Abstract
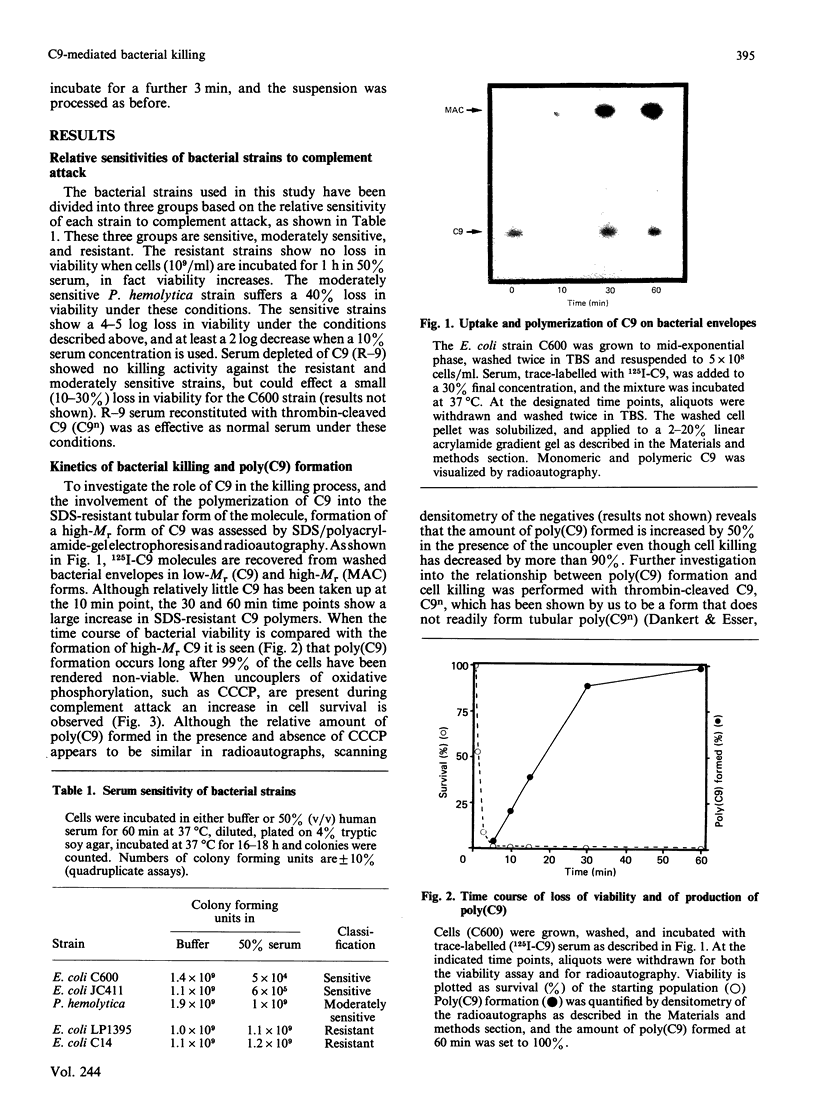
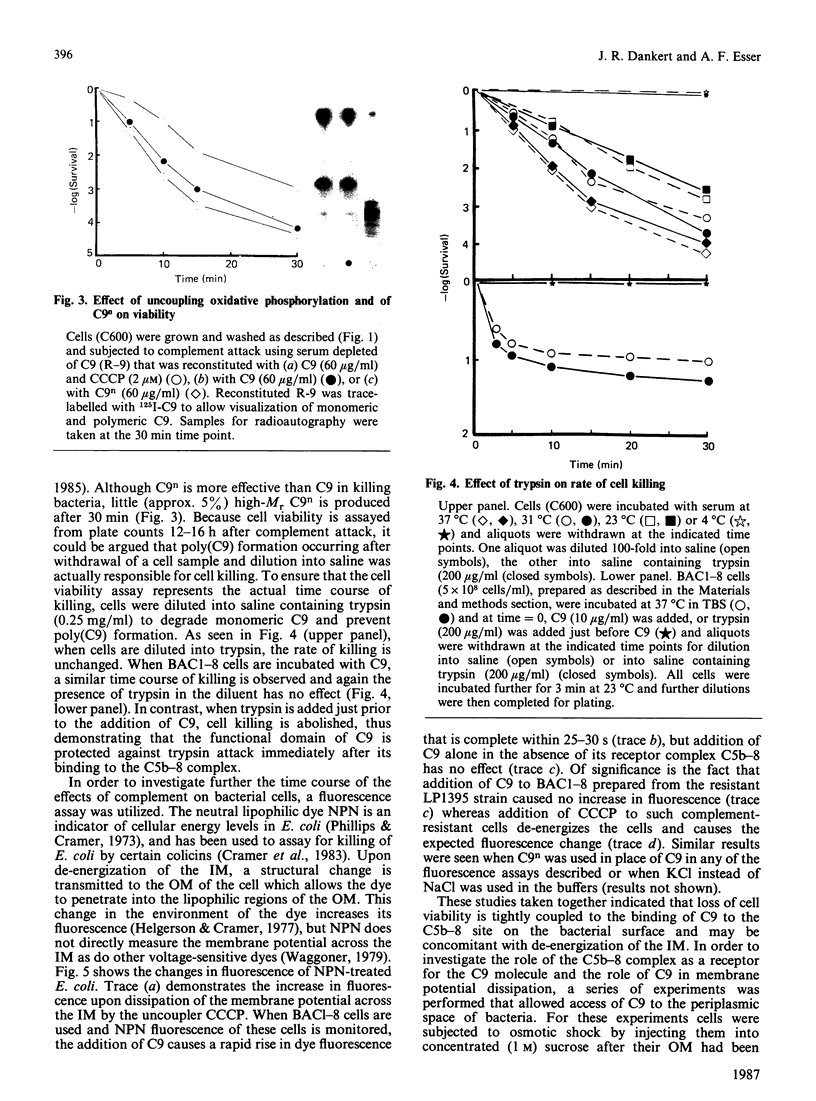
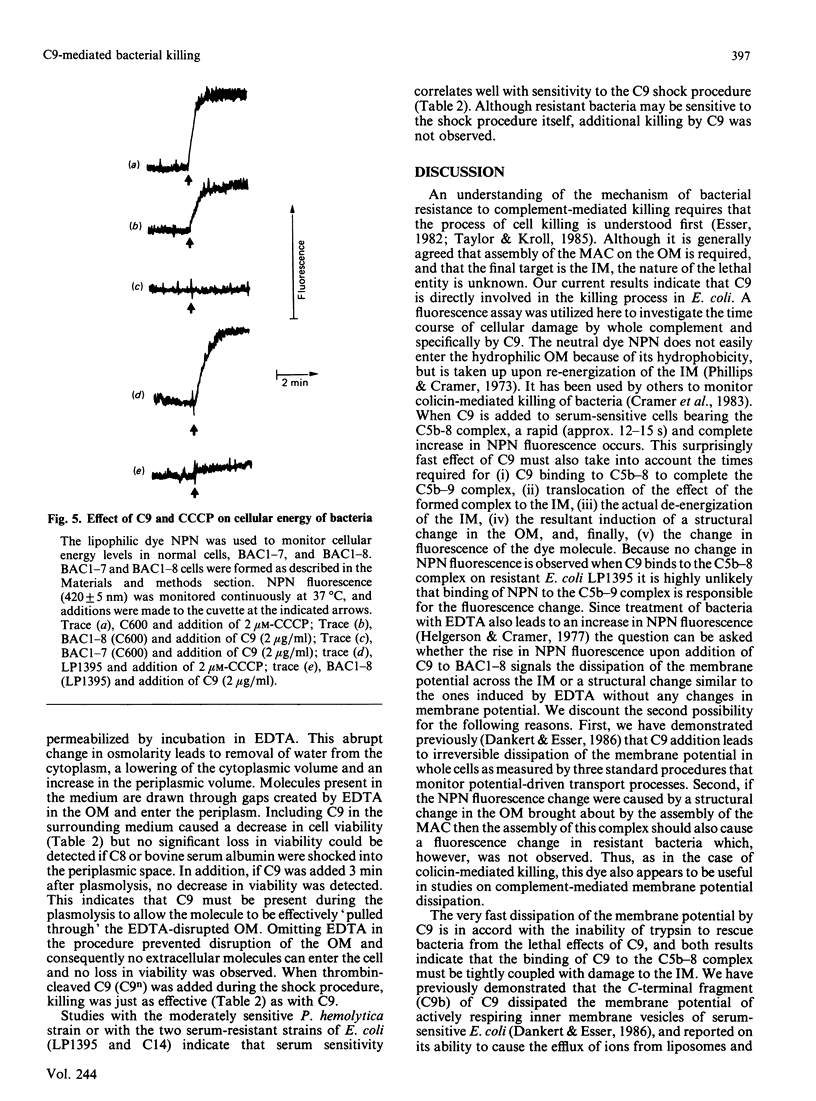
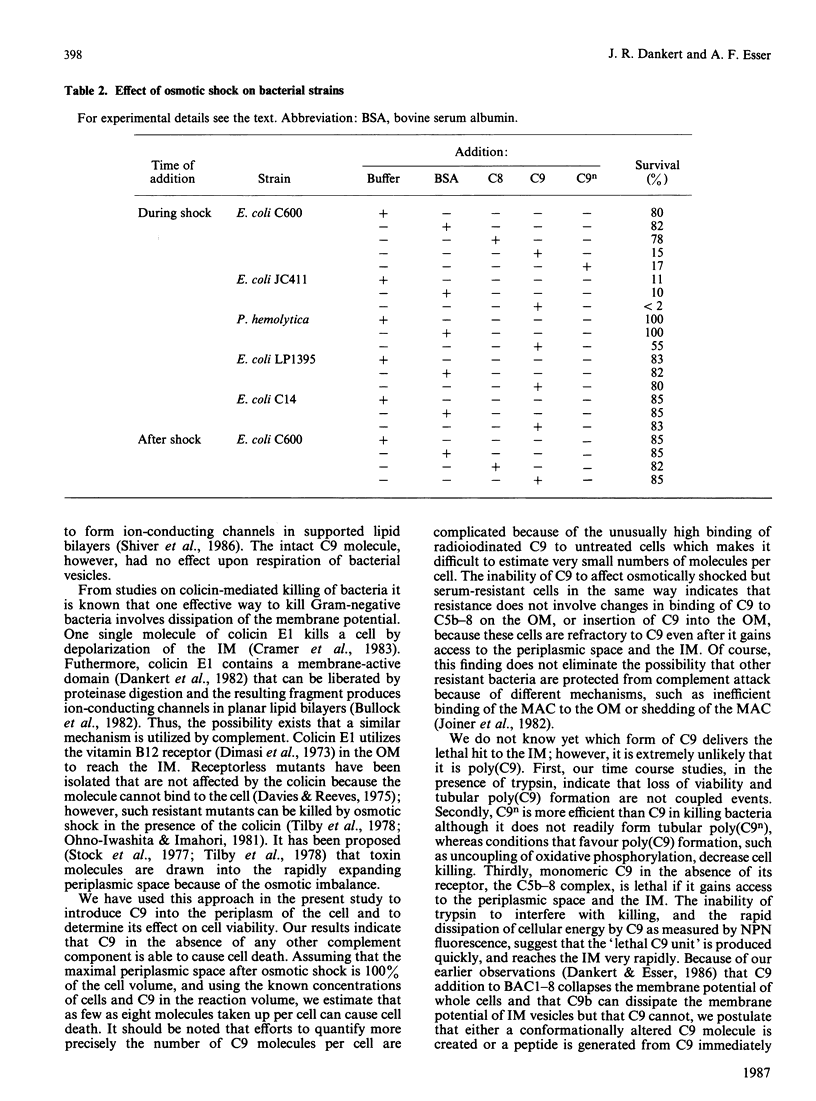
The ability of serum complement to kill Gram-negative bacteria requires assembly of the membrane attack complex (MAC) on the cell surface. The molecular events that lead to cell killing after MAC assembly are unknown. We have investigated the effect of C9 on bacterial survival in the presence and absence of its receptor, the C5b-8 complex, on the outer membrane. A fluorescence assay of the membrane potential across the inner bacterial membrane revealed that addition of C9 to cells bearing the performed C5b-8 complex caused a rapid and complete dissipation of the membrane potential. No fluorescence change was observed in serum-resistant strains of Escherichia coli. Addition of trypsin, after C9 was bound to C5b-8, did not rescue the cells from the lethal effects of C9. Furthermore, assays of cell killing kinetics and C9 binding indicate that formation of tubular poly(C9) is not required for killing. When C9 was introduced into the periplasmic space in the absence of its receptor by means of an osmotic shock procedure, cell killing occurred. Other proteins, such as C8 or serum albumin, were not toxic, and C9 was ineffective against two resistant strains. The results presented here and previously [Dankert & Esser (1986) Biochemistry 25, 1094-1100], when considered together, indicate that the 'lethal unit' in complement killing of some Gram-negative bacteria is a C9-derived product that acts by dissipation of cellular energy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cramer W. A., Dankert J. R., Uratani Y. The membrane channel-forming bacteriocidal protein, colicin El. Biochim Biophys Acta. 1983 Mar 21;737(1):173–193. doi: 10.1016/0304-4157(83)90016-3. [DOI] [PubMed] [Google Scholar]
- Dankert J. R., Esser A. F. Complement-mediated killing of Escherichia coli: dissipation of membrane potential by a C9-derived peptide. Biochemistry. 1986 Mar 11;25(5):1094–1100. doi: 10.1021/bi00353a023. [DOI] [PubMed] [Google Scholar]
- Dankert J. R., Esser A. F. Proteolytic modification of human complement protein C9: loss of poly(C9) and circular lesion formation without impairment of function. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2128–2132. doi: 10.1073/pnas.82.7.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankert J. R., Shiver J. W., Esser A. F. Ninth component of complement: self-aggregation and interaction with lipids. Biochemistry. 1985 May 21;24(11):2754–2762. doi: 10.1021/bi00332a024. [DOI] [PubMed] [Google Scholar]
- Dankert J. R., Uratani Y., Grabau C., Cramer W. A., Hermodson M. On a domain structure of colicin E1. A COOH-terminal peptide fragment active in membrane depolarization. J Biol Chem. 1982 Apr 10;257(7):3857–3863. [PubMed] [Google Scholar]
- Dankert J. R., Uratani Y., Grabau C., Cramer W. A., Hermodson M. On a domain structure of colicin E1. A COOH-terminal peptide fragment active in membrane depolarization. J Biol Chem. 1982 Apr 10;257(7):3857–3863. [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group B. J Bacteriol. 1975 Jul;123(1):96–101. doi: 10.1128/jb.123.1.96-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Masi D. R., White J. C., Schnaitman C. A., Bradbeer C. Transport of vitamin B12 in Escherichia coli: common receptor sites for vitamin B12 and the E colicins on the outer membrane of the cell envelope. J Bacteriol. 1973 Aug;115(2):506–513. doi: 10.1128/jb.115.2.506-513.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the lethal event in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2127–2131. doi: 10.1128/jb.96.6.2127-2131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths E. Metabolically controlled killing of Pasteurella septica by antibody and complement. Biochim Biophys Acta. 1974 Oct 8;362(3):598–601. doi: 10.1016/0304-4165(74)90157-3. [DOI] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A. Changes in Escherichia coli cell envelope structure and the sites of fluorescence probe binding caused by carbonyl cyanide p-trifluoromethoxyphenylhydrazone. Biochemistry. 1977 Sep 6;16(18):4109–4117. doi: 10.1021/bi00637a026. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Schmetz M. A., Sanders M. E., Murray T. G., Hammer C. H., Dourmashkin R., Frank M. M. Multimeric complement component C9 is necessary for killing of Escherichia coli J5 by terminal attack complex C5b-9. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4808–4812. doi: 10.1073/pnas.82.14.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. The membrane attack complex. Springer Semin Immunopathol. 1984;7(2-3):93–141. doi: 10.1007/BF01893017. [DOI] [PubMed] [Google Scholar]
- Phillips S. K., Cramer W. A. Properties of the fluorescence probe response associated with the transmission mechanism of colicin E1. Biochemistry. 1973 Mar 13;12(6):1170–1176. doi: 10.1021/bi00730a024. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Circular polymerization of the ninth component of complement. Ring closure of the tubular complex confers resistance to detergent dissociation and to proteolytic degradation. J Biol Chem. 1982 Dec 25;257(24):15204–15212. [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Polymerization of the ninth component of complement (C9): formation of poly(C9) with a tubular ultrastructure resembling the membrane attack complex of complement. Proc Natl Acad Sci U S A. 1982 Jan;79(2):574–578. doi: 10.1073/pnas.79.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiver J. W., Dankert J. R., Donovan J. J., Esser A. F. The ninth component of human complement (C9). Functional activity of the b fragment. J Biol Chem. 1986 Jul 25;261(21):9629–9636. [PubMed] [Google Scholar]
- Sims P. J. Complement pores in erythrocyte membranes. Analysis of C8/C9 binding required for functional membrane damage. Biochim Biophys Acta. 1983 Aug 10;732(3):541–552. doi: 10.1016/0005-2736(83)90230-4. [DOI] [PubMed] [Google Scholar]
- Stewart J. L., Monahan J. B., Brickner A., Sodetz J. M. Measurement of the ratio of the eighth and ninth components of human complement on complement-lysed membranes. Biochemistry. 1984 Aug 28;23(18):4016–4022. doi: 10.1021/bi00313a002. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Taylor P. W., Kroll H. P. Effect of lethal doses of complement on the functional integrity of target enterobacteria. Curr Top Microbiol Immunol. 1985;121:135–158. doi: 10.1007/978-3-642-45604-6_7. [DOI] [PubMed] [Google Scholar]
- Taylor P. W., Kroll H. P. Interaction of human complement proteins with serum-sensitive and serum-resistant strains of Escherichia coli. Mol Immunol. 1984 Jul;21(7):609–620. doi: 10.1016/0161-5890(84)90046-4. [DOI] [PubMed] [Google Scholar]
- Taylor P. W., Kroll H. P. Killing of an encapsulated strain of Escherichia coli by human serum. Infect Immun. 1983 Jan;39(1):122–131. doi: 10.1128/iai.39.1.122-131.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilby M., Hindennach I., Henning U. Bypass of receptor-mediated resistance to colicin E3 in Escherichia coli K-12. J Bacteriol. 1978 Dec;136(3):1189–1191. doi: 10.1128/jb.136.3.1189-1191.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Podack E. R., Müller-Eberhard H. J. The membrane attack complex of complement: C5b-8 complex as accelerator of C9 polymerization. J Immunol. 1985 Jan;134(1):495–499. [PubMed] [Google Scholar]
- Waggoner A. S. Dye indicators of membrane potential. Annu Rev Biophys Bioeng. 1979;8:47–68. doi: 10.1146/annurev.bb.08.060179.000403. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Levine R. P. How complement kills E. coli. I. Location of the lethal lesion. J Immunol. 1981 Sep;127(3):1146–1151. [PubMed] [Google Scholar]
- Wright S. D., Levine R. P. How complement kills E. coli. II. The apparent two-hit nature of the lethal event. J Immunol. 1981 Sep;127(3):1152–1156. [PubMed] [Google Scholar]




