.1086/315074. [DOI] [PubMed] [Google Scholar]
- 29.Laub O, Rall L B, Truett M, Shaul Y, Standring D N, Valenzuela P, Rutter W J. Synthesis of hepatitis B surface antigen in mammalian cells: expression of the entire gene and the coding region. J Virol. 1983;48:271–280. doi: 10.1128/jvi.48.1.271-280.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazinski D W, Taylor J M. Expression of hepatitis delta virus RNA deletions: cis and trans requirements for self-cleavage, ligation, and RNA packaging. J Virol. 1994;68:2879–2888. doi: 10.1128/jvi.68.5.2879-2888.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee I-H, Kim C-H, Ryu W-S. Presentation of the hydrophilic domains of hepatitis C viral E2 envelope glycoprotein on hepatitis B surface antigen particles. J Med Virol. 1996;50:145–151. doi: 10.1002/(SICI)1096-9071(199610)50:2<145::AID-JMV7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 32.Liu C C, Yansura D, Levinson A D. Direct expression of hepatitis B surface antigen in monkey cells from an SV40 vector. DNA. 1982;1:213–221. doi: 10.1089/dna.1.1982.1.213. [DOI] [PubMed] [Google Scholar]
- 33.Michel M-L, Mancini M, Sobczak E, Favier V, Guetard D, Bahraoui E M, Tiollais P. Induction of anti-human immunodeficiency virus (HIV) neutralizing antibodies in rabbits immunized with recombinant HIV-hepatitis B surface antigen particles. Proc Natl Acad Sci USA. 1988;85:7957–7961. doi: 10.1073/pnas.85.21.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriarty A M, Hoyer B H, Shih J W, Gerin J L, Hamer D H. Expression of the hepatitis B virus surface antigen gene in cell culture by using a simian virus 40 vector. Proc Natl Acad Sci USA. 1981;78:2606–2610. doi: 10.1073/pnas.78.4.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cell lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858–3863. [PubMed] [Google Scholar]
- 36.Nelson D R, Marousis C G, Davis G L, Rice C M, Wong J, Houghton M, Lau J Y. The role of hepatitis C virus-specific cytotoxic T lymphocytes in chronic hepatitis. J Immunol. 1997;158:1473–1481. [PubMed] [Google Scholar]
- 37.Puntoriero G, Meola A, Lahm A, Zucchelli S, Ercole B B, Tafi R, Pezzanera M, Mondelli M U, Cortese R, Tramontano A, Galfré G, Nicosia A. Towards a solution for hepatitis C virus hypervariability: mimotopes of the hypervariable region 1 can induce antibodies cross-reacting with a large number of viral variants. EMBO J. 1998;17:3521–3533. doi: 10.1093/emboj/17.13.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scarselli E, Cerino A, Esposito G, Silini E, Mondelli M U, Traboni C. Occurrence of antibodies reactive with more than one variant of the putative envelope glycoprotein (gp70) hypervariable region 1 in viremic hepatitis C virus-infected patients. J Virol. 1995;69:4407–4412. doi: 10.1128/jvi.69.7.4407-4412.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schirmbeck R, Melber K, Kuhröber A, Janowicz Z A, Reimann J. Immunization with soluble hepatitis B virus surface protein elicits murine H-2 class I-restricted CD8+ cytotoxic T lymphocyte responses in vivo. J Immunol. 1994;152:1110–1119. [PubMed] [Google Scholar]
- 40.Schirmbeck R, Melber K, Mertens T, Reimann J. Antibody and cytotoxic T-cell responses to soluble hepatitis B virus (HBV) S antigen in mice: implication for the pathogenesis of HBV-induced hepatitis. J Virol. 1994;68:1418–1425. doi: 10.1128/jvi.68.3.1418-1425.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schirmbeck R, Böhm W, Reimann J. Virus-like particles induce MHC class I-restricted T-cell responses. Intervirology. 1996;39:111–119. doi: 10.1159/000150482. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu Y K, Hijikata M, Iwamoto A, Alter H J, Purcell R H, Yoshikura H. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J Virol. 1994;68:1494–1500. doi: 10.1128/jvi.68.3.1494-1500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimizu Y K, Igarashi H, Kiyohara T, Cabezon T, Farci P, Purcell R H, Yoshikura H. A hyperimmune serum against a synthetic peptide corresponding to the hypervariable region 1 of hepatitis C virus can prevent viral infection in cell cultures. Virology. 1996;223:409–412. doi: 10.1006/viro.1996.0497. [DOI] [PubMed] [Google Scholar]
- 44.Shirai M, Arichi T, Chen M, Nishioka M, Ikeda K, Takahashi H, Enomoto N, Saito T, Major M E, Nakazawa T, Akatsuka T, Feinstone S M, Berzofsky J A. T cell recognition of hypervariable region-1 from hepatitis C virus envelope protein with multiple class II MHC molecules in mice and humans: preferential help for induction of antibodies to the hypervariable region. J Immunol. 1999;162:568–576. [PubMed] [Google Scholar]
- 45.Stirk H J, Thornton J M, Howard C R. A topological model for hepatitis B surface antigen. Intervirology. 1992;33:148–158. doi: 10.1159/000150244. [DOI] [PubMed] [Google Scholar]
- 46.Taniguchi S, Okamoto H, Sakamoto M, Kojima M, Tsuda F, Tanaka T, Munekata E, Muchmore E E, Peterson D A, Mishiro S. A structurally flexible and antigenically variable N-terminal domain of the hepatitis C virus E2/NS1 protein: implication for an escape from antibody. Virology. 1993;195:297–301. doi: 10.1006/viro.1993.1378. [DOI] [PubMed] [Google Scholar]
- 47.Trowbridge R, Gowans E J. Molecular cloning of an Australian isolate of hepatitis C virus. Arch Virol. 1998;143:501–511. doi: 10.1007/s007050050306. [DOI] [PubMed] [Google Scholar]
- 48.Ulrich R, Nassal M, Meisel H, Krüger D H. Core particles of hepatitis B virus as carrier for foreign epitopes. Adv Virus Res. 1998;50:141–182. doi: 10.1016/s0065-3527(08)60808-8. [DOI] [PubMed] [Google Scholar]
- 49.Valenzuela P, Coit D, Medina-Selby M A, Huo C H, van Nest G, Burke R L, Bull P, Urdea M S, Graves P V. Antigen engineering in yeast: synthesis and assembly of hybrid hepatitis B surface antigen-herpes simplex 1 gD particles. Bio/Technology. 1985;3:323–326. [Google Scholar]
- 50.Von Brunn A, Früh K, Müller H-M, Zentgraf H-W, Bujard H. Epitopes of the human malaria parasite P. falciparum carried on the surface of HBsAg particles elicit an immune response against parasite. Vaccine. 1991;9:477–484. doi: 10.1016/0264-410x(91)90032-2. [DOI] [PubMed] [Google Scholar]
- 51.Wang C-J, Chen P-J, Wu J-C, Patel D, Chen D-S. Small-form hepatitis B surface antigen is sufficient to help in the assembly of hepatitis delta virus-like particles. J Virol. 1991;65:6630–6636. doi: 10.1128/jvi.65.12.6630-6636.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiner A J, Geysen H M, Christopherson C, Hall J E, Mason T J, Saracco G, Bonino F, Crawford K, Marion C D, Crawford K A, Brunetto M, Barr P J, Miyamura T, McHutchinson J, Houghton M. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc Natl Acad Sci USA. 1992;89:3468–3472. doi: 10.1073/pnas.89.8.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ziebert A, Schreier E, Roggendorf M. Antibodies in human sera specific to hypervariable region 1 of hepatitis C virus can block viral attachment. Virology. 1995;208:653–661. doi: 10.1006/viro.1995.1196. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Y H, Moriyama M, Esumi M. Multiple-sequence-reactive antibodies induced by a single peptide immunization with hypervariable region 1 of hepatitis C virus. Virology. 1999;256:360–370. doi: 10.1006/viro.1999.9635. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Y H, Shimizu Y K, Esumi M. Monoclonal antibodies to the hypervariable region 1 of hepatitis C virus capture virus and inhibit virus adsorption to susceptible cells in vitro. Virology. 2000;269:276–283. doi: 10.1006/viro.2000.0227. [DOI] [PubMed] [Google Scholar]