Abstract
Background and Aims
Infections are a safety concern in patients with ulcerative colitis [UC]. Etrasimod is an oral, once daily [QD], selective sphingosine 1-phosphate [S1P]1,4,5 receptor modulator for the treatment of moderately to severely active UC. It leads to selective and reversible lymphocyte sequestration and partial peripheral lymphocyte count decrease. We report infection events from the phase 3 ELEVATE programme.
Methods
Proportions, incidence rates [IRs; per 100 patient-years], and descriptive analyses of all serious, severe, herpes zoster and opportunistic infections are reported in the Pivotal UC cohort [ELEVATE UC 52 and ELEVATE UC 12]. Cox regression models evaluated potential baseline risk factors.
Results
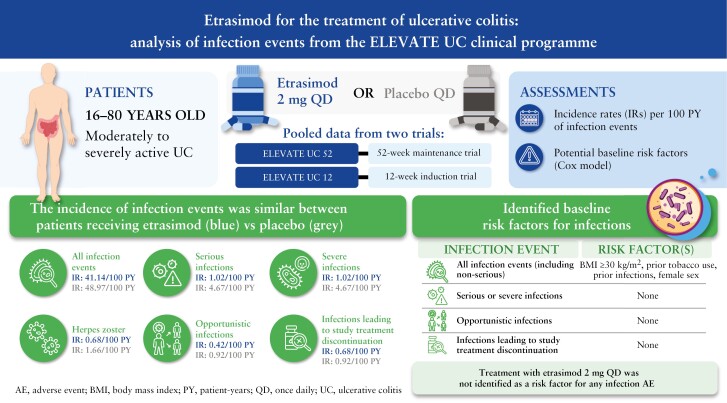
In this analysis [n = 787], proportions [IRs] of all infection events were similar for patients receiving etrasimod 2 mg QD (18.8% [41.1]) or placebo (17.7% [49.0]). Serious infections occurred in three [0.6%] and five [1.9%] patients receiving etrasimod and placebo, respectively. Two herpes zoster events were reported in each group [etrasimod: 0.4%; placebo: 0.8%], all localised and non-serious. One opportunistic infection event was reported in each group. No patient with an absolute lymphocyte count [ALC] < 0.2 × 109/L reported serious/severe or opportunistic infections; no baseline risk factors were identified for such events. No deaths occurred.
Conclusions
Patients receiving etrasimod demonstrated no increased risk of infection. The incidence of serious infections and herpes zoster was similar in each group. Among patients receiving etrasimod, no association between ALC < 0.5 × 109/L and infection events was observed. Longer-term follow-up will further characterise the etrasimod safety profile.
Clinicaltrials.gov: NCT03945188; NCT03996369
Keywords: Etrasimod, infections, ulcerative colitis
Graphical Abstract
Graphical Abstract.
1. Introduction
Ulcerative colitis [UC] is an immune-mediated, chronic disease of the colon, characterised by mucosal and submucosal inflammation.1,2 Mesalamines are used to treat UC; however, more than 70% of patients do not achieve control of inflammation with these therapies.3 Corticosteroids and immunomodulators, such as azathioprine, are common, well-established therapies for the management of UC, yet they are associated with a higher risk of infections.4 The development of newer therapies, such as biologic agents and small molecules, has provided more treatment options for patients with UC; however, some of these treatments are also associated with an increased risk of serious infections, opportunistic infections, or herpes zoster.5,6 Notably, a low risk of severe infections was one of the most important treatment attributes in a study that assessed the preferences of patients with inflammatory bowel disease [IBD].7 Therefore, there is a need for new and effective oral therapeutic options that address these safety concerns for patients with UC.
Etrasimod is an oral, once daily [QD], selective sphingosine 1-phosphate [S1P]1,4,5 receptor modulator for the treatment of moderately to severely active UC. The biological effect of etrasimod leads to selective, reversible, and partial lymphocyte sequestration in lymph nodes, with a decrease in peripheral lymphocyte count. Its pharmacokinetic profile includes a half-life of approximately 30 h, a wash-out period of 1 week, and a lack of active metabolites.8,9
In the ELEVATE UC clinical programme, the efficacy and safety of etrasimod in patients with moderately to severely active UC were demonstrated in a 52-week phase 3 study with a treat-through design [ELEVATE UC 52; NCT03945188], and a 12-week phase 3 induction study [ELEVATE UC 12; NCT03996369].8 Greater proportions of patients receiving etrasimod 2 mg QD vs placebo achieved the primary endpoint of clinical remission at both Weeks 12 and 52 in ELEVATE UC 52, and at Week 12 in ELEVATE UC 12. All key secondary efficacy endpoints were met in both trials. Etrasimod demonstrated a favourable safety profile consistent with previous studies.8
In this current study, we performed an integrated post hoc analysis to evaluate the incidence and clinical features of infections, and potential risk factors associated with infections, in patients with moderately to severely active UC who received etrasimod 2 mg QD or placebo in the ELEVATE UC clinical programme. These data can help guide treatment decisions for patients with UC and their providers.
2. Methods
2.1. Study design and patients
ELEVATE UC 52 and ELEVATE UC 12 were randomised, multicentre, double-blind, placebo-controlled, phase 3 studies. Full study design details have been previously described.8 Briefly, patients were aged 16–80 years with moderately to severely active UC (a modified Mayo score [MMS] of 4–9, a centrally read endoscopic subscore ≥ 2, and a rectal bleeding subscore ≥ 1) were randomised 2:1 to receive etrasimod 2 mg QD or placebo. Randomisation was stratified by prior biologic/Janus kinase inhibitor [JAKi] use, baseline corticosteroid use, and baseline MMS. Patients had a documented history of inadequate response, loss of response, or intolerance of at least one therapy approved for the treatment of UC, and were permitted to continue concomitant treatment with stable doses of oral 5-aminosalicylates [5-ASA] and/or oral corticosteroids (prednisone [≤20 mg/day], budesonide [≤9 mg/day], or equivalent), provided the dose was stable for ≥2 weeks prior to randomisation or ≥4 weeks prior to screening endoscopy assessment, respectively. Investigators were directed to taper corticosteroids in ELEVATE UC 52 after the Week 12 assessment. A documented presence of varicella zoster virus [VZV] immunoglobulin G [IgG] antibody or complete VZV vaccination was not required for inclusion in the ELEVATE UC clinical programme.
Both studies were approved by the institutional review board at each participating centre, and were conducted in compliance with the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice Guidelines. All patients provided written informed consent.
2.2. Infection events
This post hoc analysis used data from the Pivotal UC cohort, comprising pooled data from the ELEVATE UC 52 and ELEVATE UC 12 trials, to assess the incidence and risk factors of infections in patients receiving etrasimod 2 mg QD or placebo. Infection adverse events [AEs] included all infection events, serious infections, severe infections, herpes infections [herpes zoster and herpes simplex], and opportunistic infections. In addition, infection events leading to study treatment discontinuation are reported. Infection AEs were classified according to Medical Dictionary for Regulatory Activities [MedDRA], version 24.1. Infections were considered adverse events of special interest [AESI] if they were severe infections (Common Terminology Criteria for Adverse Events [CTCAE] Grade ≥ 3), opportunistic infections (including tuberculosis [TB]), or herpes infections [herpes simplex and herpes zoster]. Opportunistic infections were identified using Standardized MedDRA Query under narrow definition. The risk period for infection AEs was defined as the time from baseline to the date of the last study dose or date of the last study visit, whichever was later; only infection AEs within the risk period were counted.
In a per-protocol exploratory analysis, complete blood count [CBC] with differential was analysed at all visits in ELEVATE UC 52 and ELEVATE UC 12 for assessment of absolute lymphocyte count [ALC] and immunotyping. If ALC was confirmed as <0.2 × 109/L, study treatment was interrupted. Investigators were instructed to repeat CBC until ALC was >0.5 × 109/L. At this point, re-initiation of study treatment could be considered.
2.3. Statistical analyses
Demographics and baseline characteristics were summarised descriptively.
All infection events, serious, severe, herpes zoster, and opportunistic infections, as well as infection events leading to study treatment discontinuation, were evaluated as proportions (n [%]) and incidence rates [IRs], calculated as the number of unique patients with events per 100 patient-years [PY] adjusted to study stratification [ELEVATE UC 52 and ELEVATE UC 12]. The Cochran–Mantel–Haenszel [CMH] weight method was used with 95% confidence intervals [CIs], and was derived using normal approximation to the Poisson model. IRs for all infection events, serious, severe, herpes zoster, and opportunistic infections, as well as infection events leading to study treatment discontinuation, were reported overall, by subgroup [baseline characteristic] and by two time intervals (0–12 weeks [Day 1 to Day 99] and 12 weeks or after [Day ≥ 100]). Baseline characteristics that were evaluated included: age, sex, race, geographical region, body mass index [BMI], disease severity [MMS 4–6 vs 7–9], corticosteroid use, prior biologic or JAKi use, prior thiopurine use, use of proton pump inhibitors, tobacco use, duration of UC since diagnosis, history of infections, history of herpes zoster, history of diabetes, and baseline high-sensitivity C‑reactive protein [hsCRP]. Baseline ALC, absolute CD4 T cell count, absolute B cell count, and absolute neutrophil count [ANC] were also evaluated. Descriptive data stratified by last available ALC, absolute CD4 T cell count, and absolute B cell count prior to infection were also reported. The effect of etrasimod on circulating immune cells in adults with moderately to severely active UC was also assessed, reported by mean (standard error [SE]) percent change from baseline. Change and percentage change from baseline in lymphocyte counts were assessed per visit. Thresholds used in this analysis were based on CTCAE version 5 grades for ALC [<0.2, ≥0.2 to <0.5, ≥0.5 to <0.8, ≥0.8 × 109/L] and CD4 T cells [<50, ≥50 to <200, ≥200 to <441, ≥441 × 106/L].
Simple Cox model was used for each infection event on one baseline risk factor at a time, adjusting to treatment group and study stratification. Baseline risk factors with p < 0.1 from simple Cox models were subject to backward selection in a multivariable Cox model with stay criterion of p < 0.05, adjusting to treatment group and study stratification. Hazard ratios [HRs], 95% CIs, and p values were generated for each retained potential baseline risk factor in the final multivariable Cox model; p values were reported without adjustment to multiple comparisons. Additional details regarding the baseline characteristics are provided in Supplementary Table 1.
3. Results
3.1. Patient disposition and baseline characteristics
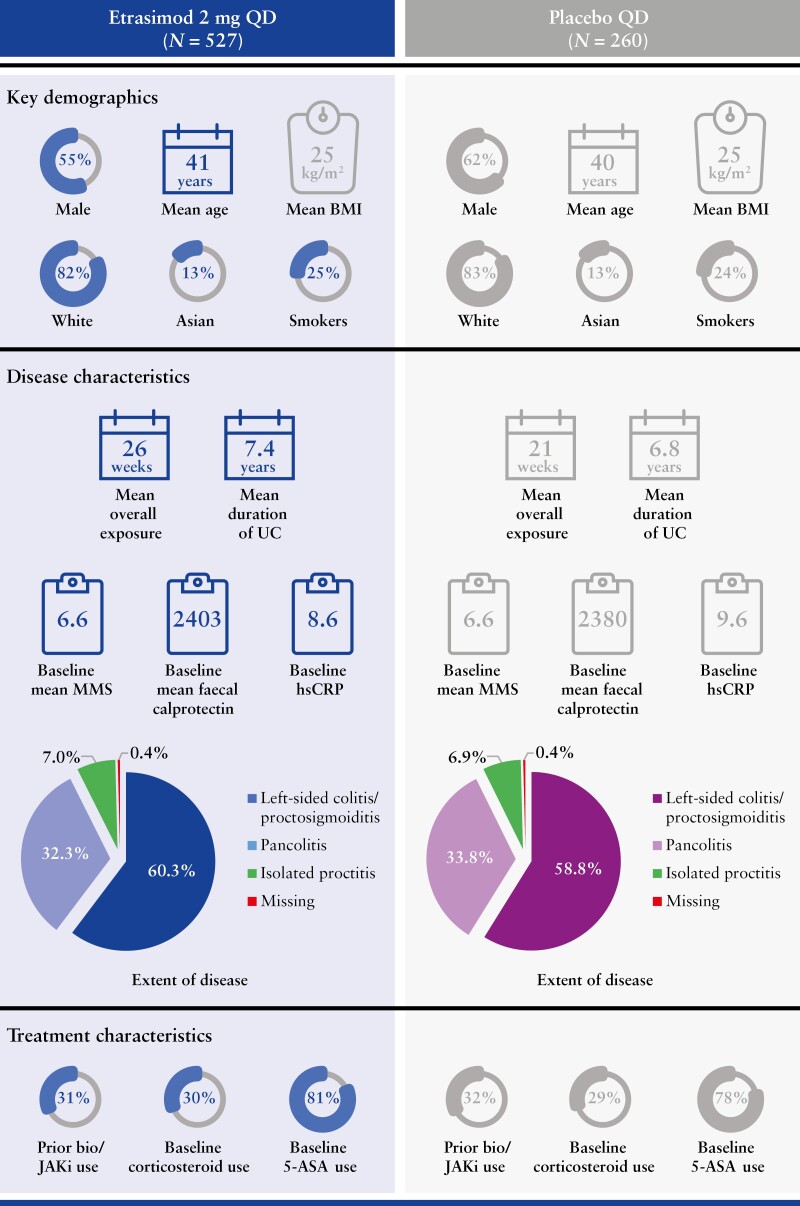
The Pivotal UC cohort included 787 patients [ELEVATE UC 52, n = 433; ELEVATE UC 12, n = 354]. Of these patients, 527 were randomised and received ≥1 dose of etrasimod [265.6 PY of exposure], and 260 patients were randomised to receive placebo [103.0 PY of exposure]. Patient demographics and baseline characteristics were generally similar between the treatment groups [Figure 1].
Figure 1.
Patient demographics and baseline disease characteristics in the pooled phase 3 ELEVATE UC 52 and ELEVATE UC 12 trials. 5-ASA, 5-aminosalicylates; BMI, body mass index; hsCRP, high-sensitivity C-reactive protein; JAKi, Janus kinase inhibitor; MMS, modified Mayo score; QD, once daily; UC, ulcerative colitis.
3.2. Infection events in the overall population
The proportions and IRs of infection events in the Pivotal UC cohort are shown in Table 1. Overall, infection rates were similar between the treatment groups, with infection events occurring in 99 [18.8%] patients in the etrasimod group (IR [95% CI] 41.14 [32.98, 49.30]) and 46 [17.7%] patients in the placebo group (IR [95% CI] 48.97 [34.80, 63.13]) during the risk period. COVID-19 was the most frequently reported infection in patients receiving etrasimod (n = 23 [4.4%]; unadjusted IR [95% CI] 8.55 [5.05, 12.04]) and in patients receiving placebo (n = 12 [4.6%]; unadjusted IR [95% CI] 11.85 [5.15, 18.56]). Infection events occurred in different organs/systems, and no specific aetiology was predominant among infection events.
Table 1.
Infection events evaluated in the pooled phase 3 ELEVATE UC 52 and ELEVATE UC 12 trials.
| Placebo QD [N = 260] |
Etrasimod 2 mg QD [N = 527] |
|||
|---|---|---|---|---|
| n [%]a | IR [95% CI] per 100 PYb | n [%]a | IR [95% CI] per 100 PYb | |
| Infections and infestationsc | 46 [17.7] | 48.97 [34.80, 63.13] | 99 [18.8] | 41.14 [32.98, 49.30] |
| Most frequent infections occurring in ≥1% of patients | ||||
| COVID-19c | 12 [4.6] | 11.85 [5.15, 18.56] | 23 [4.4] | 8.55 [5.05, 12.04] |
| Urinary tract infectionc | 3 [1.2] | 2.87 [0.00, 6.11] | 10 [1.9] | 3.68 [1.40, 5.95] |
| Nasopharyngitisc | 6 [2.3] | 5.76 [1.15, 10.37] | 6 [1.1] | 2.18 [0.44, 3.93] |
| Respiratory tract infection viralc | 2 [0.8] | 1.90 [0.00, 4.54] | 6 [1.1] | 2.19 [0.44, 3.95] |
| Serious infections | 5 [1.9] | 4.67 [0.42, 8.92] | 3 [0.6] | 1.02 [0.00, 2.31] |
| Infection AESId | ||||
| Severe infections | 5 [1.9] | 4.67 [0.42, 8.92] | 3 [0.6] | 1.02 [0.00, 2.31] |
| Herpes zoster | 2 [0.8] | 1.66 [0.00, 4.29] | 2 [0.4] | 0.68 [0.00, 1.78] |
| Herpes simplexc | 0 | N/Ae | 1 [0.2] | 0.36 [0.00, 1.07] |
| Oral herpesc | 1 [0.4] | 0.94 [0.00, 2.79] | 3 [0.6] | 1.09 [0.00, 2.32] |
| Opportunistic infectionsf | 1 [0.4] | 0.92 [0.00, 3.03] | 1 [0.2] | 0.42 [0.00, 1.38] |
| Infections leading to study treatment discontinuationg | 1 [0.4] | 0.92 [0.00, 3.03] | 2 [0.4] | 0.68 [0.00, 1.78] |
AESI, adverse event of special interest; CI, confidence interval; CMH, Cochran–Mantel–Haenszel; CMV, cytomegalovirus infection; CTCAE, Common Terminology Criteria for Adverse Events; IR, incidence rate; PY, patient-years; QD, once daily; TB, tuberculosis.
aMedDRA version 24.1 System Organ Class and Preferred Terms. Opportunistic infections were identified using Standardized MedDRA Query ‘Opportunistic Infection’ [narrow scope].
bIR [95% CI] was calculated adjusted to the study stratification using CMH weighting method at each level of a categorical baseline risk factor; 95% CI per 100 PY was estimated using normal approximation to Poisson model, adjusted per 100 PY. If n > 0 but the lower 95% CI limit <0, it was set to 0.
cIR [95% CI] was not study-adjusted.
dInfections were considered AESI if they were severe [CTCAE Grade ≥ 3], opportunistic infections, or herpes infections.
eIR per 100 PY and 95% CI were not calculated due to 0 patients with event.
fOne event of CMV was reported in the etrasimod 2 mg QD group; this was determined to be mild in severity and was detected by stool testing as a result of increased bowel movement frequency. One case of TB was reported in the placebo group.
gA total of three patients discontinued the study treatment due to infections; one patient each discontinued due to Clostridioides difficile infection and COVID-19 in the etrasimod group, and one patient in the placebo group discontinued due to TB. The patient who discontinued due to C. difficile infection had a positive stool pathogen test for C. difficile toxin at screening and was erroneously randomised in the study due to an oversight, which was considered a protocol deviation.
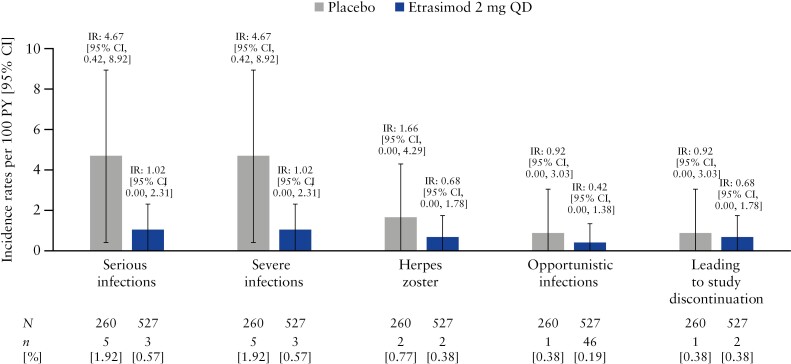
Among patients treated with etrasimod who had infections, the majority were non-serious (n = 96 [97.0%]) and did not lead to study treatment discontinuation (n = 97 [98.0%]). The proportion of patients with serious infections was higher in the placebo group (n = 5 [1.9%]) compared with the etrasimod group (n = 3 [0.6%]) and the IRs of serious infections were similar between the treatment groups (etrasimod: IR [95% CI] 1.02 [0.00, 2.31]; placebo: IR [95% CI] 4.67 [0.42, 8.92]; Figure 2) with overlapping 95% CIs [Table 1]. Infection AESIs were infrequent in both the etrasimod and placebo group [Table 1]. The proportions and incidence rates of herpes infections, including herpes zoster, oral herpes, and herpes simplex, were similar in both treatment groups, with overlapping 95% CIs for IRs [Table 1 and Figure 2]. Notably, all herpes zoster infection events were localised and non-serious. Only two opportunistic infections occurred in the Pivotal UC cohort [Table 1]. One event of TB was reported in the placebo group, and one event of cytomegalovirus infection [CMV] was reported in the etrasimod group. The patient with CMV withdrew from the study and discontinued treatment on Day 20, received rectal budesonide 2 mg from Days 20 to 40, and experienced a non-serious moderate AESI of CMV on Day 36 [stool antigen testing was performed for worsening of bowel movement frequency].
Figure 2.
Incidence rates per 100 PY for infection events [Pivotal UC cohort]. N, number of patients in the analysis set; n, number of patients with infection events; PY, patient-years; QD, once daily; UC, ulcerative colitis.
Overall, three events of infection led to study treatment discontinuation: two in the etrasimod group [both mild in severity], and one in the placebo group [moderate severity]. In the etrasimod treatment group, one patient discontinued due to a mild event of COVID-19 and one discontinued due to a mild event of Clostridioides [C.] difficile infection. This patient had a positive stool pathogen test for C. difficile toxin at screening and was erroneously randomised due to an oversight, which was considered a protocol deviation. No patients who received etrasimod discontinued the study due to serious infections, severe infections, herpes zoster infections, or opportunistic infections. One patient receiving placebo discontinued study treatment due to a moderate, non-serious event of TB. No events of progressive multifocal leukoencephalopathy [PML] were reported in patients treated with etrasimod in the ELEVATE UC clinical programme. No deaths were reported.
3.3. Infection events stratified by baseline characteristics
The IRs for all infection events were similar in the etrasimod and placebo treatment groups, both in the overall population and accounting for all baseline characteristics and subgroups that were assessed [Supplementary Material].
Among patients treated with etrasimod, the IR [95% CI] for all infection events in tobacco users at baseline was 78.32 [53.17, 103.47]; the IR [95% CI] for all infection events in non-tobacco users at baseline was 31.57 [23.60, 39.54]. In those with a history of infections [based on medical history] at baseline, the IR [95% CI] was 72.40 [48.80, 96.00], and in those without a history of infections at baseline, the IR [95% CI] was 32.76 [24.56, 40.97; Supplementary Table 2]. The IRs were similar in all other subgroups assessed.
CIs for severe infections in the etrasimod and placebo groups overlapped [or 95% CI were not calculated if no events occurred] for all subgroups [data not shown]. Consistent with the overall population previously described [Figure 1], among patients treated with etrasimod who had an infection, 30.0% [n = 29] were on concomitant corticosteroids at baseline. There were no events of severe infections among patients treated with etrasimod and corticosteroids at baseline. No patients treated with etrasimod with a medical history of diabetes had a serious or severe infection (3.61% [n = 19) of enrolled patients receiving etrasimod had a medical history of diabetes].
3.4. Infection incidence rates over time
Similar IRs for all infection events were observed in the etrasimod and placebo groups in both time intervals [0–12 weeks and after 12 weeks], with overlapping 95% CIs. The IR [95% CI] of infections [all events] was higher during the 0–12-week induction period in both treatment groups (etrasimod: IR [95% CI] 143.55 [108.67, 178.43]; placebo: IR [95% CI] 81.29 [51.81, 110.77]), compared with after 12 weeks (etrasimod: IR [95% CI] 15.85 [10.18, 21.53]; placebo: IR [95% CI] 27.15 [13.82, 40.47]). The IRs declined with continued treatment. In the first 12 weeks of treatment with etrasimod, there was one event of serious infection; no severe infections or herpes zoster infections were reported.
3.5. Assessment of potential risk factors
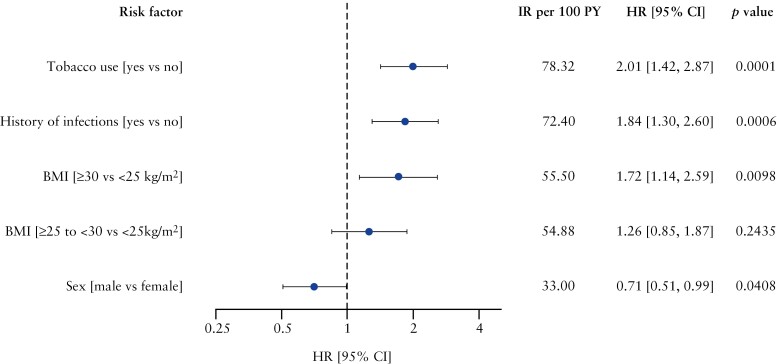
Results from the simple Cox regression models for all infection events, serious infections, severe infections, herpes zoster, opportunistic infections, and infections leading to study treatment discontinuation are shown in Supplementary Table 3. The final multivariable Cox model for all infection events [including non-serious events] identified a history of tobacco use vs no tobacco use (HR [95% CI] 2.01 [1.42, 2.87]; p = 0.0001), a history of infections vs no history of infections (HR [95% CI] 1.84 [1.30, 2.60]; p = 0.0006), body mass index [BMI] ≥ 30 kg/m2 vs BMI < 25 kg/m2 (HR [95% CI] 1.72 [1.14, 2.59]; p = 0.0098), and female sex [male sex vs female sex (HR [95% CI] 0.71 [0.51, 0.99]; p = 0.0408) as risk factors for all infection events [Figure 3]. These risk factors were identified for patients receiving etrasimod 2 mg QD and placebo. Regarding serious infections, severe infections, opportunistic infections, and infections leading to study treatment discontinuation, no baseline risk factors were identified using the multivariable Cox models due to few infection events that were reported during these trials. Treatment with etrasimod 2 mg QD was not identified as a risk factor for any of the aforementioned events.
Figure 3.
Multivariable Cox regression model of risk factors predicting all infection events [including non-serious] among patients receiving etrasimod 2 mg QD and placebo in the ELEVATE UC clinical programme. HRs [95% CI] are plotted on a logarithmic scale. A backward model selection algorithm was used on a multivariable Cox model that included effects for study [ELEVATE UC 52 and ELEVATE UC 12], treatment group [etrasimod 2 mg QD and placebo] and a set of candidate baseline risk factors selected from the corresponding simple Cox table [at least one comparison p-value < 0.1; Supplementary Table 3]. This candidate set of baseline risk factors was subject to backward model selection, except the study and treatment group which was mandatorily included in the model. No potential risk factors for serious, severe, opportunistic infections, or infection events leading to study treatment discontinuation were retained in the multivariable Cox model. BMI, body mass index; CI, confidence interval; HR, hazard ratio; IR, incidence rate; PY, patient-years; QD, once daily; UC, ulcerative colitis.
3.6. Effect of etrasimod on circulating lymphocyte subsets
Among patients treated with etrasimod, rapid mean percentage reductions from baseline to Week 2 were observed in total T cells [CD3+], T helper cells [CD3+CD4+], cytotoxic T cells [CD3+CD8+], and B cells [CD3-CD19+], with nadir and near nadir changes from baseline reached by Week 4 [Supplementary Figure 1A–D]. Reductions were maintained through Week 52 in ELEVATE UC 52 and Week 12 in ELEVATE UC 12. No clinically significant changes in natural killer cell [CD3-CD56+CD16+] or monocyte [CD14+] levels were observed during the ELEVATE UC clinical programme [Supplementary Figure 1E–F]. No patients permanently discontinued treatment with etrasimod due to decreased ALC.
3.7. Distribution of infection events according to lymphocyte counts
Of the 519 patients treated with etrasimod for whom ALC values were available, 39.7% [n = 206] had ALC < 0.5 × 109/L at any time post-baseline; in this same group, 3.5% [n = 18] had ALC < 0.2 × 109/L at any time post-baseline [Table 2]. Among the few events of serious infections and infection AESIs reported in the etrasimod group, most occurred in patients whose minimum ALC did not drop below 0.5 × 109/L at any time post-baseline [Table 2]. Conversely, among the 206 patients treated with etrasimod who had ALC < 0.5 × 109/L at any time post-baseline, only one had a serious/severe infection but was able to continue study treatment [Table 2]. All serious/severe, and opportunistic infections in the placebo group occurred in patients with ALC ≥ 0.5 × 109/L at any time post-baseline [Table 2]. All but one event of herpes zoster occurred in patients with ALC ≥ 0.5 × 109/L at any time post-baseline; one event [localised and non-serious] was reported in one patient with ALC < 0.5 × 109/L in the etrasimod group.
Table 2.
Infection events according to last ALC prior to infection event.
| Placebo QD [N = 260] |
Etrasimod 2 mg QD [N = 527] |
|
|---|---|---|
| Minimum ALC at any time post-baseline; n [%] | 254 [97.69] | 519 [98.48] |
| ALC < 0.2 × 109/L; n [%]a | 0 [0.0] | 18 [3.5] |
| Serious/severe infections; n | 0 | 0 |
| Opportunistic infections; n | 0 | 0 |
| Herpes zoster; n | 0 | 0 |
| Infections leading to discontinuation; n | 0 | 0 |
| ALC < 0.5 × 109/L; n [%]a | 5 [2.0] | 206 [39.7] |
| Serious/severe infections; n | 0 | 1 |
| Opportunistic infections; n | 0 | 0 |
| Herpes zoster; n | 0 | 1 |
| Infections leading to discontinuation; n | 0 | 0 |
| ALC ≥ 0.5 × 109/L; n [%]a | 249 [98.0] | 295 [56.8] |
| Serious/severe infections; n | 5 | 2 |
| Opportunistic infections; n | 1 | 1 |
| Herpes zoster; n | 2 | 1 |
| Infections leading to discontinuation; n | 1 | 2 |
ALC, absolute lymphocyte count; N, number of patients in the Pivotal UC cohort; n, number of patients with evaluable data; QD, once daily.
aPercentages are based on the number of patients with minimum ALC readings at any time post-baseline.
In the etrasimod group, the majority of patients for whom CD4 T cell counts were available [n = 518] had an absolute CD4 T cell count < 200 × 106/L at any time post-baseline (n = 418 [80.7%]). Among the 80 patients [15.4%] who had CD4 T cell counts dropping below 50 × 106/L at any time post-baseline, none subsequently reported a serious/severe, herpes zoster, or opportunistic infection. The incidence of serious infections and infection AESIs was low in patients with a CD4 T cell count < 200 × 106/L at any time post-baseline (n = 5 [1.2%]). In the placebo group, all serious/severe, herpes zoster, and opportunistic infections occurred in patients with normal [≥ 200 × 106/L] CD4 T cell count.
Among patients with an ANC value, the overall proportion of patients with abnormally low ANC [< 1 × 109/L] at any time post-baseline was similar in the etrasimod (n = 9 [1.7%]) and placebo (n = 4 [1.6%]) groups, and the majority of patients in both treatment groups had a normal ANC [≥ 1 × 109/L] at any time post-baseline. No patient with an abnormally low ANC [< 1 × 109/L] subsequently reported a serious/severe or herpes zoster infection.
4. Discussion
In this analysis of safety data from the ELEVATE UC clinical programme, we evaluated the incidence and risk factors for infection events in patients with UC receiving treatment with etrasimod 2 mg QD or placebo. We demonstrated that the overall incidence of all infection events, serious infections, and herpes zoster was similar between the treatment groups. Most cases were non-serious, study treatment discontinuations due to infections were infrequent, and the incidence of infections decreased over time. Notably, no patients treated with etrasimod discontinued the study due to serious, severe, herpes zoster, or opportunistic infections.
The identification of potential risk factors for infection events is relevant for clinical practice. In this analysis, the multivariable Cox model did not identify any potential risk factors for clinically relevant events of severe or opportunistic infections. This may have been partially due to the low number of events that were reported. Interestingly, demographics, such as age or exposure to corticosteroids, biologicals, or JAKi, were not associated with an increased risk of infections in the ELEVATE UC clinical programme, although these factors have previously been associated with infection risk in patients treated with other advanced therapies.10,11 It is also important to note that treatment with etrasimod was not identified as a risk factor for any infection event. In the ELEVATE UC pivotal trials, no patients treated with etrasimod and concomitant corticosteroids at baseline had a serious or severe infection. This is of interest given the well-known risk of infection associated with corticosteroid use.12,13
A selective modulator of S1P1,4,5, etrasimod induces rapid, reversible, and partial sequestration of circulating lymphocyte subsets, which in turn reduces the number of lymphocytes available to migrate to inflamed tissue in the colon.14,15 Despite this biological effect, we found no correlation between ALC < 0.5 × 109/L and infection events in patients receiving etrasimod, as only one serious/severe infection was reported among the 206 patients with low ALC [< 0.5 × 109/L] post-baseline. This could be explained by the selective mechanism of action, as etrasimod had no notable effect on innate immune cells, such as natural killer cells and monocytes, which are important components of immunosurveillance. There was also no clinically relevant effect on ANC. The effect of etrasimod is also partial, which means that a proportion of adaptive immune cells also remains in circulation.14,15 These factors may offer a potential explanation for the low incidence of infections observed in this study. Furthermore, unlike some existing S1P receptor modulators, such as ozanimod, etrasimod has no active metabolite and a short half-life of approximately 30 h, which leads to a 1-week wash-out period.16,17 In both ELEVATE UC 52 and ELEVATE UC 12, ALC returned to the normal range for around 80% of patients within 2 weeks after cessation of treatment.8 Therefore, lymphocyte recovery is relatively quick following cessation of treatment with etrasimod for most patients.
Our analysis regarding serious infections showed results consistent with those previously observed with other assessments of patients with multiple sclerosis or UC receiving a S1P receptor modulator, including etrasimod. A meta-analysis that included 1617 patients exposed to an S1P receptor modulator for the treatment of different immune-mediated diseases identified only one serious infection.18 With regards to herpes zoster infections, the findings from the current analysis appear to differ from previous studies of S1P receptor modulators19,20 and JAKi21 in UC. Our results show no imbalance between the etrasimod and placebo groups when it comes to herpes zoster infections, and enrolled patients were not required to have a documented presence of VZV IgG antibody or complete VZV vaccination. However, as a result of a low frequency of infection events in most of these studies, definitive safety comparisons with regards to herpes zoster infection cannot be made. It is also important to consider that, where available, the recombinant herpes zoster vaccine is recommended for all patients with IBD receiving advanced therapies, due to the increased risk of herpes zoster infection.5
We observed that the results of our analysis are consistent with those reported for the α4β7 integrin antibody vedolizumab. In the GEMINI 1 phase 3 study, 1.9% of patients treated with vedolizumab had a serious infection.22 These data help contextualise the proportions of patients with serious infections in our study, which was 0.6% in patients treated with etrasimod [vs 1.9% in those receiving placebo].
The incidence of opportunistic infections was also low in patients receiving etrasimod [0.4 per 100 PY; only one event of non-serious CMV infection, detected in the stool approximately 2 weeks after treatment discontinuation, was reported]. These results are in line with those reported in a meta-analysis that assessed the risk of opportunistic infections among patients receiving advanced UC therapies, including the S1P receptor modulator ozanimod, with an IR of 0.42/100 PY.23 By comparison, IRs for opportunistic infections were of similar magnitude to vedolizumab [an IR of 0.7/100 PY] in pooled data from phase 3 clinical trials.24 No events of PML or TB were reported in patients treated with etrasimod.
Our analysis has limitations. Although efforts have been made to make these studies similar to clinical practice, for example by including patients with diabetes, it is well known that patient populations from clinical trials may not be fully representative of everyday patients. The low number of patients with serious infections, severe infections, opportunistic infections, and infection events, leading to discontinuation, limited our assessment of baseline risk factors. In addition, ALC data close to the start of the infection events may not have been available, as these were collected in protocol-defined visits only. This is a post hoc analysis of two phase 3 clinical trials with 12 and 52 weeks of duration, respectively. However, it is worth noting that the 2.5-year pooled safety data from the etrasimod clinical development programme in UC showed similar results concerning the proportion of patients with infections.25 This suggests that continued treatment with etrasimod does not lead to an increased risk of infections; 5-year follow-up data will be provided from the ongoing open-label extension study [NCT03950232], which will further establish the long-term safety profile of etrasimod.
5. Conclusions
In this analysis of safety data from the ELEVATE UC clinical programme, the incidence of all infection events was similar in patients treated with etrasimod vs those who received placebo, and the incidence of infections did not increase over time; almost all infections were non-serious and did not lead to discontinuation. The incidence of herpes zoster was not increased in patients treated with etrasimod when compared with placebo. Although further research is needed, our results provide insight on the clinical relevance of the pharmacodynamic effects of etrasimod, showing that, although lymphocyte counts are reduced with treatment, immunosurveillance is maintained with no increased incidence of infection events. This analysis also expands upon the knowledge of the infection risk among patients treated with etrasimod, which is one of the key factors to consider when assessing the benefit-risk profile of advanced therapies.
Supplementary Data
Supplementary data are available at ECCO-JCC online.
Acknowledgements
The authors would like to thank the patients, investigators, and study teams who were involved in the ELEVATE UC clinical programme. This study was sponsored by Pfizer. Medical writing support, under the direction of the authors, was provided by Megan Melody, MSc, CMC Connect, a division of IPG Health Medical Communications, and was funded by Pfizer, New York, NY, USA, in accordance with Good Publication Practice [GPP 2022] guidelines.26
Contributor Information
Miguel Regueiro, Department of Gastroenterology, Hepatology, and Nutrition, Cleveland Clinic, Cleveland, OH, USA.
Britta Siegmund, Medizinische Klinik für Gastroenterologie, Infektiologie, Rheumatologie, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Andres J Yarur, Inflammatory Bowel Disease Center and Division of Gastroenterology and Hepatology, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Flavio Steinwurz, Unit of Inflammatory Bowel Disease, Hospital Israelita Albert Einstein, São Paulo, Brazil.
Krisztina B Gecse, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Amsterdam, The Netherlands.
Martina Goetsch, Pfizer AG, Zürich, Switzerland.
Abhishek Bhattacharjee, Pfizer Healthcare India Pvt Ltd., Chennai, India.
Joseph Wu, Pfizer Inc, Cambridge, MA, USA.
Jesse Green, Pfizer Inc, C, ollegeville, PA, USA.
Aoibhinn McDonnell, Pfizer Ltd, Sandwich, Kent, UK.
Catherine Crosby, Pfizer Inc, La Jolla, CA, USA.
Krisztina Lazin, Pfizer AG, Zürich, Switzerland.
Diogo Branquinho, Pfizer Inc, Ne, w York, NY, USA.
Irene Modesto, Pfizer Inc, Ne, w York, NY, USA.
Maria T Abreu, Department of Medicine, Division of Gastroenterology, Crohn’s and Colitis Center, University of Miami Miller School of Medicine, Miami, FL, USA.
Funding
This work was supported by Pfizer.
Conflict of Interest
MR reports grants and research support from AbbVie, Bristol Myers Squibb, Celgene, Genentech, Gilead, Janssen, Pfizer, Takeda, and UCB; consultancy fees from AbbVie, ALFASIGMA, S.p.A., Allergan, Amgen, Bristol Myers Squibb, Celgene, Genentech, Gilead, Janssen, Lilly, Miraca Labs, Pfizer, Prometheus, Salix, Seres, Takeda, TARGET Pharma Solutions, and UCB; and holds stock in Wolters Kluwer Health as an Author/Editor of UpToDate. BS reports research support from Pfizer; lecture fees from AbbVie, CED Service GmbH, Chiesi, Falk, Ferring, Gilead, Janssen, Lilly, Materia Prima, Pfizer, and Takeda; and consultancy fees from AbbVie, Abivax, Arena Pharma, Bristol Myers Squibb, Boehringer, CED Service GmbH, Celgene, CT-Scout, Endpoint Health, Falk, Forga Software, Galapagos, Gilead, Janssen, Lilly, Materia Prima, Pfizer, Pharma Insight, PredictImmune, PsiCro, and Takeda. AJY reports consultancy fees from Arena, Bristol Myers Squibb, Pfizer, and Takeda; and lecture/speaker fees from Bristol Myers Squibb. FS reports consultancy/speaker fees from AbbVie, Amgen, Celltrion, Eurofarma, Ferring, Janssen, Pfizer, Sandoz, and Takeda. KBG reports research support from Celltrion, Galapagos, and Pfizer; and speakers’ honoraria and/or consultancy fees from AbbVie, Celltrion, Ferring Pharmaceuticals, Immunic Therapeutics, Janssen, Novartis, Pfizer Inc, Samsung Bioepis, Takeda, and Tillotts. MG is an employee and shareholder of Pfizer AG. AB is an employee of Pfizer Healthcare India Pvt; and shareholder of Pfizer Inc. JW, JG, CC, DB, and IM are employees and shareholders of Pfizer Inc. AM is an employee of Pfizer Ltd, and a shareholder of Pfizer. KL is an employee of Pfizer AG, and a shareholder of Pfizer. MTA reports consulting fees from AbbVie, Bristol Myers Squibb, Celsius, Eli Lilly, Gilead, Janssen, Janssen Biotech, Pfizer, Prometheus, Prometheus Bioscience, Takeda, and UCB Biopharma.
Author Contributions
FS, JG, CC, DB, and MTA contributed to the study concept and design; BS contributed to patient recruitment; AB, JW, and DB contributed to data acquisition; BS, FS, AB, JW, AM, CC, DB, and MTA contributed to data analysis. All authors contributed to the development of the manuscript and all authors approved the final version. All authors agree to be accountable for all aspects of the work.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See[https://www.pfizer.com/science/clinical-trials/data-and-result] for more information.
References
- 1. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel J-F.. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gordon IO, Agrawal N, Goldblum JR, Fiocchi C, Rieder F.. Fibrosis in ulcerative colitis: mechanisms, features, and consequences of a neglected problem. Inflamm Bowel Dis 2014;20:2198–206. [DOI] [PubMed] [Google Scholar]
- 3. Murray A, Nguyen TM, Parker CE, Feagan BG, MacDonald JK.. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 2020;8:CD000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wheat CL, Ko CW, Clark-Snustad K, Grembowski D, Thornton TA, Devine B.. Inflammatory Bowel Disease [IBD] pharmacotherapy and the risk of serious infection: a systematic review and network meta-analysis. BMC Gastroenterol 2017;17:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kucharzik T, Ellul P, Greuter T, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis 2021;15:879–913. [DOI] [PubMed] [Google Scholar]
- 6. Hoisnard L, Lebrun-Vignes B, Maury S, et al. Adverse events associated with JAK inhibitors in 126,815 reports from the WHO pharmacovigilance database. Sci Rep 2022;12:7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schoefs E, Vermeire S, Ferrante M, et al. What are the unmet needs and most relevant treatment outcomes according to patients with inflammatory bowel disease? A qualitative patient preference study. J Crohns Colitis 2023;17:379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sandborn WJ, Vermeire S, Peyrin-Biroulet L, et al. Etrasimod as induction and maintenance therapy for ulcerative colitis [ELEVATE]: two randomised, double-blind, placebo-controlled, phase 3 studies. Lancet 2023;401:1159–71. [DOI] [PubMed] [Google Scholar]
- 9. Lee CA, Oh DA, Tang Y, et al. Disposition and mass balance of etrasimod in healthy subjects and in vitro determination of the enzymes responsible for its oxidative metabolism. Clin Pharmacol Drug Dev 2023;12:553–71. [DOI] [PubMed] [Google Scholar]
- 10. Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014;8:443–68. [DOI] [PubMed] [Google Scholar]
- 11. Kirchgesner J, Desai RJ, Beaugerie L, Schneeweiss S, Kim SC.. Risk of serious infections with vedolizumab versus tumor necrosis factor antagonists in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2022;20:314–24.e16. [DOI] [PubMed] [Google Scholar]
- 12. Blackwell J, Selinger C, Raine T, Parkes G, Smith MA, Pollok R.. Steroid use and misuse: a key performance indicator in the management of IBD. Frontline Gastroenterol 2021;12:207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Waljee AK, Wiitala WL, Govani S, et al. Corticosteroid use and complications in a US inflammatory bowel disease cohort. PLoS One 2016;11:e0158017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Komori K, Lee C, Acevedo L, Gilder K, Grundy J.. P045 Effect of etrasimod on circulating lymphocyte subsets: data from a randomized phase 1 study in healthy Japanese and Caucasian men. Am J Gastroenterol 2020;115:S12. [DOI] [PubMed] [Google Scholar]
- 15. Danese S, Komori K, Ryan R, et al. S928 Effect of etrasimod on circulating lymphocytes in patients with moderately to severely active ulcerative colitis: data from the phase 3 ELEVATE UC 52 and ELEVATE UC 12 trials. Am J Gastroenterol 2022;117:e673. [Google Scholar]
- 16. Surapaneni S, Yerramilli U, Bai A, et al. Absorption, metabolism, and excretion, in vitro pharmacology, and clinical pharmacokinetics of ozanimod, a novel sphingosine 1-phosphate receptor modulator. Drug Metab Dispos 2021;49:405–19. [DOI] [PubMed] [Google Scholar]
- 17. Peyrin-Biroulet L, Morgan M, Christopher R, et al. P-179 Safety, pharmacokinetics and pharmacodynamics of etrasimod [APD334], an oral selective S1P receptor modulator, after dose-escalation, in healthy volunteers [abstract P-179]. Inflamm Bowel Dis 2017;23:S60–1. [Google Scholar]
- 18. Lasa JS, Olivera PA, Bonovas S, Danese S, Peyrin-Biroulet L.. Safety of S1P modulators in patients with immune-mediated diseases: a systematic review and meta-analysis. Drug Saf 2021;44:645–60. [DOI] [PubMed] [Google Scholar]
- 19. Sandborn WJ, Feagan BG, D’Haens G, et al.; True North Study Group. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2021;385:1280–91. [DOI] [PubMed] [Google Scholar]
- 20. Sandborn WJ, Feagan BG, Wolf DC, et al.; TOUCHSTONE Study Group. Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med 2016;374:1754–62. [DOI] [PubMed] [Google Scholar]
- 21. Olivera PA, Lasa JS, Bonovas S, Danese S, Peyrin-Biroulet L.. Safety of Janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology 2020;158:1554–73.e12. [DOI] [PubMed] [Google Scholar]
- 22. Feagan BG, Rutgeerts P, Sands BE, et al.; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 23. Olivera PA, Lasa JS, Zubiaurre I, et al. Opportunistic infections in patients with inflammatory bowel disease treated with advanced therapies: a systematic review and meta-analysis of randomized controlled trials. J Crohns Colitis 2023;17:199–210. [DOI] [PubMed] [Google Scholar]
- 24. Ng SC, Hilmi IN, Blake A, et al. Low frequency of opportunistic infections in patients receiving vedolizumab in clinical trials and post-marketing setting. Inflamm Bowel Dis 2018;24:2431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vermeire S, Peyrin-Biroulet L, Panés J, et al. P490 Etrasimod for the treatment of ulcerative colitis: up to 2.5 years of pooled safety data from global clinical trials [abstract P490]. J Crohns Colitis 2023;17:i619–20. [Google Scholar]
- 26. DeTora LM, Toroser D, Sykes A, et al. Good Publication Practice [GPP] guidelines for company-sponsored biomedical research: 2022 update. Ann Intern Med 2022;175:1298–304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See[https://www.pfizer.com/science/clinical-trials/data-and-result] for more information.