Abstract
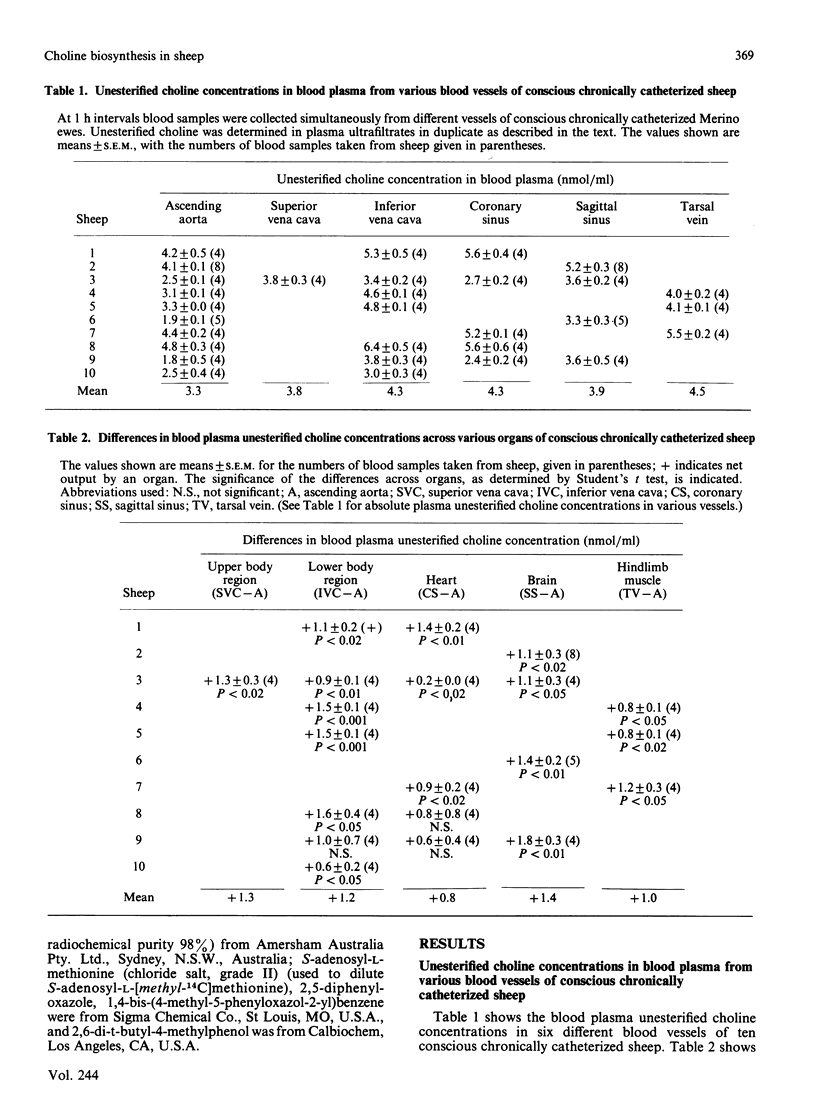
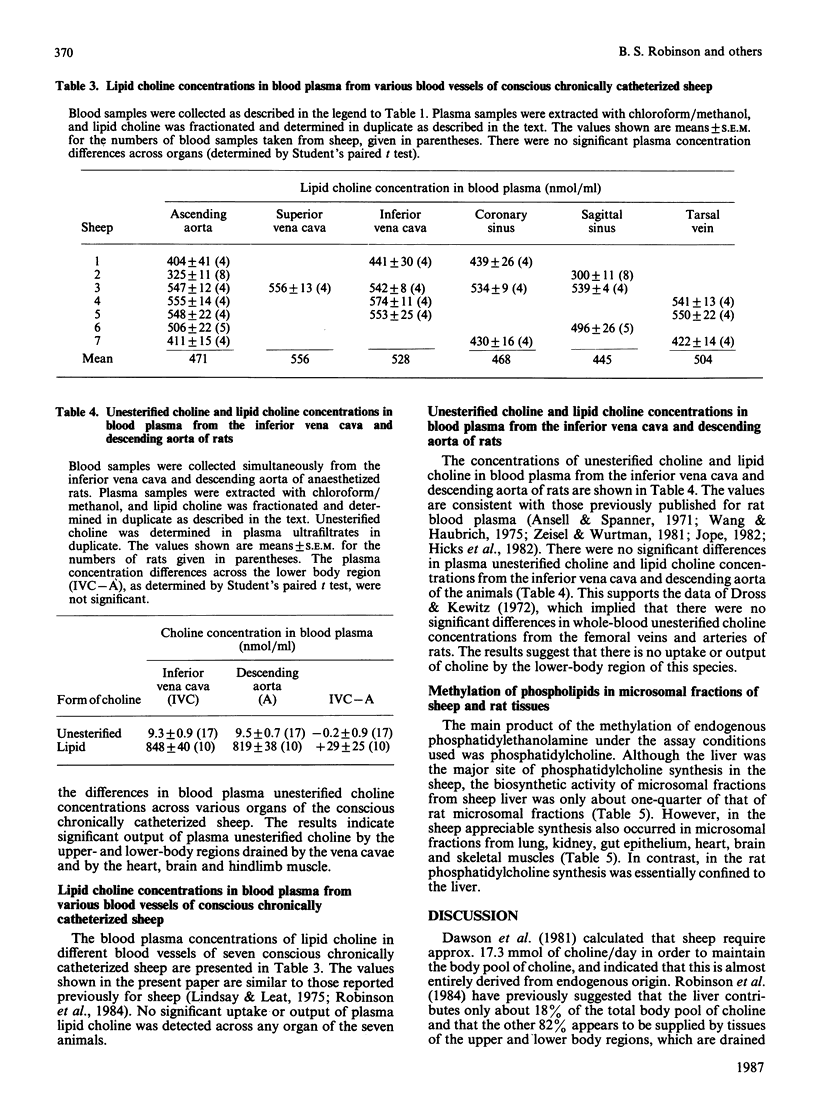
1. Choline production by various tissues of the sheep was measured by determining venous and arterial free choline concentrations in blood samples taken from various vessels in conscious multicannulated sheep. 2. Significant production of free choline occurred in the upper and lower body regions, and specifically in the heart, brain and hind-limb muscles of sheep, but there was no significant uptake or output of phosphatidylcholine across these tissues, as determined by arterio-venous differences. 3. In contrast, in the rat there were no significant arterio-venous differences in the concentrations of free choline or phosphatidylcholine across the hind-body. 4. Synthesis of phosphatidylcholine from endogenous phosphatidylethanolamine and S-adenosyl-L-[methyl-14C]methionine was measured in experiments in vitro using microsomal preparations from a variety of sheep and rat tissues. 5. The biosynthetic activity was highest in liver from sheep and rats, although the activity in sheep microsomal preparations was about one-quarter of that in rat microsomal preparations. 6. Microsomal preparations from sheep lung, kidney, gut epithelium, brain, heart and skeletal muscles also showed considerable biosynthetic activity, but in the rat the activity was virtually confined to the liver. 7. Overall, the results show a significant production of choline in extrahepatic tissues of the sheep, with skeletal muscle contributing some 60% of this extrahepatic activity. Thus the extrahepatic production of choline in the sheep, together with the extensive reutilization of bile choline, can explain the maintenance of the large endogenous body pool of choline in this species.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansell G. B., Spanner S. Studies on the origin of choline in the brain of the rat. Biochem J. 1971 May;122(5):741–750. doi: 10.1042/bj1220741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audubert F., Vance D. E. Pitfalls and problems in studies on the methylation of phosphatidylethanolamine. J Biol Chem. 1983 Sep 10;258(17):10695–10701. [PubMed] [Google Scholar]
- Bjørnstad P., Bremer J. In vivo studies on pathways for the biosynthesis of lecithin in the rat. J Lipid Res. 1966 Jan;7(1):38–45. [PubMed] [Google Scholar]
- Blusztajn J. K., Wurtman R. J. Choline biosynthesis by a preparation enriched in synaptosomes from rat brain. Nature. 1981 Apr 2;290(5805):417–418. doi: 10.1038/290417a0. [DOI] [PubMed] [Google Scholar]
- Blusztajn J. K., Zeisel S. H., Wurtman R. J. Synthesis of lecithin (phosphatidylcholine) from phosphatidylethanolamine in bovine brain. Brain Res. 1979 Dec 28;179(2):319–327. doi: 10.1016/0006-8993(79)90447-5. [DOI] [PubMed] [Google Scholar]
- Choi R. L., Freeman J. J., Jenden D. J. Kinetics of plasma choline in relation to turnover of brain choline and formation of acetylcholine. J Neurochem. 1975 Apr;24(4):735–741. [PubMed] [Google Scholar]
- Dawson R. M., Grime D. W., Lindsay D. B. On the insensitivity of sheep to the almost complete microbial destruction of dietary choline before alimentary-tract absorption. Biochem J. 1981 May 15;196(2):499–504. doi: 10.1042/bj1960499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanski A., Linksay D. B., Setchell B. P. Proceedings: Blood flow and substrate uptake and oxidation in the hind limb muscles of sheep. J Physiol. 1974 Oct;242(2):28–29P. [PubMed] [Google Scholar]
- Dross K., Kewitz H. Concentration and origin of choline in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(1):91–106. doi: 10.1007/BF00501010. [DOI] [PubMed] [Google Scholar]
- Erfle J. D. Hydroxylation of gamma-butyrobetaine by rat and ovine tissues. Biochem Biophys Res Commun. 1975 May 19;64(2):553–557. doi: 10.1016/0006-291x(75)90357-5. [DOI] [PubMed] [Google Scholar]
- Hicks P., Rolsten C., Taylor D., Samorajski T. Plasma choline and blood cholinesterases in aged rats. Gerontology. 1982;28(2):104–107. doi: 10.1159/000212518. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Hirata F., Viveros O. H., Diliberto E. J., Jr, Axelrod J. Identification and properties of two methyltransferases in conversion of phosphatidylethanolamine to phosphatidylcholine. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1718–1721. doi: 10.1073/pnas.75.4.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman D. R., Cornatzer W. E. Microsomal phosphatidylethanolamine methyltransferase: some physical and kinetic properties. Lipids. 1981 Jul;16(7):533–540. doi: 10.1007/BF02535052. [DOI] [PubMed] [Google Scholar]
- Hoffman D. R., Haning J. A., Cornatzer W. E. Microsomal phosphatidylethanolamine methyltransferase: effect of altered S-adenosylmethionine-S-adenosylhomocysteine ratios in rat liver. Int J Biochem. 1981;13(6):745–748. doi: 10.1016/0020-711x(81)90045-8. [DOI] [PubMed] [Google Scholar]
- Hübscher G., West G. R., Brindley D. N. Studies on the fractionation of mucosal homogenates from the small intestine. Biochem J. 1965 Dec;97(3):629–642. doi: 10.1042/bj0970629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Jope R. S. Effects of phosphatidylcholine administration to rats on choline in blood and choline and acetylcholine in brain. J Pharmacol Exp Ther. 1982 Feb;220(2):322–328. [PubMed] [Google Scholar]
- Moore S. L., Godley W. C., van Vliet G., Lewis J. P., Boyd E., Huisman T. H. The production of hemoglobin C in sheep carrying the gene for hemoglobin A: hematologic aspects. Blood. 1966 Sep;28(3):314–329. [PubMed] [Google Scholar]
- Neill A. R., Grime D. W., Dawson R. M. Conversion of choline methyl groups through trimethylamine into methane in the rumen. Biochem J. 1978 Mar 15;170(3):529–535. doi: 10.1042/bj1700529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill A. R., Grime D. W., Snoswell A. M., Northrop A. J., Lindsay D. B., Dawson R. M. The low availability of dietary choline for the nutrition of the sheep. Biochem J. 1979 Jun 15;180(3):559–565. doi: 10.1042/bj1800559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajares M. A., Alemany S., Varela I., Marin Cao D., Mato J. M. Purification and photoaffinity labelling of lipid methyltransferase from rat liver. Biochem J. 1984 Oct 1;223(1):61–66. doi: 10.1042/bj2230061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquini J. M., Krawiec L., Soto E. F. Turnover of phosphatidylcholine in cell membranes of adult rat brain. J Neurochem. 1973 Sep;21(3):647–653. doi: 10.1111/j.1471-4159.1973.tb06009.x. [DOI] [PubMed] [Google Scholar]
- Pethick D. W., Lindsay D. B., Barker P. J., Northrop A. J. Acetate supply and utilization by the tissues of sheep in vivo. Br J Nutr. 1981 Jul;46(1):97–110. doi: 10.1079/bjn19810013. [DOI] [PubMed] [Google Scholar]
- Robinson B. S., Snoswell A. M., Runciman W. B., Upton R. N. Uptake and output of various forms of choline by organs of the conscious chronically catheterized sheep. Biochem J. 1984 Jan 15;217(2):399–408. doi: 10.1042/bj2170399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runciman W. B., Ilsley A. H., Mather L. E., Carapetis R., Rao M. M. A sheep preparation for studying interactions between blood flow and drug disposition. I: Physiological profile. Br J Anaesth. 1984 Sep;56(9):1015–1028. doi: 10.1093/bja/56.9.1015. [DOI] [PubMed] [Google Scholar]
- Sastry B. V., Statham C. N., Axelrod J., Hirata F. Evidence for two methyltransferase involved in the conversion of phosphatidylethanolamine to phosphatidylcholine in the rat liver. Arch Biochem Biophys. 1981 Oct 15;211(2):762–773. doi: 10.1016/0003-9861(81)90513-0. [DOI] [PubMed] [Google Scholar]
- Spanner S., Hall R. C., Ansell G. B. Arterio-venous differences of choline and choline lipids across the brain of rat and rabbit. Biochem J. 1976 Jan 15;154(1):133–140. doi: 10.1042/bj1540133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance J. E., Vance D. E. A deazaadenosine-insensitive methylation of phosphatidylethanolamine is involved in lipoprotein secretion. FEBS Lett. 1986 Aug 18;204(2):243–246. doi: 10.1016/0014-5793(86)80820-1. [DOI] [PubMed] [Google Scholar]
- Wang F. L., Haubrich D. R. A simple, sensitive, and specific assay for free choline in plasma. Anal Biochem. 1975 Jan;63(1):195–201. doi: 10.1016/0003-2697(75)90204-3. [DOI] [PubMed] [Google Scholar]
- Zeisel S. H. Formation of unesterified choline by rat brain. Biochim Biophys Acta. 1985 Jul 9;835(2):331–343. doi: 10.1016/0005-2760(85)90289-9. [DOI] [PubMed] [Google Scholar]
- Zeisel S. H., Wurtman R. J. Developmental changes in rat blood choline concentration. Biochem J. 1981 Sep 15;198(3):565–570. doi: 10.1042/bj1980565. [DOI] [PMC free article] [PubMed] [Google Scholar]


