Abstract
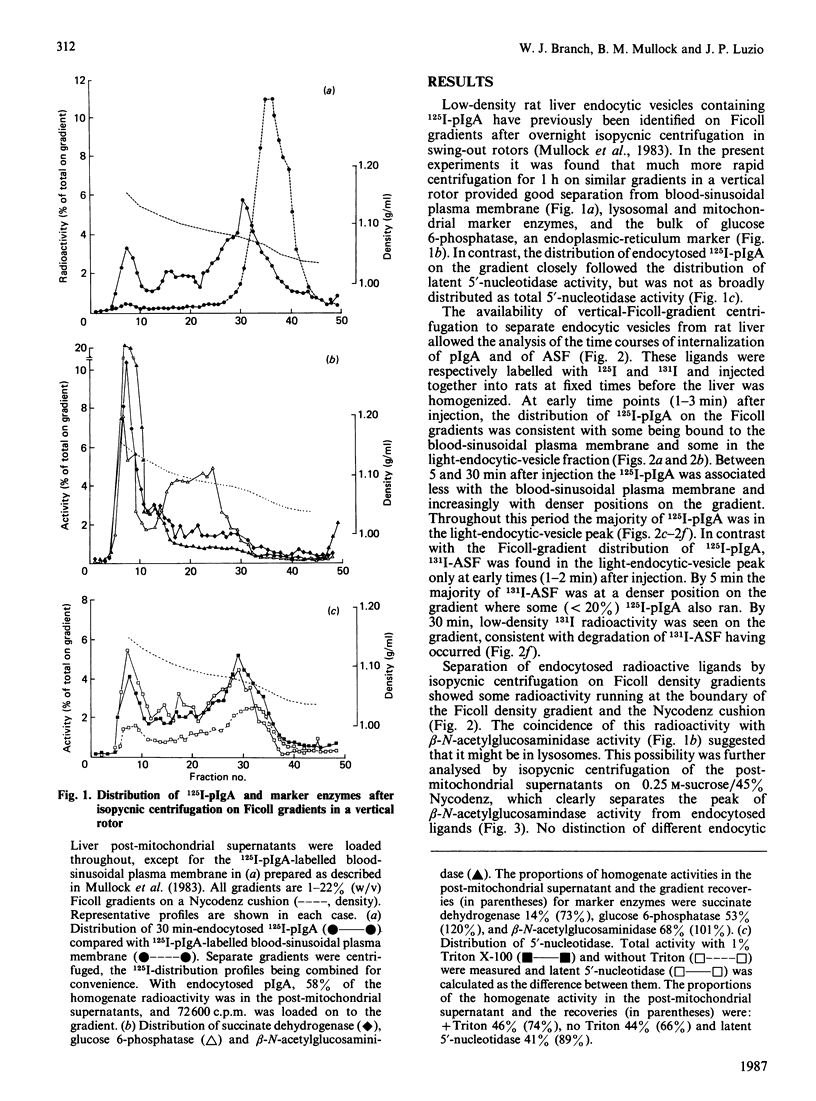
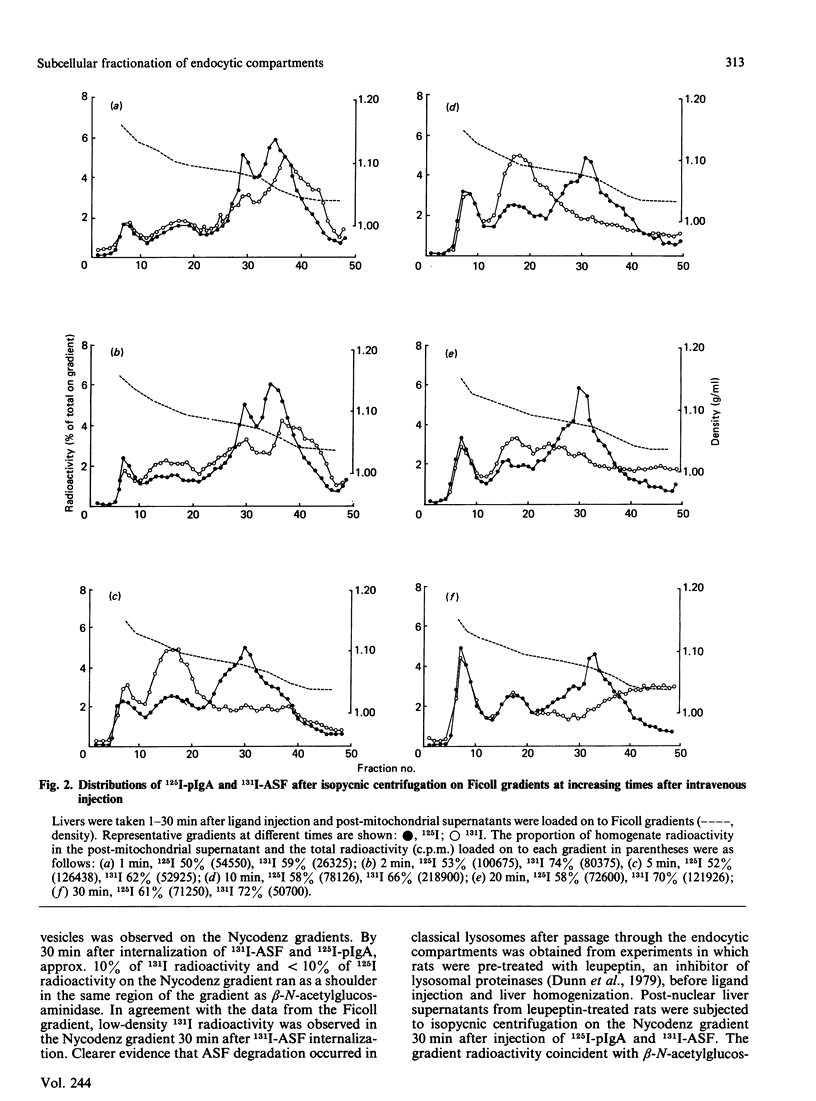
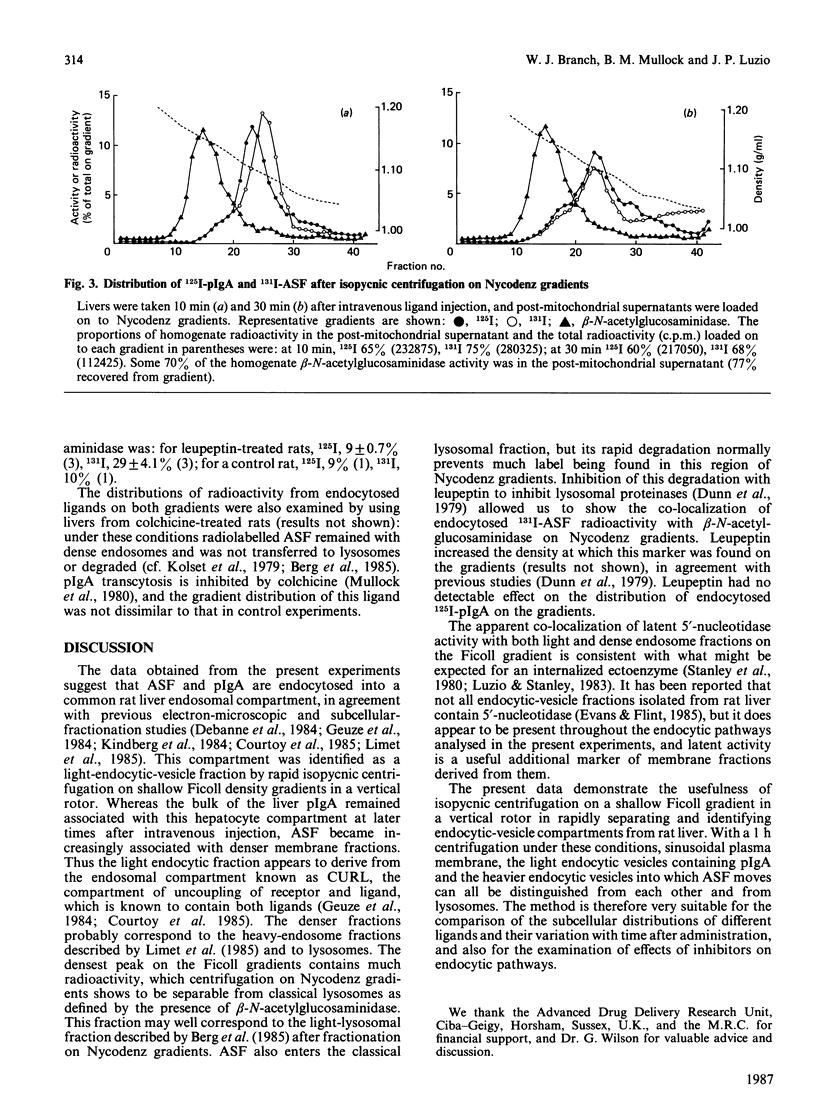
The distributions of two endocytosed radiolabelled ligands (polymeric immunoglobulin A and asialofetuin) in rat liver endocytic compartments were investigated by using rapid subcellular fractionation of post-mitochondrial supernatants on vertical density gradients of Ficoll or Nycodenz. Two endocytic compartments were identified, both ligands being initially associated with a light endocytic-vesicle fraction on Ficoll gradients, asialofetuin then accumulating in denser endosomes before transfer to lysosomes for degradation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg T., Kindberg G. M., Ford T., Blomhoff R. Intracellular transport of asialoglycoproteins in rat hepatocytes. Evidence for two subpopulations of lysosomes. Exp Cell Res. 1985 Dec;161(2):285–296. doi: 10.1016/0014-4827(85)90086-2. [DOI] [PubMed] [Google Scholar]
- DeBanne M. T., Bolyos M., Gauldie J., Regoeczi E. Two populations of prelysosomal structures transporting asialoglycoproteins in rat liver. Proc Natl Acad Sci U S A. 1984 May;81(10):2995–2999. doi: 10.1073/pnas.81.10.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. A., LaBadie J. H., Aronson N. N., Jr Inhibition of 125I-asialofetuin catabolism by leupeptin in the perfused rat liver and in vivo. J Biol Chem. 1979 May 25;254(10):4191–4196. [PubMed] [Google Scholar]
- Evans W. H., Flint N. Subfractionation of hepatic endosomes in Nycodenz gradients and by free-flow electrophoresis. Separation of ligand-transporting and receptor-enriched membranes. Biochem J. 1985 Nov 15;232(1):25–32. doi: 10.1042/bj2320025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Peppard J., von Figura K., Hasilik A., Schwartz A. L. Intracellular receptor sorting during endocytosis: comparative immunoelectron microscopy of multiple receptors in rat liver. Cell. 1984 May;37(1):195–204. doi: 10.1016/0092-8674(84)90315-5. [DOI] [PubMed] [Google Scholar]
- Kindberg G. M., Ford T., Blomhoff R., Rickwood D., Berg T. Separation of endocytic vesicles in Nycodenz gradients. Anal Biochem. 1984 Nov 1;142(2):455–462. doi: 10.1016/0003-2697(84)90489-5. [DOI] [PubMed] [Google Scholar]
- Kolset S. O., Tolleshaug H., Berg T. The effects of colchicine and cytochalasin B on uptake and degradation of asialo-glycoproteins in isolated rat hepatocytes. Exp Cell Res. 1979 Aug;122(1):159–167. doi: 10.1016/0014-4827(79)90570-6. [DOI] [PubMed] [Google Scholar]
- Limet J. N., Quintart J., Schneider Y. J., Courtoy P. J. Receptor-mediated endocytosis of polymeric IgA and galactosylated serum albumin in rat liver. Evidence for intracellular ligand sorting and identification of distinct endosomal compartments. Eur J Biochem. 1985 Feb 1;146(3):539–548. doi: 10.1111/j.1432-1033.1985.tb08685.x. [DOI] [PubMed] [Google Scholar]
- Luzio J. P., Stanley K. K. The isolation of endosome-derived vesicles from rat hepatocytes. Biochem J. 1983 Oct 15;216(1):27–36. doi: 10.1042/bj2160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K. E., Simister N. E. Transcytosis. Cell. 1985 Dec;43(2 Pt 1):389–390. doi: 10.1016/0092-8674(85)90166-7. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Jones R. S., Peppard J., Hinton R. H. Effect of colchicine on the transfer of IgA across hepatocytes into bile in isolated perfused rat livers. FEBS Lett. 1980 Nov 3;120(2):278–282. doi: 10.1016/0014-5793(80)80316-4. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Luzio J. P., Hinton R. H. Preparation of a low-density species of endocytic vesicle containing immunoglobulin A. Biochem J. 1983 Sep 15;214(3):823–827. doi: 10.1042/bj2140823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlans E., Peppard J., Reynolds J., Hall J. Rapid active transport of immunoglobulin A from blood to bile. J Exp Med. 1978 Feb 1;147(2):588–592. doi: 10.1084/jem.147.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff J. M., Fisher M. M., Underdown B. J. Receptor-mediated biliary transport of immunoglobulin A and asialoglycoprotein: sorting and missorting of ligands revealed by two radiolabeling methods. J Cell Biol. 1984 Jan;98(1):79–89. doi: 10.1083/jcb.98.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Edwards M. R., Luzio J. P. Subcellular distribution and movement of 5'-nucleotidase in rat cells. Biochem J. 1980 Jan 15;186(1):59–69. doi: 10.1042/bj1860059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolleshaug H., Berg T., Holte K. Kinetics of internalization and degradation of asialo-glycoproteins in isolated rat hepatocytes. Eur J Cell Biol. 1980 Dec;23(1):104–109. [PubMed] [Google Scholar]
- Tolleshaug H., Berg T., Nilsson M., Norum K. R. Uptake and degradation of 125I-labelled asialo-fetuin by isolated rat hepatocytes. Biochim Biophys Acta. 1977 Aug 25;499(1):73–84. doi: 10.1016/0304-4165(77)90230-6. [DOI] [PubMed] [Google Scholar]



