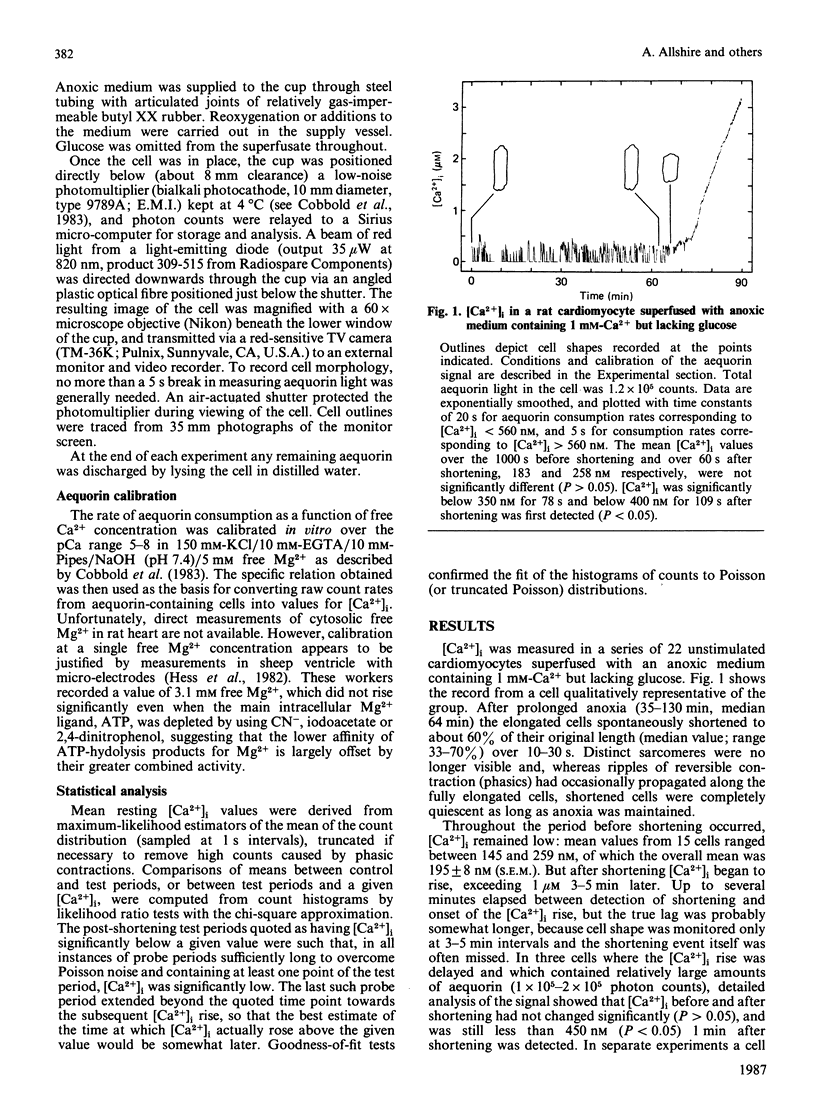
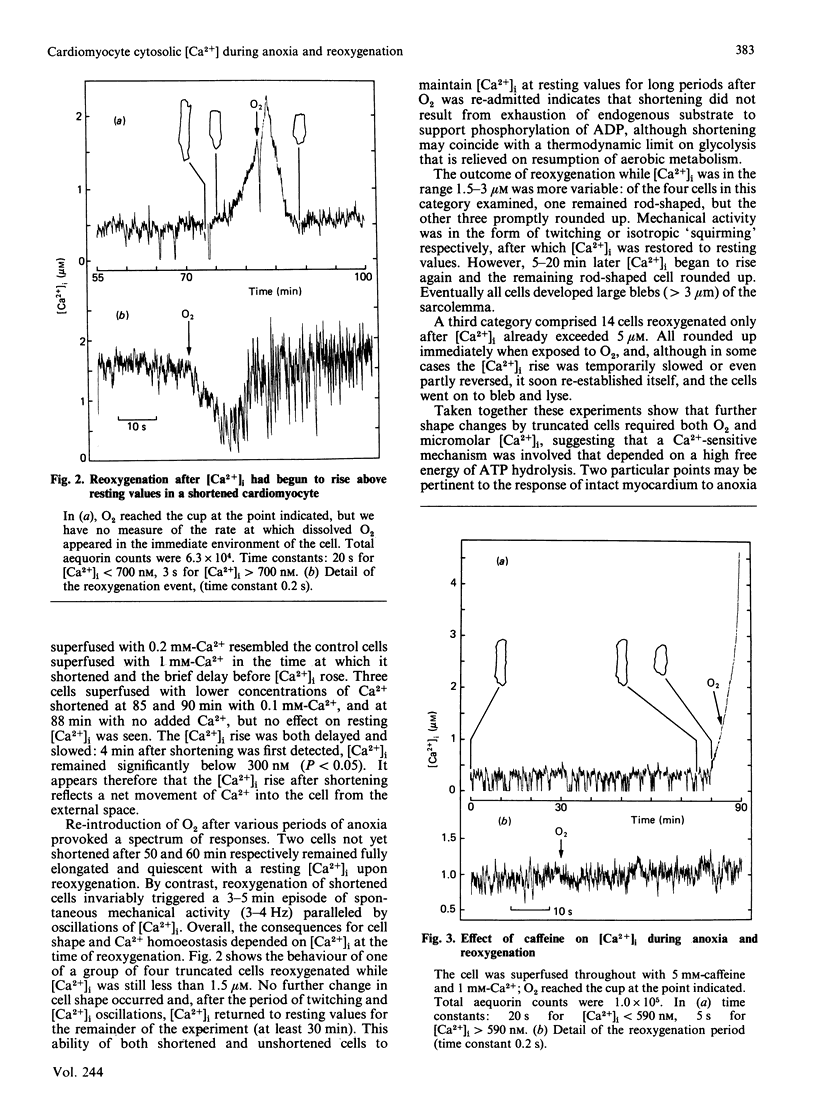
Abstract
Free Ca2+ in the cytosol ([Ca2+]i) of individual rat ventricle cells injected with aequorin was measured under anoxia. In glucose-free medium myocytes spontaneously shortened after about 60 min, although [Ca2+]i was still at or near resting levels. However, within minutes a net inward movement of Ca2+ across the sarcolemma developed and [Ca2+]i began to rise. Provided oxygen was readmitted before [Ca2+]i exceeded 2-3 microM, cells were able to restore [Ca2+]i to resting levels through caffeine-sensitive sequestration of Ca2+ in the sarcoplasmic reticulum. We suggest that Ca2+-independent shortening of anoxic cardiomyocytes reflects onset of rigor which triggers loss of [Ca2+]i homoeostasis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschuld R. A., Wenger W. C., Lamka K. G., Kindig O. R., Capen C. C., Mizuhira V., Vander Heide R. S., Brierley G. P. Structural and functional properties of adult rat heart myocytes lysed with digitonin. J Biol Chem. 1985 Nov 15;260(26):14325–14334. [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Cobbold P. H., Bourne P. K. Aequorin measurements of free calcium in single heart cells. 1984 Nov 29-Dec 5Nature. 312(5993):444–446. doi: 10.1038/312444a0. [DOI] [PubMed] [Google Scholar]
- Cobbold P. H., Cuthbertson K. S., Goyns M. H., Rice V. Aequorin measurements of free calcium in single mammalian cells. J Cell Sci. 1983 May;61:123–136. doi: 10.1242/jcs.61.1.123. [DOI] [PubMed] [Google Scholar]
- Haworth R. A., Hunter D. R., Berkoff H. A. Contracture in isolated adult rat heart cells. Role of Ca2+, ATP, and compartmentation. Circ Res. 1981 Nov;49(5):1119–1128. doi: 10.1161/01.res.49.5.1119. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Garlick P. B., Humphrey S. M. Ischemic contracture of the myocardium: mechanisms and prevention. Am J Cardiol. 1977 Jun;39(7):986–993. doi: 10.1016/s0002-9149(77)80212-9. [DOI] [PubMed] [Google Scholar]
- Hess P., Metzger P., Weingart R. Free magnesium in sheep, ferret and frog striated muscle at rest measured with ion-selective micro-electrodes. J Physiol. 1982 Dec;333:173–188. doi: 10.1113/jphysiol.1982.sp014447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holubarsch C., Alpert N. R., Goulette R., Mulieri L. A. Heat production during hypoxic contracture of rat myocardium. Circ Res. 1982 Dec;51(6):777–786. doi: 10.1161/01.res.51.6.777. [DOI] [PubMed] [Google Scholar]
- Kammermeier H., Schmidt P., Jüngling E. Free energy change of ATP-hydrolysis: a causal factor of early hypoxic failure of the myocardium? J Mol Cell Cardiol. 1982 May;14(5):267–277. doi: 10.1016/0022-2828(82)90205-x. [DOI] [PubMed] [Google Scholar]
- Kentish J. C., Allen D. G. Is force production in the myocardium directly dependent upon the free energy change of ATP hydrolysis? J Mol Cell Cardiol. 1986 Sep;18(9):879–884. doi: 10.1016/s0022-2828(86)80001-3. [DOI] [PubMed] [Google Scholar]
- Piper H. M., Probst I., Schwartz P., Hütter F. J., Spieckermann P. G. Culturing of calcium stable adult cardiac myocytes. J Mol Cell Cardiol. 1982 Jul;14(7):397–412. doi: 10.1016/0022-2828(82)90171-7. [DOI] [PubMed] [Google Scholar]
- Piper H. M., Schwartz P., Spahr R., Hütter J. F., Spieckermann P. G. Absence of reoxygenation damage in isolated heart cells after anoxic injury. Pflugers Arch. 1984 May;401(1):71–76. doi: 10.1007/BF00581535. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson P. A., Harding D. P., Bourdillon P. D., Tones M. A. Calcium out of control. J Mol Cell Cardiol. 1984 Feb;16(2):175–187. doi: 10.1016/s0022-2828(84)80706-3. [DOI] [PubMed] [Google Scholar]
- Stern M. D., Chien A. M., Capogrossi M. C., Pelto D. J., Lakatta E. G. Direct observation of the "oxygen paradox" in single rat ventricular myocytes. Circ Res. 1985 Jun;56(6):899–903. doi: 10.1161/01.res.56.6.899. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R., Vassort G. Rigor tension during metabolic and ionic rises in resting tension in rat heart. J Mol Cell Cardiol. 1981 Jun;13(6):551–561. doi: 10.1016/0022-2828(81)90326-6. [DOI] [PubMed] [Google Scholar]
- Williamson J. R. Glycolytic control mechanisms. II. Kinetics of intermediate changes during the aerobic-anoxic transition in perfused rat heart. J Biol Chem. 1966 Nov 10;241(21):5026–5036. [PubMed] [Google Scholar]


