Abstract
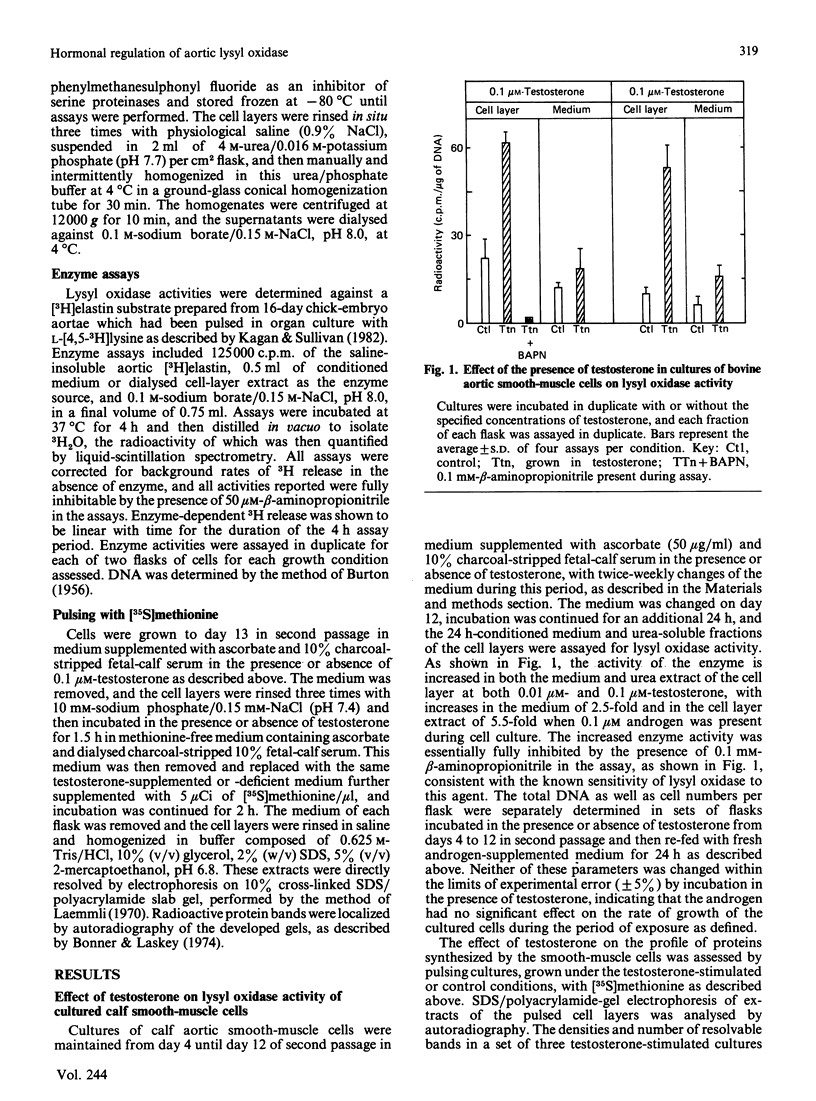
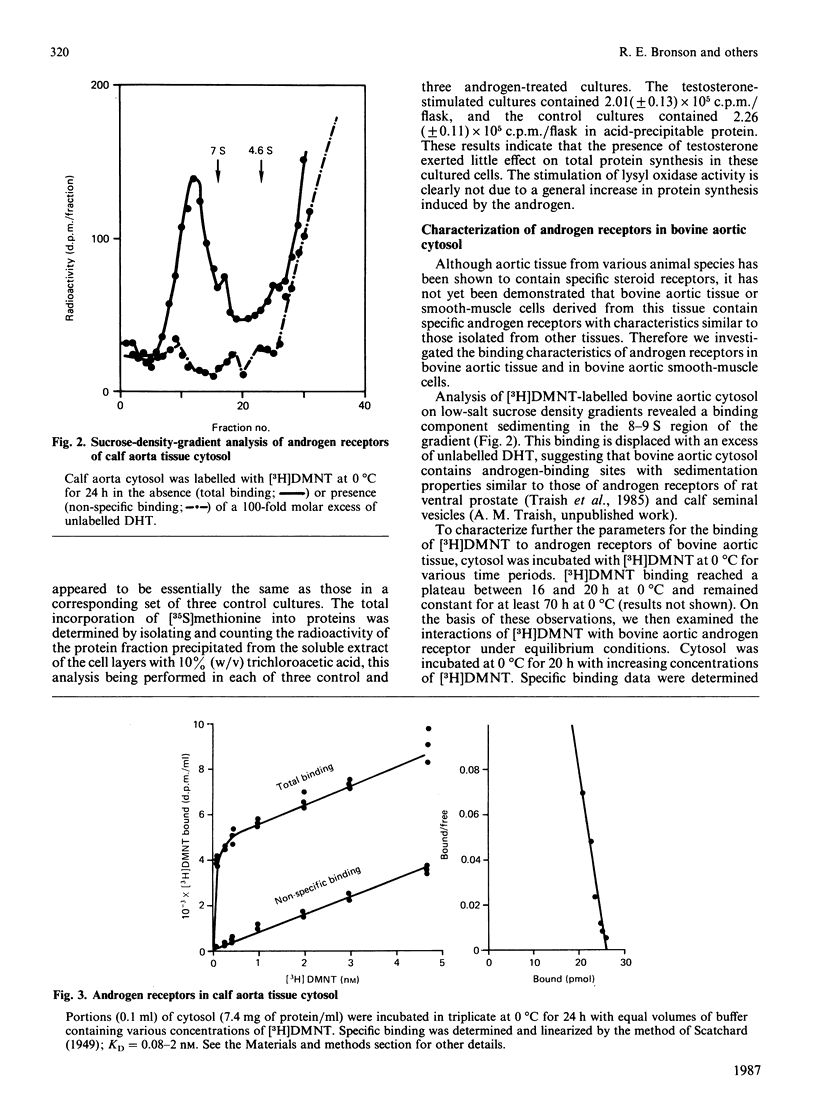
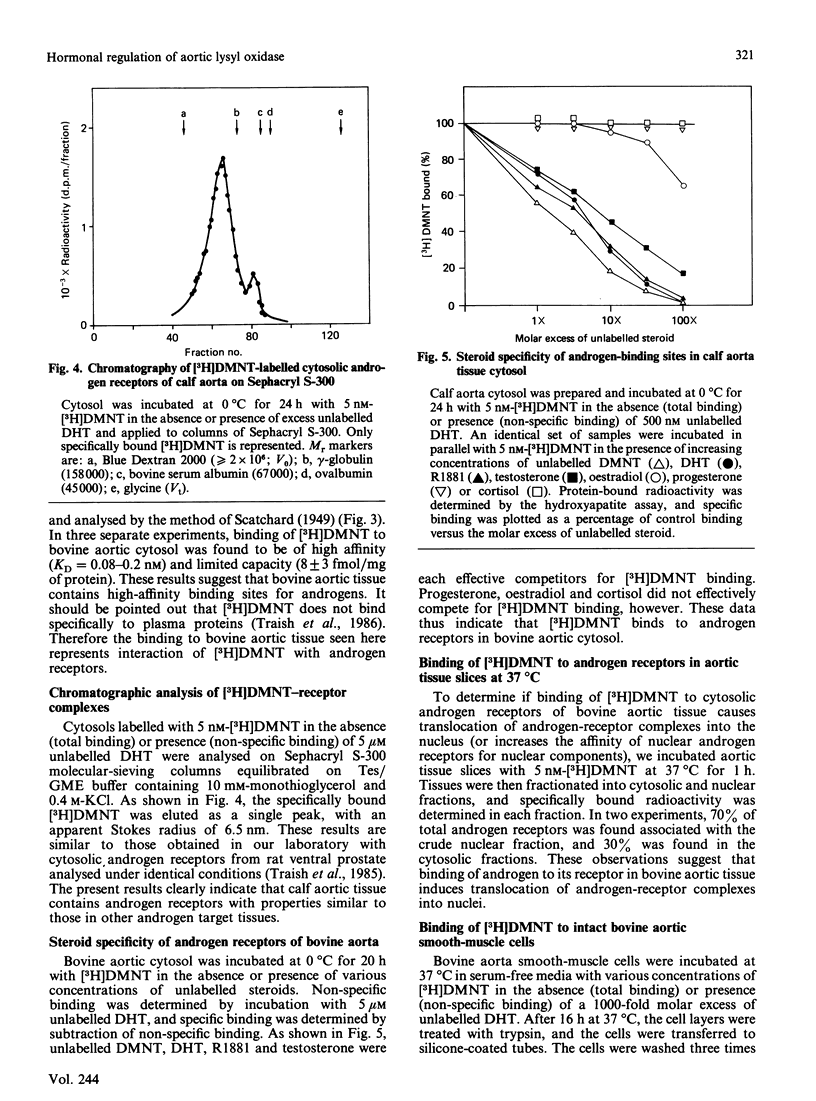
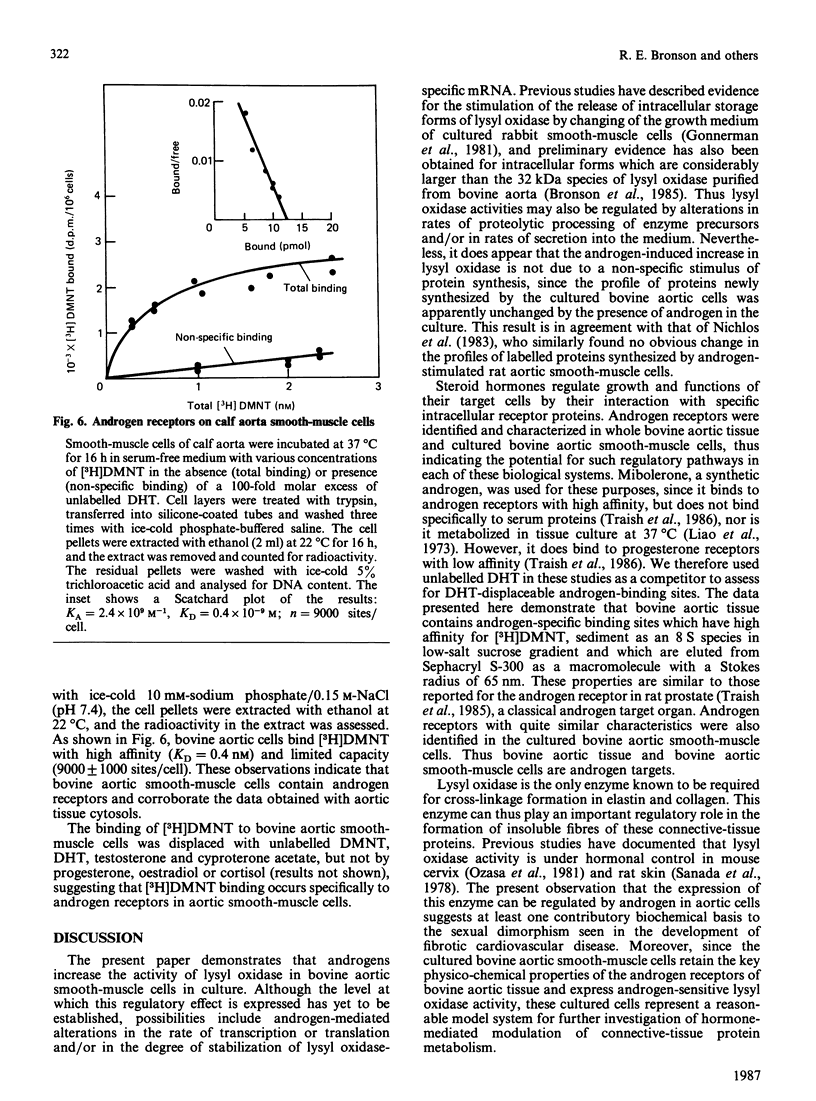
Previous studies have indicated a greater incidence of atherosclerotic cardiovascular disease in men than in women of child-bearing age, suggesting that vascular interactions with sex steroids may effect pathogenesis in these cases. In the present study, it was found that the presence of 10-100 nM-testosterone in the growth medium of calf aortic smooth-muscle cells in culture stimulates lysyl oxidase activity approx. 2.5-fold in the medium and 5.5-fold in the fraction bound to the cell layer. Androgen receptors were identified in these cultured smooth-muscle cells, and their properties were very similar to those in the cytosolic fraction of whole bovine aortic tissue. These receptors appeared to be specific for androgen, of high affinity (Kd = 0.4 nM) and of low capacity (9000 sites/cell). The present results indicate that the aortic smooth-muscle cell is a cellular target for androgens, and thus raise the possibility that the development of fibrotic arterial lesions involving the deposition of excess collagen may in part be regulated by androgen-mediated stimulation of collagen cross-linkage formation as catalysed by lysyl oxidase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldekas J. C., Smith B., Gerstenfeld L. C., Sonenshein G. E., Franzblau C. Effects of 17 beta-estradiol on the biosynthesis of collagen in cultured bovine aortic smooth muscle cells. Biochemistry. 1981 Apr 14;20(8):2162–2167. doi: 10.1021/bi00511a014. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Paz M. A., Gallop P. M. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- Fischer G. M., Swain M. L. In vivo effects of sex hormones on aortic elastin and collagen dynamics in castrated and intact male rats. Endocrinology. 1978 Jan;102(1):92–97. doi: 10.1210/endo-102-1-92. [DOI] [PubMed] [Google Scholar]
- Gonnerman W. A., Ferrera R., Franzblau C. Measurement of medium lysyl oxidase activity in aorta smooth muscle cells. Effects of multiple medium changes and inhibition of protein synthesis. Biochemistry. 1981 Jun 23;20(13):3864–3867. doi: 10.1021/bi00516a030. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Horwitz L. D. Canine vascular tissues are targets for androgens, estrogens, progestins, and glucocorticoids. J Clin Invest. 1982 Apr;69(4):750–758. doi: 10.1172/JCI110513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan H. M., Sullivan K. A. Lysyl oxidase: preparation and role in elastin biosynthesis. Methods Enzymol. 1982;82(Pt A):637–650. doi: 10.1016/0076-6879(82)82092-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liao S., Liang T., Fang S., Castañeda E., Shao T. C. Steroid structure and androgenic activity. Specificities involved in the receptor binding and nuclear retention of various androgens. J Biol Chem. 1973 Sep 10;248(17):6154–6162. [PubMed] [Google Scholar]
- Lin A. L., Gonzalez R., Jr, Shain S. A. Androgen directs apparent cytoplasmic and nuclear distribution of rat cardiovascular androgen receptors. Arteriosclerosis. 1985 Nov-Dec;5(6):659–667. doi: 10.1161/01.atv.5.6.659. [DOI] [PubMed] [Google Scholar]
- Lin A. L., McGill H. C., Jr, Shain S. A. Hormone receptors of the baboon cardiovascular system. Biochemical characterization of aortic and myocardial cytoplasmic progesterone receptors. Circ Res. 1982 May;50(5):610–616. doi: 10.1161/01.res.50.5.610. [DOI] [PubMed] [Google Scholar]
- Lin A. L., Shain S. A. Estrogen-mediated cytoplasmic and nuclear distribution of rat cardiovascular estrogen receptors. Arteriosclerosis. 1985 Nov-Dec;5(6):668–677. doi: 10.1161/01.atv.5.6.668. [DOI] [PubMed] [Google Scholar]
- Lin A. L., Shain S. A. Sexual dimorphism characterizes steroid hormone modulation of rat aortic steroid hormone receptors. Endocrinology. 1986 Jul;119(1):296–302. doi: 10.1210/endo-119-1-296. [DOI] [PubMed] [Google Scholar]
- McGill H. C., Jr, Sheridan P. J. Nuclear uptake of sex steroid hormones in the cardiovascular system of the baboon. Circ Res. 1981 Feb;48(2):238–244. doi: 10.1161/01.res.48.2.238. [DOI] [PubMed] [Google Scholar]
- Müller R. E., Traish A. M., Beebe D. A., Wotiz H. H. Reversible inhibition of estrogen receptor activation by molybdate. J Biol Chem. 1982 Feb 10;257(3):1295–1300. [PubMed] [Google Scholar]
- Nichols N. R., Olsson C. A., Funder J. W. Steroid effects on protein synthesis in cultured smooth muscle cells from rat aorta. Endocrinology. 1983 Sep;113(3):1096–1101. doi: 10.1210/endo-113-3-1096. [DOI] [PubMed] [Google Scholar]
- Ozasa H., Tominaga T., Nishimura T., Takeda T. Lysyl oxidase activity in the mouse uterine cervix is physiologically regulated by estrogen. Endocrinology. 1981 Aug;109(2):618–621. doi: 10.1210/endo-109-2-618. [DOI] [PubMed] [Google Scholar]
- Raaka B. M., Samuels H. H. The glucocorticoid receptor in GH1 cells. Evidence from dense amino acid labeling and whole cell studies for an equilibrium model explaining the influence of hormone on the intracellular distribution of receptor. J Biol Chem. 1983 Jan 10;258(1):417–425. [PubMed] [Google Scholar]
- Sanada H., Shikata J., Hamamoto H., Ueba Y., Yamamuro T., Takeda T. Changes in collagen cross-linking and lysyl oxidase by estrogen. Biochim Biophys Acta. 1978 Jul 3;541(3):408–413. doi: 10.1016/0304-4165(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Tomita T., Yonekura I., Umegaki K., Okada T., Hayashi E. Sex differences in aortic cholesterol esterase activity in rats, and changes of the activity following castration and gonadal hormone treatment. Atherosclerosis. 1982 Jun;43(2-3):405–415. doi: 10.1016/0021-9150(82)90039-9. [DOI] [PubMed] [Google Scholar]
- Traish A. M., Müller R. E., Wotiz H. H. Binding of 7 alpha, 17 alpha-dimethyl-19-nortestosterone (mibolerone) to androgen and progesterone receptors in human and animal tissues. Endocrinology. 1986 Apr;118(4):1327–1333. doi: 10.1210/endo-118-4-1327. [DOI] [PubMed] [Google Scholar]
- Traish A. M., Müller R. E., Wotiz H. H. Differences in the physicochemical characteristics of androgen-receptor complexes formed in vivo and in vitro. Endocrinology. 1984 May;114(5):1761–1769. doi: 10.1210/endo-114-5-1761. [DOI] [PubMed] [Google Scholar]
- Traish A. M., Müller R. E., Wotiz H. H. Resolution of non-activated and activated androgen receptors based on differences in their hydrodynamic properties. J Steroid Biochem. 1985 May;22(5):601–609. doi: 10.1016/0022-4731(85)90212-2. [DOI] [PubMed] [Google Scholar]
- Wolinsky H. Effects of androgen treatment on the male rat aorta. J Clin Invest. 1972 Oct;51(10):2552–2555. doi: 10.1172/JCI107071. [DOI] [PMC free article] [PubMed] [Google Scholar]



