Abstract
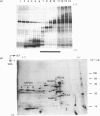
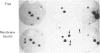
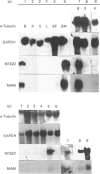
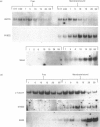
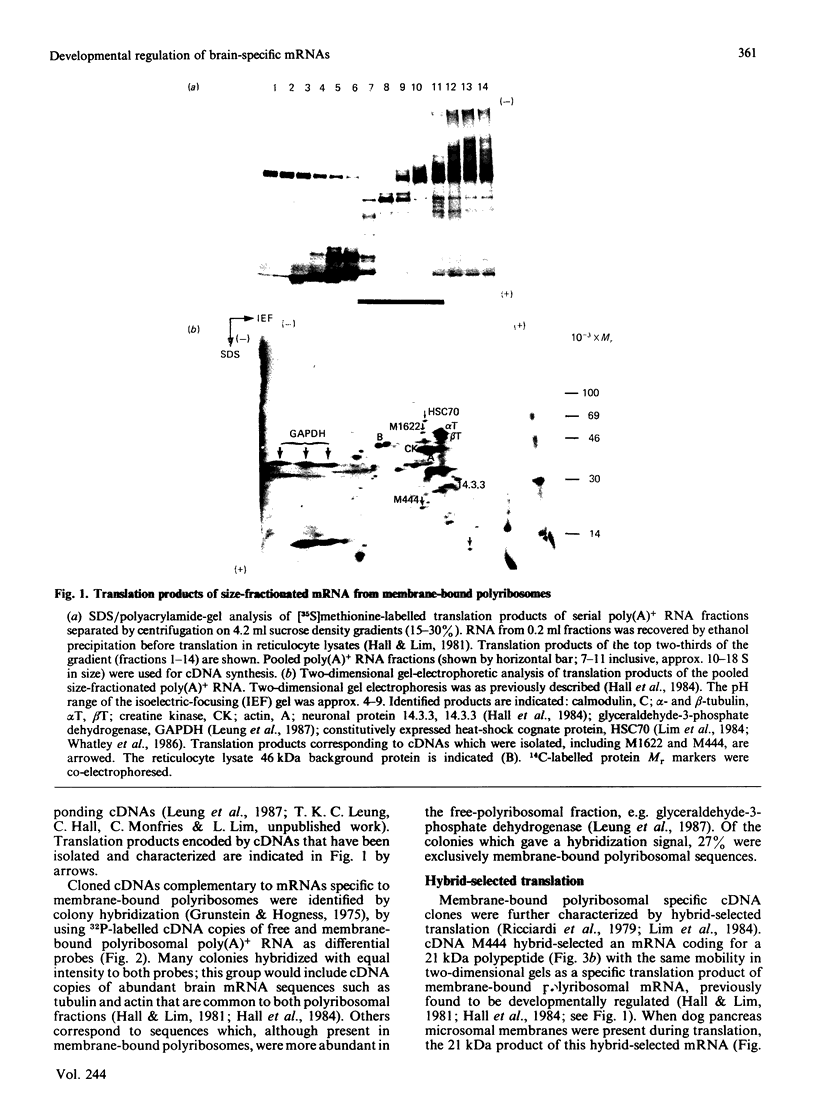
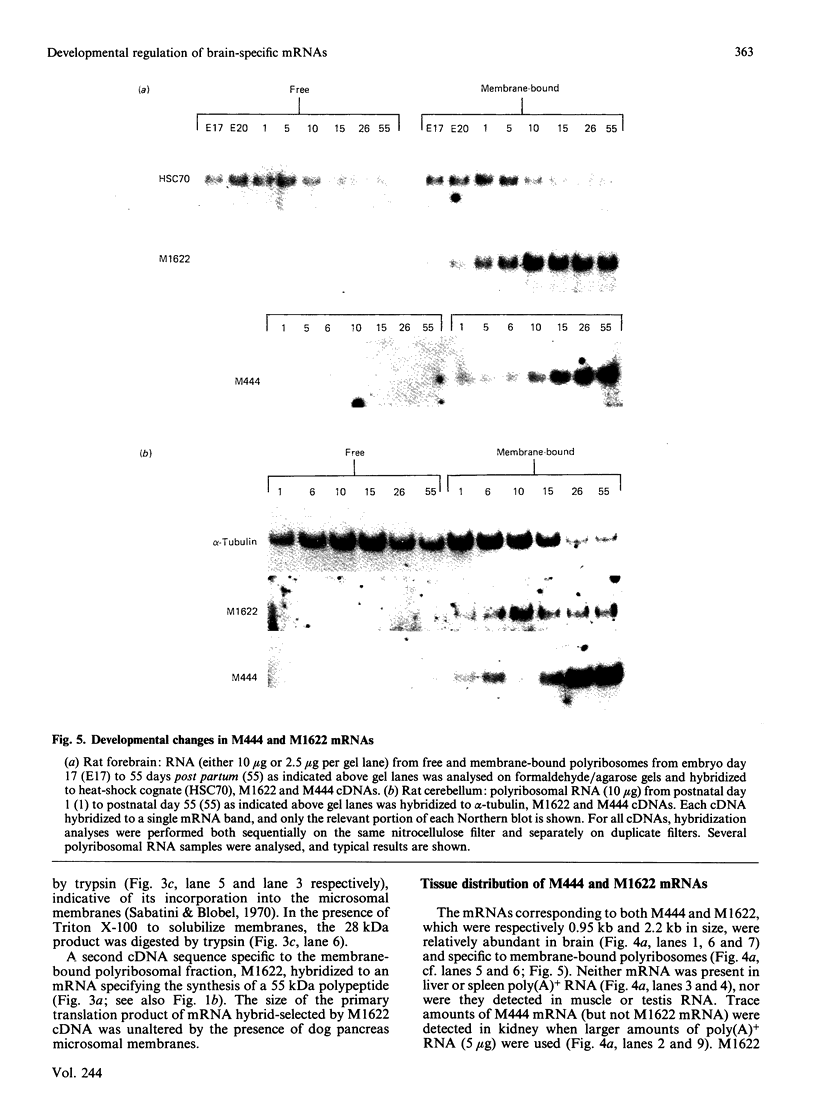
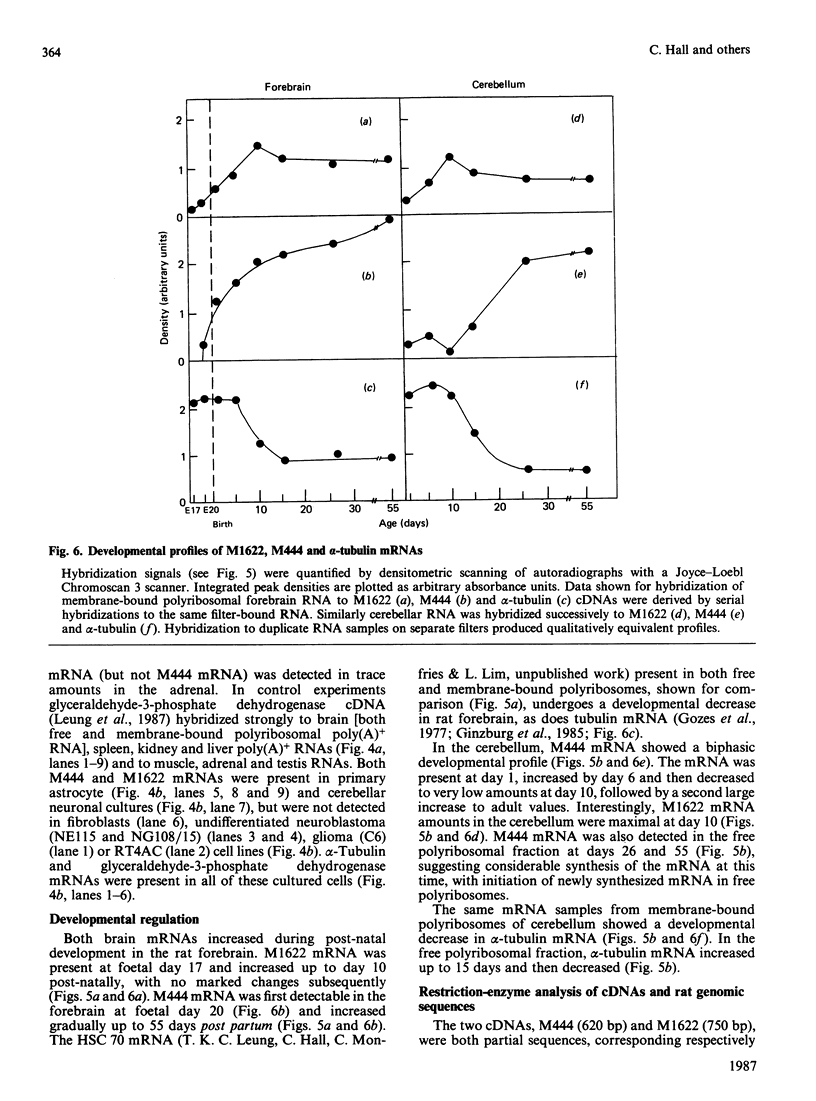
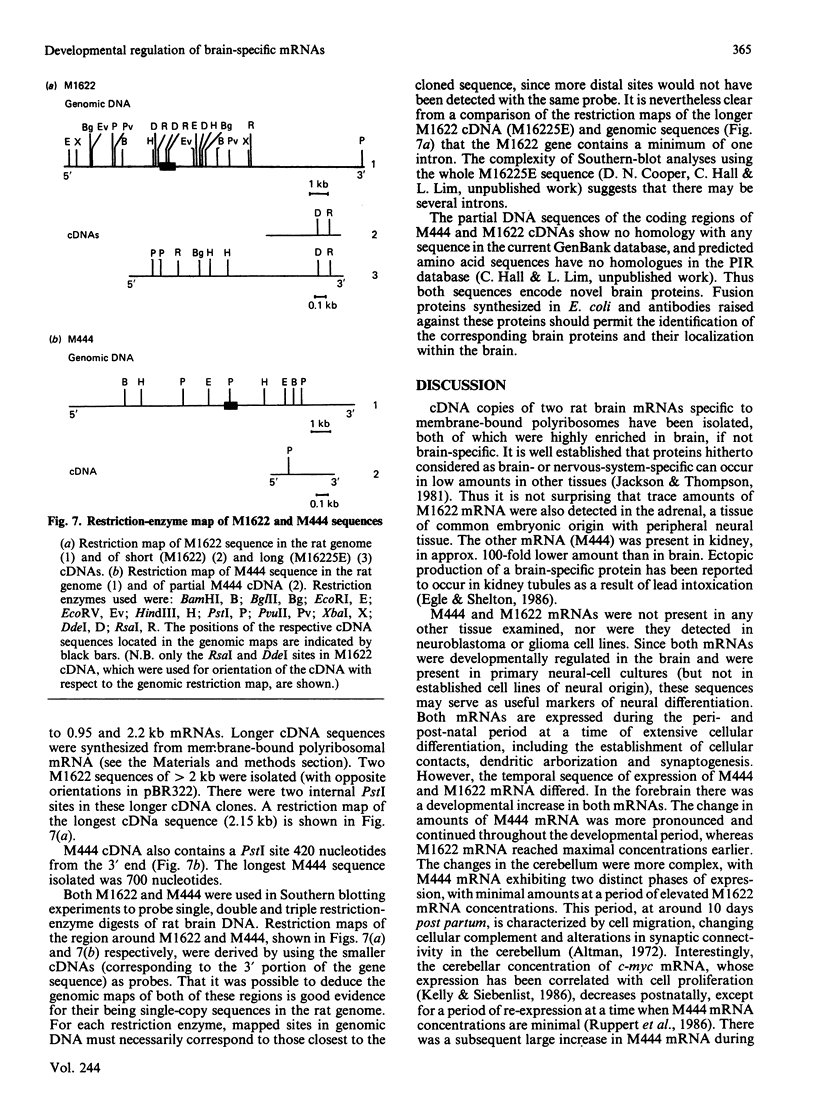
Translation in vitro of membrane-bound polyribosomal mRNAs from rat brain has shown several to be developmentally regulated [Hall & Lim (1981) Biochem. J. 196, 327-336]. Here we describe the isolation and characterization of cDNAs corresponding to two such brain mRNAs. One cDNA (M444) hybrid-selected a 0.95 kb mRNA directing the synthesis in vitro of a 21 kDa pI-6.3 polypeptide, which was processed in vitro by microsomal membranes. A second cDNA (M1622) hybridized to a 2.2 kb mRNA directing the synthesis of a 55 kDa pI-5.8 polypeptide. Both mRNAs were specific to membrane-bound polyribosomes. Restriction maps of the corresponding genomic DNA sequences are consistent with both being single copy. The two mRNAs were present in astrocytic and neuronal cultures, but not in liver or spleen or in neuroblastoma or glioma cells. The two mRNAs were differently regulated during brain development. In the developing forebrain there was a gradual and sustained increase in M444 mRNA during the first 3 weeks post partum, whereas M1622 mRNA appeared earlier and showed no further increase after day 10. In the cerebellum the developmental increase in M444 mRNA was biphasic. After a small initial increase there was a decrease in this mRNA at day 10, coincident with high amounts of M1622 mRNA. This was followed by a second, larger, increase in M444 mRNA, when amounts of M1622 mRNA were constant. The contrasting changes in these two mRNAs in the developing cerebellum are of particular interest, since they occur during an intensive period of cell proliferation, migration and altering neural connectivity. As these mRNAs are specific to differentiated neural tissue, they represent useful molecular markers for studying brain differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972 Jul;145(3):353–397. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Chikaraishi D. M., Deeb S. S., Sueoka N. Sequence complexity of nuclear RNAs in adult rat tissues. Cell. 1978 Jan;13(1):111–120. doi: 10.1016/0092-8674(78)90142-3. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Smith B. A., Cooke H. J., Niemann S., Schmidtke J. An estimate of unique DNA sequence heterozygosity in the human genome. Hum Genet. 1985;69(3):201–205. doi: 10.1007/BF00293024. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Hall L., Parker D., Campbell P. N. The construction, identification and partial characterization of plasmids containing guinea-pig milk protein complementary DNA sequences. Biochem J. 1981 Mar 15;194(3):989–998. doi: 10.1042/bj1940989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egle P. M., Shelton K. R. Chronic lead intoxication causes a brain-specific nuclear protein to accumulate in the nuclei of cells lining kidney tubules. J Biol Chem. 1986 Feb 15;261(5):2294–2298. [PubMed] [Google Scholar]
- Ginzburg I., Behar L., Givol D., Littauer U. Z. The nucleotide sequence of rat alpha-tubulin: 3'-end characteristics, and evolutionary conservation. Nucleic Acids Res. 1981 Jun 25;9(12):2691–2697. doi: 10.1093/nar/9.12.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg I., Teichman A., Dodemont H. J., Behar L., Littauer U. Z. Regulation of three beta-tubulin mRNAs during rat brain development. EMBO J. 1985 Dec 30;4(13B):3667–3673. doi: 10.1002/j.1460-2075.1985.tb04133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. M., MacDonald R. J. Cloning of hormone genes from a mixture of cDNA molecules. Methods Enzymol. 1979;68:75–90. doi: 10.1016/0076-6879(79)68007-2. [DOI] [PubMed] [Google Scholar]
- Gozes I., Walker M. D., Kaye A. M., Littauer U. Z. Synthesis of tubulin and actin by neuronal and glial nuclear preparations from devloping rat brain. J Biol Chem. 1977 Mar 10;252(5):1819–1825. [PubMed] [Google Scholar]
- Grouse L., Chilton M. D., McCarthy B. J. Hybridization of ribonucleic acid with unique sequences of mouse deoxyribonucleic acid. Biochemistry. 1972 Feb 29;11(5):798–805. doi: 10.1021/bi00755a019. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hall C., Lim L. Developmental changes in the composition of polyadenylated RNA isolated from free and membrane-bound polyribosomes of the rat forebrain, analysed by translation in vitro. Biochem J. 1981 Apr 15;196(1):327–336. doi: 10.1042/bj1960327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C., Mahadevan L., Whatley S., Biswas G., Lim L. Characterization of translation products of the polyadenylated RNA of free and membrane-bound polyribosomes of rat forebrain. Biochem J. 1984 May 1;219(3):751–761. doi: 10.1042/bj2190751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada M., Sueoka N. Clonal sublines of rat neurotumor RT4 and cell differentiation. I. Isolation and characterization of cell lines and cell type conversion. Dev Biol. 1978 Sep;66(1):97–108. doi: 10.1016/0012-1606(78)90276-2. [DOI] [PubMed] [Google Scholar]
- Jackson P., Thompson R. J. The demonstration of new human brain-specific proteins by high-resolution two-dimensional polyacrylamide gel electrophoresis. J Neurol Sci. 1981 Mar;49(3):429–438. doi: 10.1016/0022-510x(81)90032-0. [DOI] [PubMed] [Google Scholar]
- Kelly K., Siebenlist U. The regulation and expression of c-myc in normal and malignant cells. Annu Rev Immunol. 1986;4:317–338. doi: 10.1146/annurev.iy.04.040186.001533. [DOI] [PubMed] [Google Scholar]
- Klee W. A., Nirenberg M. A neuroblastoma times glioma hybrid cell line with morphine receptors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3474–3477. doi: 10.1073/pnas.71.9.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Hall C., Leung T., Mahadevan L., Whatley S. Neurone-specific enolase and creatine phosphokinase are protein components of rat brain synaptic plasma membranes. J Neurochem. 1983 Oct;41(4):1177–1182. doi: 10.1111/j.1471-4159.1983.tb09069.x. [DOI] [PubMed] [Google Scholar]
- Lim L., Hall C., Leung T., Whatley S. The relationship of the rat brain 68 kDa microtubule-associated protein with synaptosomal plasma membranes and with the Drosophila 70 kDa heat-shock protein. Biochem J. 1984 Dec 1;224(2):677–680. doi: 10.1042/bj2240677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis J. T. Fractionation of DNA fragments by polyethylene glycol induced precipitation. Methods Enzymol. 1980;65(1):347–353. doi: 10.1016/s0076-6879(80)65044-7. [DOI] [PubMed] [Google Scholar]
- Monahan J. J., Harris S. E., Woo S. L., Robberson D. L., O'Malley B. W. The synthesis and properties of the complete complementary DNA transcript of ovalbumin mRNA. Biochemistry. 1976 Jan 13;15(1):223–233. doi: 10.1021/bi00646a034. [DOI] [PubMed] [Google Scholar]
- Patel A. J., Hunt A. Observations on cell growth and regulation of glutamine synthetase by dexamethasone in primary cultures of forebrain and cerebellar astrocytes. Brain Res. 1985 Feb;350(1-2):175–184. doi: 10.1016/0165-3806(85)90262-7. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert C., Goldowitz D., Wille W. Proto-oncogene c-myc is expressed in cerebellar neurons at different developmental stages. EMBO J. 1986 Aug;5(8):1897–1901. doi: 10.1002/j.1460-2075.1986.tb04442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryffel G. U., McCarthy B. J. Complexity of cytoplasmic RNA in different mouse tissues measured by hybridization of polyadenylated RNA to complementary DNA. Biochemistry. 1975 Apr 8;14(7):1379–1385. doi: 10.1021/bi00678a006. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Blobel G. Controlled proteolysis of nascent polypeptides in rat liver cell fractions. II. Location of the polypeptides in rough microsomes. J Cell Biol. 1970 Apr;45(1):146–157. doi: 10.1083/jcb.45.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y. H., Tischfield J., Ruddle F. H. The linkage of genes for the human interferon-induced antiviral protein and indophenol oxidase-B traits to chromosome G-21. J Exp Med. 1973 Feb 1;137(2):317–330. doi: 10.1084/jem.137.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangnipon W., Kingsbury A., Webb M., Balazs R. Observations on rat cerebellar cells in vitro: influence of substratum, potassium concentration and relationship between neurones and astrocytes. Brain Res. 1983 Dec;313(2):177–189. doi: 10.1016/0165-3806(83)90215-8. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley S. A., Hall C., Davison A. N., Lim L. Alterations in the relative amounts of specific mRNA species in the developing human brain in Down's syndrome. Biochem J. 1984 May 15;220(1):179–187. doi: 10.1042/bj2200179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley S. A., Leung T., Hall C., Lim L. The brain 68-kilodalton microtubule-associated protein is a cognate form of the 70-kilodalton mammalian heat-shock protein and is present as a specific isoform in synaptosomal membranes. J Neurochem. 1986 Nov;47(5):1576–1583. doi: 10.1111/j.1471-4159.1986.tb00797.x. [DOI] [PubMed] [Google Scholar]