Abstract
Endometriosis is a prevalent gynecological condition, affecting around 10% of reproductive-age women. Inflammatory processes associated with endometriosis may contribute to endothelial dysfunction. Increased skin accumulation of advanced glycation end-products (AGEs), reflecting arterial stiffness, potentially links endometriosis with elevated risk of cardiovascular events. We hypothesized that patients with endometriosis have impaired endothelial function as well as increased arterial stiffness and AGE skin accumulation, compared to healthy controls. We compared endothelial function, arterial stiffness, and levels of AGEs in patients suffering from endometriosis and in healthy controls. The study included 45 women aged 20 to 40: 21 patients with endometriosis and 24 healthy controls, matched in terms of age, BMI, and blood pressure values. Endo-PAT 2000 device was used for non-invasive assessment of (i) endothelial function, expressed as Reactive Hyperemia Index (RHI), and (ii) arterial stiffness, expressed as Augmentation Index (AI) and Augmentation Index at 75 heart beats/min (AI@75). Endothelial dysfunction was defined as an RHI value ≤ 1.67. AGE Reader device was used for non-invasive evaluation of skin AGE level accumulation. Patients with endometriosis had lower mean RHI values (1.69 ± 0.54 vs. 2.02 ± 0.48, p = 0.037) and a higher prevalence of endothelial dysfunction, (52.4% vs. 20.8%, p = 0.027) compared to healthy controls. Skin AGE level was higher in patients with endometriosis, compared to controls (2.00 ± 0.57 vs. 1.70 ± 0.24, p = 0.013). There were no significant differences in AI and AI@75 between the two groups. Patients with endometriosis have impaired endothelial function and higher AGE skin accumulation, which are well-established preclinical manifestations of increased cardiovascular risk. There is a great need for comprehensive cardiovascular risk assessments in women with endometriosis to prevent the development of potential atherosclerotic-based complications.
Keywords: Endothelial dysfunction, Cardiovascular risk, Endometriosis, EndoPAT 2000, AGE reader
Subject terms: Risk factors, Cardiovascular diseases, Reproductive disorders
Introduction
Endometriosis is a chronic, inflammatory gynecological condition characterized by the presence of endometrial-like tissue outside of the uterine cavity, commonly affecting the pelvic peritoneum, the ovaries, the rectovaginal septum, the bladder, and the bowel1,2. The disease affects approximately 10% of reproductive-age women and is estimated to impact around 190 million women globally3. Endometriosis manifests with chronic pelvic pain, dysmenorrhea, dyspareunia, and gastrointestinal disturbances1,2. Severe symptoms dramatically diminish the quality of life of the affected women4,5. Furthermore, endometriosis can also lead to infertility1,2.
The pathogenesis of endometriosis remains a subject of ongoing research, with several hypotheses proposed, including retrograde menstruation, coelomic metaplasia and immune system dysfunction6. Research on the pathogenesis and mechanism underlying this condition reports elevated levels of inflammatory cytokines, including interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-alpha in serum and peritoneal fluid7. These cytokines trigger chronic inflammatory processes, causing damage to the vascular endothelium and atherosclerosis. The endothelium, an active monolayer of cells lining the lumen of vessels, is an integral regulator of vascular homeostasis through the modulation of vascular tone, smooth muscle cell proliferation, hemostasis processes, and immune cell migration8. Endothelial dysfunction refers to a condition where there is reduced production or availability of nitric oxide, or an imbalance between endothelium-derived relaxing and contracting factors. Notably, endothelial dysfunction can be reversed with the treatment of cardiovascular risk factors and serves as an independent predictor of cardiac events9. Endothelial dysfunction as a recognized early sign of atherosclerosis, can be assessed by measuring reactive hyperemia, which indicates alterations in peripheral arterial tone and reflects arterial stiffness, thereby helping to predict future cardiovascular risk10. Except for endothelial dysfunction, skin accumulation of advanced glycation end-products (AGEs) is associated with metabolic alterations and the aging processes and may also reflect an elevated cardiovascular risk11,12. AGEs represent a diverse category of bioactive compounds resulting from the nonenzymatic interaction between reducing sugars and aminoacids found in proteins and other macromolecules13,14. AGEs formed within the body contribute to arterial stiffness through the process of cross-linking collagen within the arterial wall13,15. Simultaneously, endogenously formed AGEs activate the receptor for AGEs (RAGE), initiating a subsequent cascade that ultimately results in low-grade inflammation13,16.
A higher incidence of ischemic heart disease, cerebrovascular disease, heart failure, dyslipidemia, arrhythmias and all-cause mortality have been reported among patients with endometriosis, compared to matched healthy controls17–21,23,25. So far, endothelial cell function, arterial stiffness and AGEs skin accumulation have not been studied in women with endometriosis. We hypothesized that patients with endometriosis have impaired endothelial function, increased arterial stiffness and higher AGE skin accumulation, compared to healthy controls. We aimed to evaluate endothelial dysfunction, arterial stiffness and skin AGEs accumulation as preclinical manifestations of increased cardiovascular risk in patients with endometriosis.
Materials and methods
Patients
Study participants were recruited from the 2nd Department of Obstetrics and Gynecology of Medical University of Warsaw between December 2021 and April 2022. Eligibility criteria included (i) women between 20 and 40 years of age, (ii) a diagnosis of endometriosis confirmed at least in transvaginal ultrasound (TVUS), and (iii) no evidence of clinically overt atherosclerosis-related cardiovascular disease. The diagnostic criteria for endometriosis were based on specific sonographic features identified during TVUS. For ovarian endometriomas, the key features included: the presence of at least one unilocular (single-chambered) cyst with homogeneous, low-level, “ground-glass” echogenicity and thickened walls, typically without internal vascularity. For deep infiltrating endometriosis (DIE), sonographic features included hypoechoic nodules or masses in the rectovaginal septum, uterosacral ligaments, or posterior vaginal fornix; hypoechoic thickening or nodules in the bladder wall, particularly in the posterior aspect; and hypoechoic, irregular thickening of the bowel wall, especially in the rectosigmoid colon. In several patients, endometriosis was also confirmed through MRI or surgery. Asymptomatic patients were not included in the study. Exclusion criteria included (i) pregnancy and breastfeeding, (ii) presence of malignancy, (iii) concomitant chronic diseases affecting endothelial function (i.e., hypertension, diabetes mellitus, chronic kidney disease). The control group consisted of healthy women with no abnormalities in ultrasound, matched for age and body mass index (BMI), with the same exclusion criteria as the study group.
The study protocol was approved by the bioethics committee of the Medical University of Warsaw (no. of approval: KB/211/2021). The study was performed in accordance with the Declaration of Helsinki. All patients provided written informed consent to participate in the study.
Assessment of endothelial dysfunction and arterial stiffness
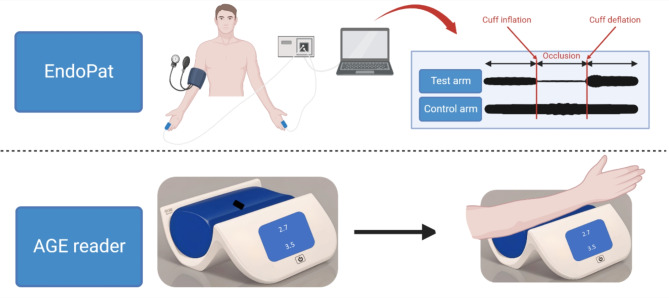
Endothelial function and arterial stiffness were assessed using a noninvasive diagnostic device, the EndoPAT 2000 (Itamar Medical Ltd., Caesarea, Israel). Participants were instructed to abstain from caffeine and smoking for at least 12 h prior to the test. Upon arrival, patients rested for 15 min, during which data on coexisting diseases, age, weight, height and blood pressure were collected. Before initiating the evaluation, the patient was instructed to remain in a supine position. Finger probes were attached to the index fingers of both hands to record the baseline pulse amplitude. A blood pressure cuff was applied to the nondominant arm to create reactive hyperemia during further measurements. The reactive hyperemia procedure consists of a 3–10 min baseline recording, 4.5–5.5 min of blood flow occlusion to one arm using an upper arm blood pressure cuff, and 3–5 min of recording after cuff release. The anticipated response involves an increase in the PAT signal amplitude following occlusion. The system’s software automatically generates the PAT score, which is essentially the ratio of the post-occlusion to pre-occlusion average signal amplitude, adjusted for baseline levels and any systemic variations. Based on pulse wave analysis of the signal, EndoPAT 200 device calculates several scores: the Reactive Hyperemia Index (RHI), Augmentation Index (AI) and Augmentation Index at 75 beats/min (AI@75) providing a quantitative assessment of endothelial dysfunction (RHI) and arterial stiffness (AI, AI@75). The RHI is determined by the ratio of post- to pre-occlusion PAT signals in the occluded arm, compared to the same ratio in the control arm, with adjustments for the baseline vascular tone of the occluded arm. The RHI categorizes endothelial function as normal with RHI > 1.67 or abnormal (endothelial dysfunction) with RHI ≤ 1.67. EndoPAT 2000 also calculates the heart rate from the PAT signals in the baseline region of interest. RHI provides a quantitative assessment of endothelial dysfunction. AI is an indicator of arterial stiffness, derived from pulse wave analysis using the EndoPAT 2000 device. Iis calculated from the PAT pulses at the base-line period of the occluded arm, by averaging multiple valid pulses and finding the systolic peak (P1) and the backward reflected peak (P2) and then using the formula: (P2-P1)/P1. Since AI is influenced by heart rate, the result is then corrected to a standard of AI at heart rate of 75BPM (AI@75)22,23.
Skin AGE level accumulation was measured using AGE Reader device (DiagnOptics Technologies BV, Groningen, The Netherlands), which utilizes fluorescence spectroscopy, emitting a specific wavelength of light onto the skin. This light causes AGEs present in the skin to fluoresce, emitting light of a different wavelength. The emitted fluorescence is then detected and analyzed by the device. Typically, the forearm is chosen as the measurement site. An arm cuff is applied to stabilize the area and block external light interference.
The measurement process typically takes a few minutes to complete. Once the measurement is complete, the device provides a numerical value representing the level of AGE accumulation in the skin. Two measurements were taken at a time, from which an average was calculated22.
Working principles of EndoPAT 2000 and AGE Reader are shown in Fig. 1.
Fig. 1.
Working principle of a device to measure endothelial function and arterial stiffness (EndoPAT 2000) and a device to measure skin accumulation of advanced glycation endproducts (AGE Reader). Modified based on22. Created with BioRender.com, licensed version (A.G.).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics (version 27.0). Shapiro-Wilk test was used to examine the normality of the distribution. Normally distributed values were compared using Student’s t-test. Non-normally distributed values were compared using Mann-Whitney U test. P-value below 0.05 was considered statistically significant.
Results
Demographics and clinical evaluation
A total of 45 patients participated in the study, 21 in the study group and 24 in the control group. Baseline characteristics of the study population are presented in Table 1. There were no differences between the groups in terms of age, BMI, average systolic blood pressure or average diastolic blood pressure.
Table 1.
Characteristics of the study population.
| Parameter | Study group (n = 21) | Control group (n = 24) | Total (n = 45) | p | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Heart rate (beats per minute) | 71.5 | 10.1 | 67.7 | 8.5 | 69.5 | 9.3 | 0.180 |
| Age (years) | 31.0 | 5.1 | 30.3 | 5.6 | 30.6 | 5.3 | 0.700 |
| Height (m) | 1.7 | 0.1 | 1.7 | 0.1 | 1.7 | 0.1 | 0.161 |
| Weight (kg) | 62.3 | 9.5 | 60.7 | 14.6 | 61.4 | 12.4 | 0.667 |
| BMI (kg/m2) | 22.0 | 3.9 | 22.0 | 4.3 | 22.0 | 4.1 | 0.986 |
| Average SBP (mmHg) | 120.5 | 9.0 | 115.4 | 10.1 | 117.8 | 9.8 | 0.079 |
| Average DBP (mmHg) | 78.4 | 8.9 | 75.8 | 8.4 | 77.0 | 8.6 | 0.305 |
BMI- Body Mass Index; SBP – systolic blood pressure; DBP- diastolic blood pressure.
Endothelial function and arterial stiffness assessment
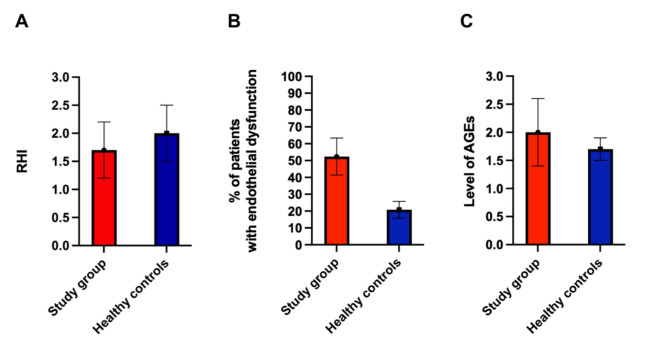
The mean RHI was lower in patients with endometriosis, compared to healthy controls (1.7 ± 0.5 vs. 2.0 ± 0.5, respectively; p = 0.037). Endothelial dysfunction, defined as an RHI value ≤ 1.67, was observed more often in the study group (n = 11, 52.4%), compared to control group (n = 5, 20.8%) (p = 0.027). The mean value of skin autofluorescence measured using AGE Reader was higher in the study group, compared to the control group (2.00 ± 0.6 vs. 1.70 ± 0.2 respectively, p = 0.013). The AI and AI@75 were comparable in both groups. The results of the assessment of endothelial function and arterial stiffness in the study group and in healthy controls are presented in Table 2. Comparison of the results between both groups is illustrated in Fig. 2.
Table 2.
Results of the assessment of endothelial function and arterial stiffness.
| Parameter | Study group (n = 21) | Healthy controls (n = 24) | p |
|---|---|---|---|
| RHI (mean, SD) | 1.7 (0.5) | 2.0 (0.5) | *0.037 |
| Endothelial dysfunction (%, n) | 52.4 (11) | 20.8 (5) | *0.027 |
| AGE Reader (mean, SD) | 2.0 (0.6) | 1.7 (0.2) | *0.013 |
| AI (mean, SD) | -4.2 (14.2) | 1.0 (13.3) | 0.215 |
| AI@75 (mean, SD) | -6.4 (12.1) | -3.6 (12.5) | 0.451 |
AGE Reader - Advanced Glycation Endproducts measurement using AGE Reader device; RHI - reactive hyperaemia index; Ln-RHI - natural logarithm of reactive hyperemia index; AI – augmentation index; AI@75 - augmentation index at 75 beats/min; endothelial dysfunction was defined as RHI equal or below 1.67.
* indicates statistical significance at p < 0.05.
Fig. 2.
Comparison of endothelial function and arterial stiffness in patients from the study group and healthy controls. A Comparison of mean RHI in patients with endometriosis and in healthy controls (p = 0.037). B Comparison of percentage of patients with endothelial dysfunction within the group of patients with endometriosis and healthy controls (p = 0.027). C Comparison of level of AGEs in patients with endometriosis and healthy controls (p = 0.013).
Discussion
To our knowledge, this is the first study evaluating endothelial function, arterial stiffness and AGEs skin accumulation in reproductive-age women with endometriosis. The results of the study demonstrate a higher prevalence of endothelial dysfunction and higher AGEs skin accumulation among patients with endometriosis, compared with healthy controls, indicating higher cardiovascular risk in this population.
Endometriosis as a chronic, inflammatory condition, continues to be a subject of ongoing research. Scientists are investigating the influence of endometriosis on several organ systems, and also mechanisms behind its development and progression. In recent years, several studies showed the increased risk of cardiovascular diseases in endometriosis. In a retrospective matched cohort study including 56 090 patients with endometriosis and 223 669 healthy controls, women with endometriosis had increased risk of atherosclerotic-based cardiovascular diseases, including ischemic heart disease and cerebrovascular disease24. Two other scientific teams also confirmed the increased cardiovascular risk among patients with endometriosis25,26. Additionally, it was revealed that women with endometriosis had worse lipid profiles, including higher serum concentrations of total cholesterol, low-density lipoproteins and triglycerides, as well as lower levels of high-density lipoproteins, compared to controls27. Women with endometriosis were more likely to develop hypercholesterolemia and hypertension, both of which are also established risk factors for atherosclerotic cardiovascular disease28,29.
Endothelial dysfunction, recognized as an early sign of atherosclerosis, is another indicator of cardiovascular risk. Multiple methods were used to assess endothelial function in patients with endometriosis, such as cardio-ankle vascular index (CAVI), laser-doppler flux (LDF) and flow-mediated dilation (FMD)30–32. For example, higher CAVI values and impaired LDF were found in women with endometriosis, indicating increased arterial stiffness and impaired endothelial microvascular function30,31. Moreover, surgical treatment of endometriosis was shown to improve FMD 2 years after the surgery, indicating improved endothelial function32. Despite using a different method to assess arterial stiffness, our results are consistent with those reported previously, indicating substantially increased cardiovascular risk in patients with endometriosis30.
We have not evaluated the pathogenesis of this increased risk, but other authors postulated that it might be due to (i) increased levels of circulating extracellular vesicles (EVs) and (ii) subclinical inflammation inside the peritoneal cavity. Increased levels of EVs released from dysfunctional endothelial cells, known for their involvement in inflammation, were found especially in patients with deeply infiltrating endometriosis (DIE). Concurrently, endometrial stem cells which migrate to the peritoneal cavity in the course of endometriosis undergo differentiation into endometrial cells, which release cytokines, growth factors and adhesive proteins33–35. Several studies described the presence of vascular endothelial growth factor (VEGF), interleukines- interleukin-1 (IL-1), IL-8, IL-10, intercellular adhesion molecule-1 (ICAM-1), monocyte chemotactic protein-1 (MCP-1), regulated upon activation, normal T-cell expressed and secreted (RANTES), insulin-like growth factor (IGF), tumor necrosis factor β (TNFβ) and metalloproteinase-9 (MMP-9) in peripheral blood and peritoneal fluid of patients with endometriosis34,36–42.
Since chronic inflammation is a hallmark of endometriosis, both elevated circulating EV levels and the presence of numerous pro-inflammatory mediators inside the peritoneal cavity underscore the systemic inflammatory state associated with the disease. Similar observations have been made in patients with atherosclerosis, who exhibit elevated levels of circulating EVs in the bloodstream. This association may elucidate the relationship between endometriosis and the preclinical manifestations of atherosclerosis, such as increased vascular stiffness, observed in our study population43,44.
AGEs promote oxidative stress, inflammation and atherosclerosis, which all are the causes of cardiovascular disease45,46. Binding of AGEs to the receptor for advanced glycation end products (RAGE) results in subsequent activation of the pro-inflammatory transcription factors and stimulates chemokine secretion at inflammatory sites47. Concurrently, the concentration of soluble form of RAGE (sRAGE) was higher in follicular and peritoneal fluids of patients with endometriosis. sRAGE is considered to be a detoxifying agent by binding agonists of RAGE such as AGEs. The authors concluded that AGEs-RAGE regulation causes a chain reaction: the failure of apoptosis in retrograded, ectopic endometrial cells, localized angiogenesis, and an immunological tissue response that leads to the development and progression of endometriosis48. It is important to emphasize that the skin reflects systemic AGE levels more thoroughly than blood measurements, making it a valuable, non-invasive marker for cardiovascular risk assessment49.
In summary, our findings are consistent with the previous evidence, indicating endothelial dysfunction and increased risk of cardiovascular disease among patients with endometriosis. Although in the described study, we have primarily focused on the cardiovascular risks associated with endometriosis, it is increasingly recognized that endometriosis is not merely a localized gynecological disorder but a systemic disease with widespread implications, largely driven by chronic inflammation. This ongoing pro-inflammatory state potentially results in widespread tissue damage. In the latest research scientists revealed potential relation between endometriosis and polycystic ovary syndrome, caused by systemic increases in kisspeptin levels, tumor necrosis factor- alpha (TNF-α), IL-6, and C-reactive protein (CRP)50. Molecular mechanisms by which endometriosis-associated inflammation may influence systemic health are still being studied. The nuclear factor-kappa B (NF-κB) pathway and mitogen-activated protein kinase (MAPK) pathway are considered to be responsible for the systemic spread of inflammation. The systemic effects of chronic inflammation in endometriosis are further supported by evidence showing that endometriosis is associated with an increased risk of autoimmune diseases, allergic disorders, and even certain cancers51–54.
Given the systemic nature of endometriosis, it is essential to consider therapeutic strategies that address both the local and systemic aspects of the disease. In conclusion, while the cardiovascular risks associated with endometriosis are significant, it is important to recognize the broader systemic effects of this disease. The chronic inflammatory state induced by endometriosis not only contributes to cardiovascular risk but also has widespread implications for overall health, potentially mediated by key inflammatory molecules and pathways. Future research should continue to explore these systemic effects, with the aim of developing comprehensive treatment strategies that address the full spectrum of endometriosis-related health risks.
Strength and limitations of our study
A strength of our study was the inclusion of a well-matched control group, meticulously selected based on age, height, weight, and blood pressure. Further, we used two independent, non-invasive methods to evaluated preclinical manifestations of atherosclerosis in patients with endometriosis. The limitations of the study include a relatively small sample size and the lack of long-term follow-up, which would allow to evaluate the association between endothelial dysfunction and/or skin AGEs accumulation and cardiovascular events. For a more comprehensive overview, it would be beneficial to also assess and compare laboratory parameters related to cardiovascular risk. Additionally, the study was conducted at a single center, which may limit the generalizability of the findings.
Conclusions
Patients with endometriosis have impaired endothelial function and increased AGEs skin accumulation, which are well-established preclinical manifestations of increased cardiovascular risk. Our results highlight the importance of comprehensive cardiovascular risk assessments and bring attention to the urgent need of comprehensive actions to prevent the development of atherosclerotic-based cardiovascular diseases in women with endometriosis. Furthermore, endometriosis, often diagnosed at a young age, may give a great opportunity for the early detection of atherosclerosis and a better risk stratification in this group of patients. There is a great need for multi-center studies involving larger cohorts of patients to validate and strengthen our findings.
Author contributions
J.M.S. funding acquisition, investigation, data analysis, writing—original draft preparation, figures preparation, tables preparation, project administration; Z.D. investigation, data analysis, writing—original draft preparation, tables preparation; M.K. investigation, data analysis, writing—original draft preparation; M.Z. software, funding acquisition; P.A. software, funding acquisition; M.G. methodology, software; R.G. conceptualization, supervision; M.G. conceptualization, supervision; A.G. conceptualization, methodology, funding acquisition, project administration, data analysis, writing—review and editing, supervision, E.R.W. conceptualization, methodology, supervision. All authors reviewed the manuscript.
Funding
This work was supported by the Medical University of Warsaw, grant number 43/M/MG/N/21. Funding source number 1WR/3/M/MG/N/21.
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Taylor, H. S., Kotlyar, A. M. & &Flores, V. A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet. 397(10276), 839–852 (2021). [DOI] [PubMed]
- 2.Saunders, P. T. K. & Horne, A. W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell. 184 (11), 2807–2824 (2021). [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Endometriosis. https://www.who.int/news-room/fact-sheets/detail/endometriosis (2023).
- 4.Missmer, S. A. et al. Impact of endometriosis on life-course potential: A narrative review. Int. J. Gen. Med.14, 9–25 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, L. H., Lo, W. C., Huang, H. Y. & Wu, H. M. A lifelong impact on endometriosis: Pathophysiology and pharmacological treatment. Int. J. Mol. Sci.24 (8), 7503 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sourial, S., Tempest, N. & Hapangama, D. K. Theories on the pathogenesis of endometriosis. Int. J. Reprod. Med. 179515. 10.1155/2014/179515 (2014). [DOI] [PMC free article] [PubMed]
- 7.Malutan, A. M. et al. Pro-inflammatory cytokines for evaluation of inflammatory status in endometriosis. Cent. Eur. J. Immunol.40 (1), 96–102 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reriani, M. K., Flammer, A. J., Jama, A., Lerman, L. O. & Lerman, A. Novel functional risk factors for the prediction of cardiovascular events in vulnerable patients following acute coronary syndrome. Circ. J.76 (4), 778–783 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadi, H. A., Carr, C. S. & Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 1 (3), 183–198 (2005). [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberry, R. & Nelson, M. D. Reactive hyperemia: A review of methods, mechanisms, and considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol.318 (3), R605–r18 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Shardlow, A. et al. The association of skin autofluorescence with cardiovascular events and all-cause mortality in persons with chronic kidney disease stage 3: A prospective cohort study. PLoS Med.17 (7), e1003163. 10.1371/journal.pmed.1003163 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIntyre, N. J., Fluck, R. J., McIntyre, C. W. & Taal, M. W. Skin autofluorescence and the association with renal and cardiovascular risk factors in chronic kidney disease stage 3. Clin. J. Am. Soc. Nephrol.6 (10), 2356–2363 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linkens, A. M. et al. Habitual intake of dietary advanced glycation end products is not associated with arterial stiffness of the aorta and carotid artery in adults: The Maastricht study. J. Nutr.151 (7), 1886–1893 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goh, S. Y. & Cooper, M. E. Clinical review: the role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab.93 (4), 1143–1152 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Sell, D. R. & Monnier, V. M. Molecular basis of arterial stiffening: Role of glycation - A mini-review. Gerontology. 58 (3), 227–237 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature. 414 (6865), 813–820 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Mu, F., Rich-Edwards, J., Rimm, E. B., Spiegelman, D. & Missmer, S. A. Endometriosis and risk of coronary heart disease. Circ. Cardiovasc. Qual. Outcomes. 9 (3), 257–264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang, H. J. et al. Risk of major adverse cardiovascular and cerebrovascular events in Taiwanese women with endometriosis. J. Formos. Med. Assoc.120 (1), 327–336 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Wei, C. H., Chang, R., Wan, Y. H., Hung, Y. M. & Wei, J. C. C. Endometriosis and newonset coronary artery disease in Taiwan: A nationwide population-based study. Front. Med.25 (8), 619664 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saavalainen, L. et al. Mortality of midlife women with surgically verified endometriosis - A cohort study including 2.5 million person-years of observation. Hum. Reprod.34 (8), 1576–1586 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Farland, L. V., Degnan, W. J., Bell, M., Rexrode, K. M. & Missmer, S. A. Laparoscopically confirmed endometriosis and risk of incident stroke: A prospective cohort study. Fertil. Steril. 116 (2021). [DOI] [PMC free article] [PubMed]
- 22.Rolek, B., Błażejowska, E., Procyk, G., Zimodro, J. M. & Gąsecka, A. Dedicated devices for non-invasive cardiovascular risk assessment - The future of cardiovascular prevention. Cardiol. J.31 (3), 496–498 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.EndoPAT. Device User Manual. Itamar Medical. REF: OM1695214. https://www.itamar-medical.com/wp-content/uploads/2019/07/OM1695214.pdf (2023).
- 24.Okoth, K. et al. Risk of cardiovascular outcomes among women with endometriosis in the United Kingdom: A retrospective matched cohort study. Bjog. 128 (10), 1598–1609 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Okoli, U. et al. Endometriosis and risk of cardiovascular disease: Systematic review and meta-analysis. J. Womens Health (Larchmt). 32 (12), 1328–1339 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Poeta do Couto, C., Policiano, C., Pinto, F. J., Brito, D. & Caldeira, D. Endometriosis and cardiovascular disease: A systematic review and meta-analysis. Maturitas. 171, 45–52 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Nahar, K., Khanam, N. N., Chowdhury, A. A., Khan, N. J. & Mohamed, Z. Association of dyslipidemia with endometriosis: A case control study. Mymensingh Med. J.32 (1), 118–124 (2023). [PubMed] [Google Scholar]
- 28.Melo, A. S. et al. Unfavorable lipid profile in women with endometriosis. Fertil. Steril.93, 2433–2436 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Mu, F. et al. Association between endometriosis and hypercholesterolemia or hypertension. Hypertension. 70, 59–65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilic, D. et al. Association between endometriosis and increased arterial stiffness. Kardiol. Pol.79 (1), 58–65 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Dillon, G. A. et al. Seven days of statin treatment improves nitric-oxide mediated endothelial-dependent cutaneous microvascular function in women with endometriosis. Microvasc. Res.144, 104421 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santoro, L. et al. Regression of endothelial dysfunction in patients with endometriosis after surgical treatment: A 2-year follow-up study. Hum. Reprod.29 (6), 1205–1210 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Munrós, J. et al. Total circulating microparticle levels are increased in patients with deep infiltrating endometriosis. Hum. Reprod.32 (2), 325–331 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Kajdos, M., Szymanski, J., Jerczynska, H., Stetkiewicz, T. & Wilczynski, J. R. Microvesicles released from ectopic endometrial foci as a potential biomarker of endometriosis. Ginekol. Pol.94 (10), 780–791 (2023). [DOI] [PubMed] [Google Scholar]
- 35.Ng, Y. H. et al. Endometrial exosomes/microvesicles in the uterine microenvironment: A new paradigm for embryo-endometrial cross talk at implantation. PLoS One. 8 (3). 10.1371/journal.pone.0058502 (2013). e58502. [DOI] [PMC free article] [PubMed]
- 36.McLaren, J. et al. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J. Clin. Invest.98 (2), 482–489 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kyama, C. M., Debrock, S. & Mwenda, J. M. D’Hooghe T. M. potential involvement of the immune system in the development of endometriosis. Reprod. Biol. Endocrinol.1 (1), 123 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Velasco, J. & Arici, A. Interleukin-8 stimulates the adhesion of endometrial stromal cells to fibronectin. Fertil. Steril.72 (2), 336–340 (1999). [DOI] [PubMed] [Google Scholar]
- 39.van der Linden, P. J. Theories on the pathogenesis of endometriosis. Hum. Reprod.11 (3), 53–65 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Jolicoeur, C. et al. Increased expression of monocyte chemotactic protein-1 in the endometrium of women with endometriosis. Am. J. Pathol.152 (1), 125–133 (1998). [PMC free article] [PubMed] [Google Scholar]
- 41.Kim, J. G. et al. Insulin-like growth factors (IGFs), IGF-binding proteins (IGFBPs), and IGFBP-3 protease activity in the peritoneal fluid of patients with and without endometriosis. Fertil. Steril.73 (5), 996–1000 (2000). [DOI] [PubMed] [Google Scholar]
- 42.Overton, C., Fernandez-Shaw, S., Hicks, B., Barlow, D. & Starkey, P. Peritoneal fluid cytokines and the relationship with endometriosis and pain. Hum. Reprod.11 (2), 380–386 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Dignat-George, F. & Boulanger, C. M. The many faces of endothelial microparticles. Arterioscler. Thromb. Vasc Biol.31 (1), 27–33 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Olejarz, W., Sadowski, K. & Radoszkiewicz, K. Extracellular vesicles in atherosclerosis: State of the art. Int. J. Mol. Sci.25 (1), 388 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasad, K., Dhar, I. & Caspar-Bell, G. Role of advanced glycation end products and its receptors in the pathogenesis of cigarette smoke-induced cardiovascular disease. Int. J. Angiol.24 (2), 75–80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ergun, T. et al. Advanced Glycation End products, a potential link between Psoriasis and Cardiovascular Disease: a case-control study. Indian J. Dermatol.64 (3), 201–206 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cataldegirmen, G. et al. RAGE limits regeneration after massive liver injury by coordinated suppression of TNF-alpha and NF-kappaB. J. Exp. Med.201 (3), 473–484 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujii, E. Y., Nakayama, M. & Nakagawa, A. Concentrations of receptor for advanced glycation end products, VEGF and CML in plasma, follicular fluid, and peritoneal fluid in women with and without endometriosis. Reprod. Sci.15 (10), 1066–1074 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Pinto, R. S., Machado, U. F. & Passarelli, M. Advanced glycation end products as biomarkers for cardiovascular disease: browning clarifying atherogenesis. Biomark. Med.14 (8), 611–614 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Salmeri, N., Viganò, P., Cavoretto, P., Marci, R. & Candiani, M. The kisspeptin system in and beyond reproduction: exploring intricate pathways and potential links between endometriosis and polycystic ovary syndrome. Rev. Endocr. Metab. Disord. 25 (2), 239–257 (2024). [DOI] [PubMed] [Google Scholar]
- 51.Kvaskoff, M. et al. Endometriosis and cancer: A systematic review and metaanalysis. Hum. Reprod.27 (2), 393–420 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Shigesi, N. et al. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod.25, 486–503 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bungum, H. F., Vestergaard, C. & Knudsen, U. B. Endometriosis and type 1 allergies/immediate type hypersensitivity: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol.179, 209–215 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Xu, Y., Deng, Z., Fei, F. & Zhou, S. An overview and comprehensive analysis of interdisciplinary clinical research in endometriosis based on trial registry. iScience. 27(3), 109298 (2024). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.