Abstract
Surface properties of ovalbumin and of its putative signal sequence, and their interactions with phospholipids at an air-water interface, have been studied. The mature protein can form an interfacial film spontaneously from its bulk solution, whereas the signal sequence cannot. Mature ovalbumin also penetrates phospholipid monolayers from the subphase (independently of the type of phospholipid present), whereas its signal sequence does not. The surface stability of a spread film of the signal sequence is, however, higher than that of a film of mature ovalbumin. Above specific threshold concentrations of signal peptide and of mature ovalbumin in mixed films with phospholipids, two separate phases are formed. In such immiscible films, the signal sequence peptide is also able to support a higher lateral surface pressure than mature ovalbumin, at corresponding areas of peptide and mature protein in the mixed monolayers. It is suggested that the differing lateral stabilities of ovalbumin and of its putative signal sequence may be relevant to the translocation of ovalbumin across the membrane of the endoplasmic reticulum, and a scheme for its translocation is proposed that is based on these properties.
Full text
PDF
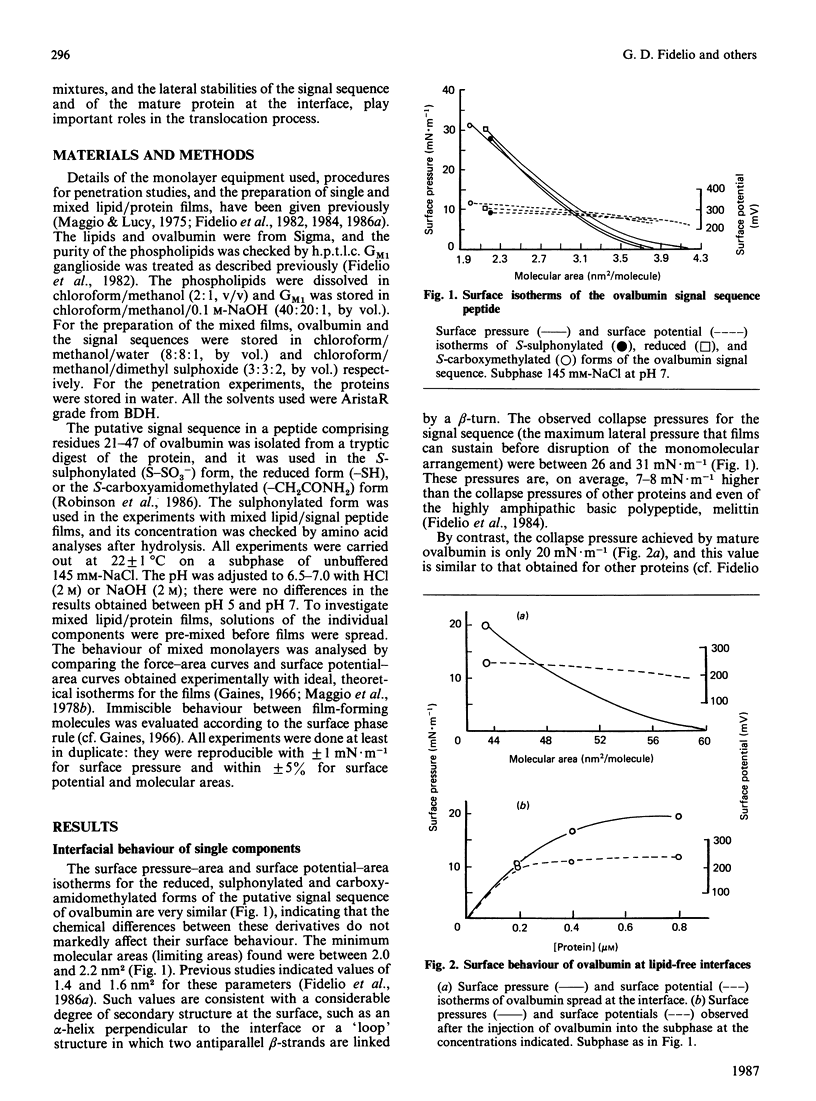
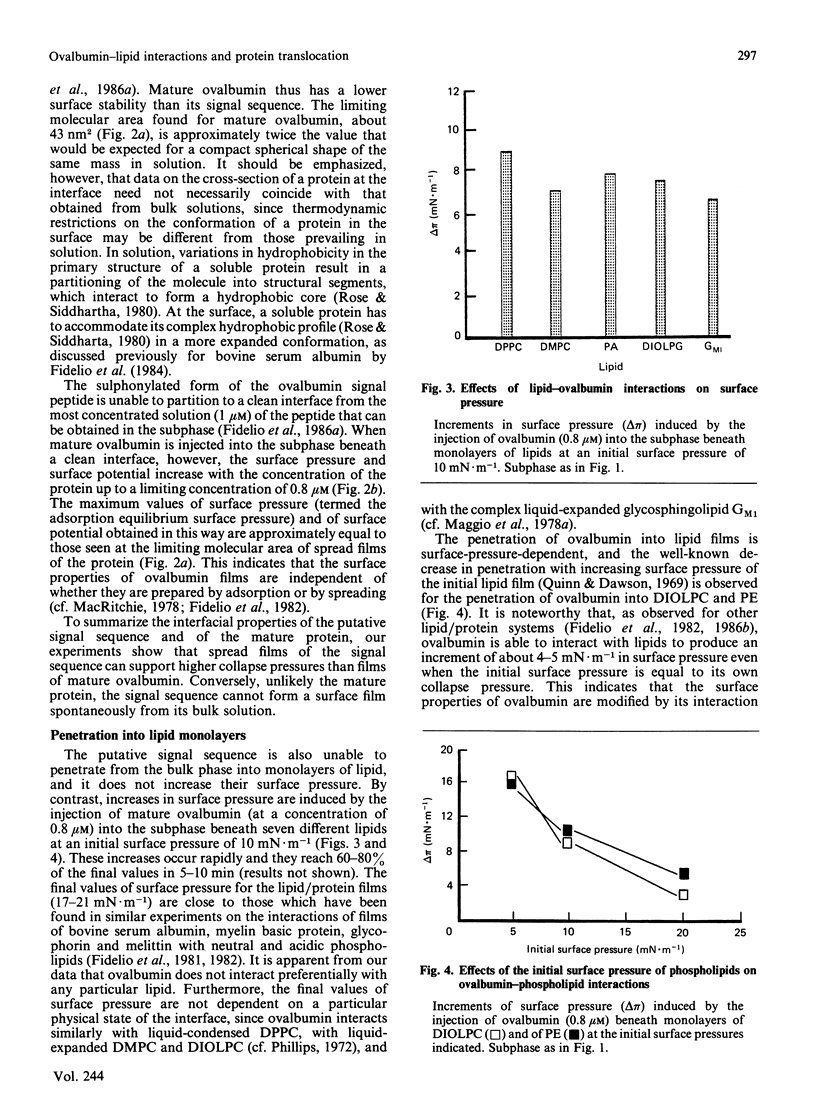
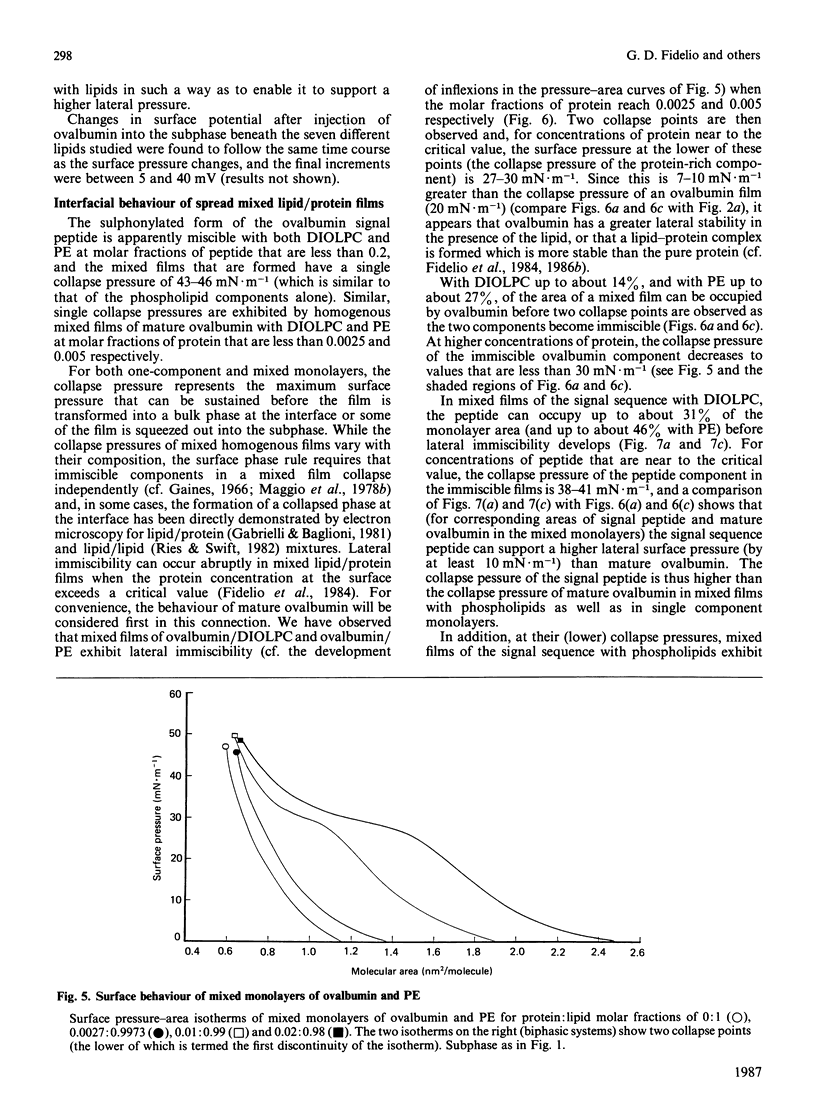
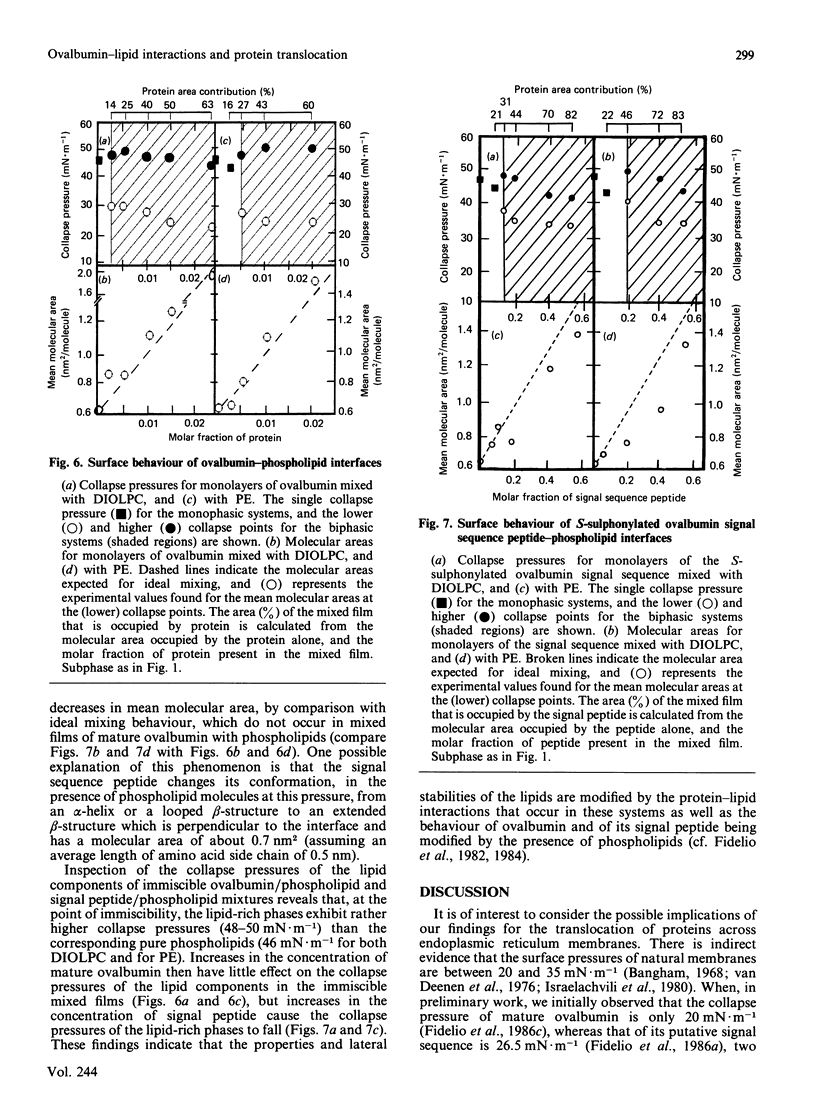


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austen B. M. Predicted secondary structures of amino-terminal extension sequences of secreted proteins. FEBS Lett. 1979 Jul 15;103(2):308–313. doi: 10.1016/0014-5793(79)81351-4. [DOI] [PubMed] [Google Scholar]
- Austen B. M., Ridd D. H. The signal peptide and its role in membrane penetration. Biochem Soc Symp. 1981;(46):235–258. [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs M. S., Gierasch L. M., Zlotnick A., Lear J. D., DeGrado W. F. In vivo function and membrane binding properties are correlated for Escherichia coli lamB signal peptides. Science. 1985 May 31;228(4703):1096–1099. doi: 10.1126/science.3158076. [DOI] [PubMed] [Google Scholar]
- Eilers M., Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986 Jul 17;322(6076):228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Silhavy T. J. Importance of secondary structure in the signal sequence for protein secretion. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4599–4603. doi: 10.1073/pnas.80.15.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Fidelio G. D., Austen B. M., Chapman D., Lucy J. A. Properties of signal-sequence peptides at an air-water interface. Biochem J. 1986 Aug 15;238(1):301–304. doi: 10.1042/bj2380301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidelio G. D., Maggio B., Cumar F. A., Caputto R. Interaction of glycosphingolipids with melittin and myelin basis protein in monolayers. Biochem J. 1981 Feb 1;193(2):643–646. doi: 10.1042/bj1930643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidelio G. D., Maggio B., Cumar F. A. Interaction of myelin basic protein, melittin and bovine serum albumin with gangliosides, sulphatide and neutral glycosphingolipids in mixed monolayers. Chem Phys Lipids. 1984 Aug;35(3):231–245. doi: 10.1016/0009-3084(84)90049-5. [DOI] [PubMed] [Google Scholar]
- Fidelio G. D., Maggio B., Cumar F. A. Interaction of soluble and membrane proteins with monolayers of glycosphingolipids. Biochem J. 1982 Jun 1;203(3):717–725. doi: 10.1042/bj2030717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Different exported proteins in E. coli show differences in the temporal mode of processing in vivo. Cell. 1981 Jul;25(1):151–157. doi: 10.1016/0092-8674(81)90239-7. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Blobel G. Chicken ovalbumin contains an internal signal sequence. Nature. 1979 Sep 13;281(5727):117–121. doi: 10.1038/281117a0. [DOI] [PubMed] [Google Scholar]
- Macritchie F. Proteins at interfaces. Adv Protein Chem. 1978;32:283–326. doi: 10.1016/s0065-3233(08)60577-x. [DOI] [PubMed] [Google Scholar]
- Maggio B., Cumar F. A., Caputto R. Interactions of gangliosides with phospholipids and glycosphingolipids in mixed monolayers. Biochem J. 1978 Dec 1;175(3):1113–1118. doi: 10.1042/bj1751113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Cumar F. A., Caputto R. Surface behaviour of gangliosides and related glycosphingolipids. Biochem J. 1978 Jun 1;171(3):559–565. doi: 10.1042/bj1710559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Lucy J. A. Studies on mixed monolayers of phospholipids and fusogenic lipids. Biochem J. 1975 Sep;149(3):597–608. doi: 10.1042/bj1490597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds L., O'Malley B. W., Nisbet A. D., Fothergill J. E., Givol D., Fields S., Robertson M., Brownlee G. G. Sequence of chicken ovalbumin mRNA. Nature. 1978 Jun 29;273(5665):723–728. doi: 10.1038/273723a0. [DOI] [PubMed] [Google Scholar]
- Meek R. L., Walsh K. A., Palmiter R. D. The signal sequence of ovalbumin is located near the NH2 terminus. J Biol Chem. 1982 Oct 25;257(20):12245–12251. [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Nesmeyanova M. A. On the possible participation of acid phospholipids in the translocation of secreted proteins through the bacterial cytoplasmic membrane. FEBS Lett. 1982 Jun 7;142(2):189–193. doi: 10.1016/0014-5793(82)80131-2. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Walsh K. A. Ovalbumin: a secreted protein without a transient hydrophobic leader sequence. Proc Natl Acad Sci U S A. 1978 Jan;75(1):94–98. doi: 10.1073/pnas.75.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. J., Dawson R. M. Interactions of cytochrome c and [14C]. Biochem J. 1969 Oct;115(1):65–75. doi: 10.1042/bj1150065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A., Meredith C., Austen B. M. Isolation and properties of the signal region from ovalbumin. FEBS Lett. 1986 Jul 28;203(2):243–246. doi: 10.1016/0014-5793(86)80751-7. [DOI] [PubMed] [Google Scholar]
- Rose G. D., Roy S. Hydrophobic basis of packing in globular proteins. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4643–4647. doi: 10.1073/pnas.77.8.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Kornberg R. D. Cell biology. An unfolding story of protein translocation. Nature. 1986 Jul 17;322(6076):209–210. doi: 10.1038/322209a0. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabe L., Krieg P., Strachan R., Jackson D., Wallis E., Colman A. Segregation of mutant ovalbumins and ovalbumin-globin fusion proteins in Xenopus oocytes. Identification of an ovalbumin signal sequence. J Mol Biol. 1984 Dec 15;180(3):645–666. doi: 10.1016/0022-2836(84)90031-7. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7112–7116. doi: 10.1073/pnas.77.12.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Gilmore R., Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984 Aug;38(1):5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]
- Wickner W. Assembly of proteins into membranes. Science. 1980 Nov 21;210(4472):861–868. doi: 10.1126/science.7001628. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Blomberg C. Trans-membrane translocation of proteins. The direct transfer model. Eur J Biochem. 1979 Jun;97(1):175–181. doi: 10.1111/j.1432-1033.1979.tb13100.x. [DOI] [PubMed] [Google Scholar]


