Abstract
Type B leukemogenic virus (TBLV) induces rapidly appearing T-cell tumors in mice. TBLV is highly related to mouse mammary tumor virus (MMTV) except that TBLV long terminal repeats (LTRs) have a deletion of negative regulatory elements and a triplication of sequences flanking the deletion. To determine if the LTR triplication represents a viral enhancer element, we inserted the triplication upstream and downstream in either orientation relative to the thymidine kinase promoter linked to the luciferase gene. These experiments showed that upregulation of reporter gene activity by the TBLV triplication was relatively orientation independent, consistent with the activity of eukaryotic enhancer elements. TBLV enhancer activity was observed in T-cell lines but not in fibroblasts, B cells, or mammary cells, suggesting that enhancer function is cell type dependent. To analyze the transcription factor binding sites that are important for TBLV enhancer function, we prepared substitution mutations in a reconstituted C3H MMTV LTR that recapitulates the deletion observed in the TBLV LTR. Transient transfections showed that a single mutation (556M) decreased TBLV enhancer activity at least 20-fold in two different T-cell lines. This mutation greatly diminished AML-1 (recently renamed RUNX1) binding in gel shift assays with a mutant oligonucleotide, whereas AML-1 binding to a wild-type TBLV oligomer was specific, as judged by competition and supershift experiments. The 556 mutation also reduced TBLV enhancer binding of two other protein complexes, called NF-A and NF-B, that did not appear to be related to c-Myb or Ets. AML-1 overexpression in a mammary cell line enhanced expression from the TBLV LTR approximately 30-fold. These data suggest that binding of AML-1 to the TBLV enhancer, likely in combination with other factors, is necessary for optimal enhancer function.
Mouse mammary tumor virus (MMTV) causes mammary carcinomas and, at lower frequency, T-cell lymphomas in mice (2, 18, 46). At least one strain of MMTV, type B leukemogenic virus (TBLV), causes exclusively T-cell tumors with a short latency (2 to 3 months) (14). TBLV is virtually identical to MMTV strains that cause mammary tumors, except in the region of the long terminal repeats (LTRs) (4). The TBLV LTRs have a 443-bp deletion that is accompanied by triplication of the 62-bp sequences flanking the deletion (4). We and others have shown that this deletion eliminates several negative regulatory elements (NREs) that bind the homeoproteins, special AT-rich binding protein 1 (SATB1) and CCAAT displacement protein, and suppress MMTV expression in lymphoid cells (9, 24, 35). Mutation of the promoter-proximal SATB1 binding site in the MMTV LTR elevates expression in lymphoid tissues of transgenic mice (35). Acquired MMTV proviruses in T-cell lymphomas invariably delete this SATB1 binding site in the LTR (4, 24, 32, 35, 45), leading to elevated transcription of the viral genome and integration near proto-oncogenes, e.g., c-myc (55). Moreover, Yanagawa et al. showed that substitution of the LTRs of a mammotropic MMTV provirus with truncated LTRs lacking the NREs resulted in a provirus that caused exclusively T-cell tumors (66). These results indicated that loss of the NREs is critical for MMTV-induced T-cell lymphomas.
Multimerization of sequences flanking the NRE deletion also is common in MMTV strains that cause T-cell tumors (4, 32, 45). Paquette et al. have shown that the TBLV LTR (containing both an NRE deletion and a triplication) was sufficient to direct c-myc or CD4 expression primarily to the thymic tissues of transgenic mice; the TBLV LTR–c-myc transgenic mice developed exclusively CD4+ CD8+ T-cell tumors (54), whereas TBLV induces both CD4+ CD8+ and CD4− CD8− lymphomas in mice (42, 48). Multimers of LTR elements have been proposed to function as T-cell-specific enhancers based on transfection experiments in cell culture (61, 67). In support of this hypothesis, results from Yanagawa et al. indicated that multimerized regions flanking the NRE deletion accelerated lymphomagenesis compared to MMTV strains that had simple LTR deletions (66). Analysis of other leukemogenic murine retroviruses also reveals the presence of repeated regions within the LTRs that are important for viral disease specificity (10, 15, 25, 33, 56). For example, replacement of the enhancer repeats in the LTRs of a thymotropic Moloney murine leukemia virus (MuLV) with the enhancer of an erythroleukemia-inducing MuLV is sufficient to switch viral disease specificity (11, 20, 26). Mutagenesis experiments have shown that several transcription factor binding sites within the MuLV enhancers are crucial for the ability of these viruses to induce T-cell lymphomas, including those for AML-1 (47) (also known as RUNX1 [36, 38], polyomavirus enhancer binding protein 2 [1], SL3 enhancer factor 1 [62], and core binding factor [39, 60]), c-Myb (51), and Ets-1 (34, 37, 51, 69).
To determine the role of the TBLV LTR in viral transcriptional specificity in T cells, we inserted the TBLV LTR enhancer region upstream of the C3H MMTV promoter in a luciferase reporter vector. The repeated region elevated MMTV promoter activity at least 100-fold in transient transfection assays of T-cell lines. The increased activity was detectable when the repeats were upstream or downstream of a herpes simplex virus (HSV) thymidine kinase (TK) promoter in either orientation, typical of eukaryotic enhancer elements, yet the enhancer activity appeared to be cell type specific. Mutagenesis experiments indicated that a region spanning the +556 site in the TBLV LTR was crucial for enhancer activity in T cells. Gel shift experiments indicated that the transcription factor AML-1, and two unknown complexes, bound to this critical region. AML-1 overexpression elevated TBLV LTR reporter gene expression approximately 30-fold in non-T cells.
MATERIALS AND METHODS
Construction of plasmids.
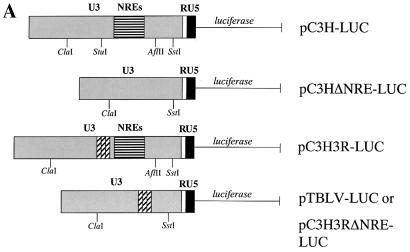
Plasmid pC3H-LUC previously described as pLC-LUC (9), has been modified by the destruction of the SstI site in the polylinker; this construct contains the MMTV C3H LTR upstream of the firefly luciferase gene. Plasmid pTBLV-LUC was engineered by replacement of the ∼760-bp ClaI-to-SstI fragment of the C3H MMTV LTR in pC3H-LUC with the ∼440-bp ClaI-to-SstI fragment of the TBLV LTR; this region of the TBLV LTR includes the triplicated enhancer sequence as well as the 443-bp deletion of the NREs (4) (Fig. 1). The pC3HΔNRE-LUC vector was created by digestion of pC3H-LUC with AflII, filling the ends, digestion with StuI, and religation. The vector pC3H3RΔNRE-LUC was made by substitution of a ∼240-bp StuI-to-SstI fragment from the TBLV LTR (generated by PCR using a 5′ primer with a Stul site) for the ∼531-bp StuI-to-SstI fragment of the C3H MMTV LTR. Plasmid pC3H3R-LUC was engineered by insertion of the StuI-to-SstI fragment of pC3H3RΔNRE-LUC into the StuI site of the pC3H-LUC vector.
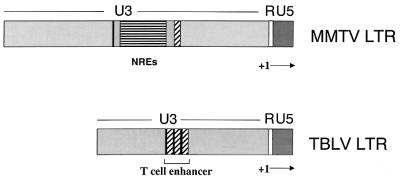
FIG. 1.
Structures of MMTV and TBLV LTRs. The U5, R, and U3 regions are shown. The start of transcription (+1) occurs at the U3/R junction. Horizontally striped regions indicate positions of the NREs in the MMTV LTR; black and diagonally hatched elements within the U3 region indicate sequences flanking the NREs in the MMTV LTR that are present in three copies in the TBLV LTR. The triplicated element is shown as the T-cell enhancer in the TBLV LTR.
The pd6 parental vector for substitution mutations was prepared using a recombinant PCR strategy (23). Using pLC-LUC as a template, two separate PCRs were performed, one using LTR 329+ (5′ CCG CAT CGA TTT TGT CCT TCA 3′) and LTR 523− (5′ CGT TTT AGG CCT TTG AGG TTG AGC GTC TCT TTC T 3′) and the other using LTR 1024+ (5′ CCT CAA AGG CCT AAA ACG AGG ATG TGA GAC AAG T 3′) and LTR1068- (5′ CTC AGA GCT CAG ATC AGA ACC TTT GAT 3′). (The added StuI site is shown in bold.) The products were purified by polyacrylamide gel electrophoresis, and equimolar amounts of the two PCRs were combined. Using LTR 329+ and LTR 1068−, the final product was amplified, resulting in the deletion of the LTR sequence from positions 523 to 1024 and the creation of a StuI site. Plasmid pC3H-LUC was partially digested with ClaI and completely digested with SstI, and the ClaI-to-SstI fragment from the C3H LTR was removed by gel purification using Prep-A-Gene matrix (Bio-Rad, Hercules, Calif.). The TBLV PCR product also was digested with ClaI and SstI and ligated into the digested vector to generate pd6. This clone was verified by sequencing. The wild-type 62-bp enhancer element was cloned into the vector pGEM-TEasy (Promega, Madison, Wis.), and the resulting plasmid (pGEM-62) was used to generate single copies of the substitution mutants by a modification of the QuikChange site-directed mutagenesis method described by Stratagene (La Jolla, Calif.). Briefly, the plasmid vector was mixed with complementary primers containing 6- to 8-bp mutations (including a BglII site) in the enhancer element; each mutation was flanked by 16 to 22 bp of the pGEM-62 sequence. After PCR using PfuTurbo (Stratagene), a mutant enhancer plasmid with staggered nicks was generated and the reaction was digested with DpnI, thus cleaving the methylated parental wild-type DNA. The undigested mutant plasmids then were recovered by transformation of Escherichia coli DH5α and screened for the presence of an appropriate BglII site. The mutations (shown in Fig. 4A) were verified by sequencing and then amplified by PCR using primers that had been treated with T4 polynucleotide kinase. The PCR products then were concatemerized, and the triplicated product was purified and cloned into the pd6 vector that had been linearized with StuI. Clones containing the triplication in the correct orientation were verified by sequencing.
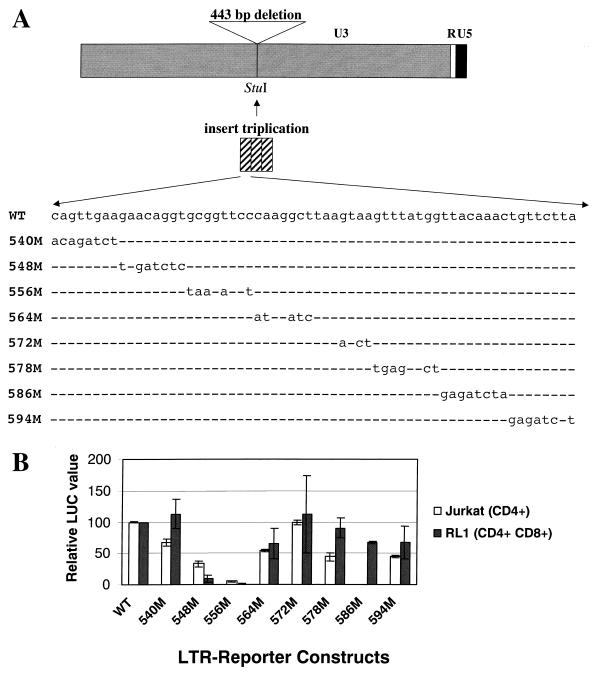
FIG. 4.
Characterization of substitution mutants in the TBLV LTR enhancer. (A) Diagram of the TBLV LTR and positions of enhancer substitution mutations. The reporter gene plasmid used as the backbone for preparation of the substitution mutations (pd6) was prepared as described in Materials and Methods. After the NREs were removed and replaced with a StuI site, triplicated regions containing the wild-type (WT) or mutant (M) sequences were inserted. (B) Reporter gene activity of mutant enhancers in transient assays in Jurkat or RL1 T cells. Means of triplicate assays with standard deviations are shown. Luciferase activity was determined as described in the legend to Fig. 2 except that values are relative to that of the pTBLV-WT-LUC vector (assigned a value of 100).
Plasmid pRL-TK (Promega) contains the HSV TK promoter upstream of the sea pansy (Renilla reniformis) luciferase gene. After digestion with either BamHI, BglII, or HindIII to linearize the plasmid, blunt ends were generated by treatment of the linear vectors with Klenow enzyme (New England Biolabs, Beverly, Mass.), and 5′ phosphates were removed using calf intestinal phosphatase (Roche Molecular Biochemicals, Mannheim, Germany). The triplicated enhancer region of the TBLV LTR was amplified by PCR using a positive-strand primer (5′ AAT AGA AAG AGA CTC TCA ACC TC 3′), a negative-strand primer (5′ AAC CAC TTG TCT CAC ATC CTC G 3′), and pTBLV-LUC as the template. The primers were treated with T4 polynucleotide kinase prior to ligation with the vector. Individual clones were verified by sequencing.
Cell culture and preparation of cell extracts for gel shift assays.
The culture of Jurkat human T cells has been described elsewhere (35). The RL1 (42), LBB.11 (49), and A20 (29) cell lines were maintained in RPMI medium (GIBCO BRL, Gaithersburg, Md.) supplemented with 7.5% fetal bovine serum (FBS); Summit Biotechnology, Fort Collins, Colo.), gentamicin sulfate (50 μg/ml), streptomycin (50 μg/ml), penicillin (100 U/ml), and 5 × 10−5 M 2-mercaptoethanol. Culture of NMuMG (53) and HC11 (5) mouse mammary cells has been described elsewhere (70). Whole-cell extracts were prepared by washing the cells with Tris-buffered saline (10 mM Tris-HCl [pH 7.4], 150 mM NaCl) prior to sonication on ice in microextraction buffer (20 mM HEPES [pH 7.4], 450 mM NaCl, 0.2 mM EDTA, 0.5 mM dithiothreitol, 25% glycerol) supplemented with 1.25 mM phenylmethylsulfonyl fluoride (Sigma, St. Louis, Mo.) and 0.2 mM pepstatin A (Sigma). After sonication, the lysates were clarified by centrifugation at 13,000 × g at 4°C, and protein concentrations were determined as previously described (70). Alternatively, whole-cell extracts were prepared by washing cells with Tris-buffered saline prior to disruption with glass beads (Sigma).
Transfections.
DNA samples for transfection were prepared as described by Bramblett et al. (9). Jurkat T cells were transfected using SuperFect transfection reagent (Qiagen, Inc., Valencia, Calif.) as specified by the manufacturer. Cells (2.5 × 106) were plated in six-well plates in a volume of 2.5 ml of complete medium on the day of transfection. In some cases, cells were cultured in 7.5% charcoal-stripped FBS to remove endogenous steroid hormones. Samples included 2 μg of pC3H-LUC or one of the substitution mutants and 0.25 μg of pRL-TK. DNA was mixed with 75 μl of RPMI medium with no additives. Superfect (10 μl) was mixed with the DNA and incubated for 10 min at room temperature. The solution then was added dropwise to the cells and mixed thoroughly. XC cells were passaged to achieve 90% confluence on the following day. The wild-type or substitution mutant plasmids (5 μg) and pRL-TK (0.5 μg) were transfected using DMRIE-C transfection reagent (GIBCO BRL) according to instructions from the manufacturer. RL1 cells were passaged to achieve 80 to 90% confluence on the following day. The wild-type or mutant plasmids (30 μg) and pRL-TK (5 μg) were transfected using a BTX (San Diego, Calif.) electroporator at 140 V and 1,900 μF in a 0.2-cm cuvette. Cells were electroporated at a concentration of 107/200 μl in RPMI medium containing 10% FBS. HC11 cells were passaged to achieve 90% confluence on the following day. Plasmids containing firefly luciferase (30 μg) and pRL-TK (2 μg) were transfected using a BTX electroporator at 165 V and 1,700 μF in a 0.2-cm cuvette. Electroporations were performed at a concentration of 107 cells/200 μl in RPMI medium containing 10% FBS. The LBB.11 cells were electroporated by the method of Knutson and Yee (30). Cells were seeded at 6 × 105 cells/ml the day prior to transfection, and 2 × 107 cells were electroporated with a test plasmid (30 μg) and a control reporter plasmid (5 μg) in 550 μl of complete medium in a 1-cm cuvette at 2,000 V and 100 μF (electroporator; University of Wisconsin Medical Electronics Laboratory). All cells (except for LBB.11) were incubated for 48 h at 37°C prior to preparation of extracts for reporter gene assays. LBB.11 cells were harvested at 24 h.
Reporter gene assays.
Assays were performed using the Dual-Luciferase reporter assay system (Promega) that independently measures Renilla and firefly luciferase activities. Briefly, cells were rinsed once with phosphate-buffered saline and subsequently disrupted using passive lysis buffer (Promega) and two to three freeze-thaw cycles. The lysates then were clarified by centrifugation at 13,000 × g for 5 min at 4°C. Luciferase activity was determined according to the manufacturer's instructions using a Turner TD-20e luminometer (Turner Designs, Inc., Sunnyvale, Calif.) after assays for protein concentration. Samples were normalized for DNA uptake using luciferase values obtained from the cotransfected pRL-TK or firefly luciferase vectors.
EMSAs.
Probes for electrophoretic mobility shift assays (EMSAs) were prepared by annealing the appropriate oligonucleotides and end labeling with Sequenase version 2.0 (Amersham Pharmacia Biotech, Piscataway, N. J.) as described previously (35). DNA binding reactions (10 to 20 μl) were performed on ice in a buffer containing 20 mM HEPES (pH 7.9), 1 mM MgCl2, 0.1 mM EGTA, 0.4 mM dithiothreitol, 200 mM KCl, 12 μg of salmon sperm DNA/ml, and 4 μg of poly(dl-dC) (Amersham Pharmacia). Reactions were analyzed using 4% nondenaturing polyacrylamide gels and TBE running buffer (22.3 mM Tris base, 22.3 mM boric acid, 0.5 mM EDTA) prior to autoradiography of the dried gel. Supershift experiments were performed with rabbit antiserum against the AML-1 peptide Arg-lle-Pro-Val-Asp-Ala-Ser-Thr-Ser-Arg-Arg-Phe-Thr-Pro-Pro-Ser as described previously (41). To verify the specificity of supershift experiments, the peptide (4 μg) was preincubated with antibody before addition to EMSAs.
RESULTS
Unique cis-acting elements in the TBLV LTR confer T-cell-specific transcriptional activity.
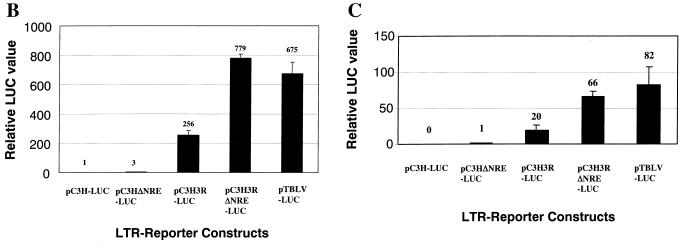
Previous experiments using TBLV LTR reporter genes in transgenic mice suggested that TBLV has a unique transcriptional control region that is preferentially active in CD4+ CD8+ T cells (54). Examination of the TBLV LTR sequence shows that there is a deletion of 443 bp of the U3 region and triplication of 62 bp flanking the deletion relative to the MMTV LTR (Fig. 1) (4). We have previously reported the presence of several NREs within the MMTV LTR that inhibit transcription in lymphoid tissues (9, 24). Therefore, the contributions of the NRE and the triplication to TBLV-mediated transcription were determined. We compared the transcriptional activity of the wild-type C3H MMTV LTR with that of an LTR containing an NRE deletion between the StuI and AflII sites (pC3HΔNRE-LUC), an LTR with an insertion of the triplicated region from TBLV into the StuI site (pC3H3R-LUC), or a C3H LTR containing either a substitution of the TBLV LTR region between the ClaI and SstI sites (pTBLV-LUC) or StuI and SstI (pC3H3RΔNRE-LUC) (Fig. 2A). Transient transfections of these constructs into Jurkat T cells showed that elimination of the NREs between the C3H MMTV StuI and AflII sites (pC3HΔNRE-LUC) elevated reporter gene expression threefold compared to pC3H-LUC (Fig. 2B). Inclusion of the TBLV triplication in the NRE-minus LTR (either pTBLV-LUC or pC3H3RΔNRE-LUC) increased expression ca. 700- to 800-fold over that observed with pC3H-LUC, whereas the triplication alone (pC3H3R-LUC) elevated expression ca. 250-fold above the activity of the C3H MMTV LTR. These assays revealed that the combined effects of the deletion and triplication were sufficient to account for the differences in the transcriptional activities of the MMTV and TBLV LTRs in Jurkat cells.
FIG. 2.
cis-acting elements in the TBLV LTR lead to high transcriptional activity in T cells. (A) Reporter gene constructs used in transient transfection assays. The positions of relevant restriction enzyme sites within the U3 region that were used for cloning are shown. The construct pTBLV-LUC was prepared by substituting the ClaI-to-SstI fragment of TBLV for the ClaI-to-SstI fragment of pC3H-LUC. The construct pC3H3RΔNRE-LUC was made by substitution of a StuI-to-SstI fragment from the TBLV LTR for the StuI-to-SstI fragment of pC3H-LUC. (B) Activities of reporter gene constructs in Jurkat cells. Luciferase (LUC) activity is given in light units/100 μg of protein normalized for DNA uptake as measured by cotransfection with the pRL-TK reporter plasmid. Luciferase activity is reported relative to that of pC3H-LUC, assigned a value of 1; standard deviations from the means of triplicate assays are shown. (C) Activities of reporter gene constructs in RL1 cells. Luciferase activity is reported relative to pC3HΔNRE-LUC (assigned a value of 1) since pC3H-LUC was not detected in these assays.
Transient transfections with MMTV and TBLV LTR constructs also were performed in RL1 T cells and other cell types. Results for RL1 cells (CD4+ CD8+) were similar to those for Jurkat cells except that we could not detect the basal activity of the MMTV promoter in RL1 cells (probably due to lower transfection efficiencies) (Fig. 2C). In contrast to results obtained with T-cell lines, there was little difference in the transcriptional activity of the C3H MMTV LTR relative to the TBLV LTR in HC11 mouse mammary cells, XC rat fibroblasts, or LBB.11 mouse B cells (Table 1). Together, these experiments showed that loss of the NREs and acquisition of the triplicated region allowed higher transcriptional activity of the MMTV LTR in T cells but not other cell types, including B cells.
TABLE 1.
Activities of MMTV and TBLV-LTR reporter gene constructs in non-T-cell lines
| Cell line | LTR promoter | Relative luciferase activitya |
|---|---|---|
| HC11 mammary cells | C3H MMTV | 1.0 ± 0.1 |
| TBLV | 0.5 ± 0.1 | |
| XC rat fibroblasts | C3H MMTV | 1.0 ± 0.2 |
| TBLV | 1.0 ± 0.1 | |
| LBB.11 B cells | C3H MMTV | 1.0 ± 0.1 |
| TBLV | 1.8 ± 0.3 |
Luciferase activity in light units/100 μg of protein extract was determined. Values for pTBLV-LUC then were calculated relative to a value of 1.0 for pC3H-LUC.
Enhancer properties and cell-type specificity of the TBLV triplication.
Because the TBLV triplication is reminiscent of other retroviral enhancer regions that have been shown to be important for viral disease specificity (10, 16, 33, 56), we amplified the entire triplicated region (three copies of the 62-bp sequence) using PCR and inserted the triplication in both orientations at three positions upstream and downstream of the HSV TK promoter in the pRL-TK reporter gene plasmid (Fig. 3A). The resulting constructs then were used in transient transfections of Jurkat T cells. Insertions of the TBLV LTR triplication upstream or downstream showed 9- to 89-fold elevation of luciferase expression from the TK promoter, and expression was relatively orientation independent (Fig. 3B). The highest level of expression was observed when the triplication was downstream of the reporter gene in the sense orientation. These results were consistent with the ability of the TBLV LTR triplication to act as a transcriptional enhancer (7, 31).
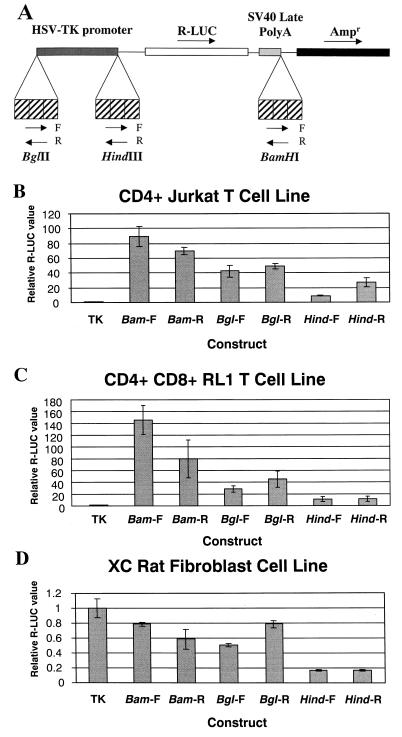
FIG. 3.
Transcriptional enhancement by the TBLV triplication is relatively independent of distance and orientation from the HSV TK promoter. (A) Structures of plasmids containing the TBLV triplication in the pRL-TK vector. The transcriptional orientations of the Renilla luciferase (R-LUC) and ampicillin resistance (Ampr) genes are shown by arrows above the plasmid construct. The TBLV triplication was inserted in three positions within the TK promoter-luciferase vector at the BglII, HindIII, and BamHI sites. The forward (F) and reverse (R) orientations of the triplication inserts are shown by arrows below the plasmid construct. (B) Transient transfections in Jurkat human T cells. (C) Transient transfections in RL1 mouse T cells. (D) Transient transfections in rat XC fibroblasts. Standard deviations from the means of triplicate assays are shown. Luciferase activity is reported as described in the legend to Fig. 2 except that values are relative to that for the pRL-TK plasmid without the TBLV enhancer (assigned a value of 1). DNA uptake was normalized using pTBLV-LUC.
To determine if the LTR enhancer activity was cell type dependent, we used the pRL-TK plasmids containing the TBLV triplication for transient transfections in a second T-cell line, RL1. Such experiments showed orientation-independent enhancement of TK promoter activity (Fig. 3C). We also used the same constructs to perform transient transfection assays in XC rat fibroblast cells (Fig. 3D). In these experiments, the TBLV triplication did not enhance transcription from the TK promoter; instead, the presence of the triplication inhibited (up to fivefold) transcriptional activity of the promoter. Similar results were obtained with HC11 mammary cells (data not shown). Together with previous results, these experiments suggest that the TBLV enhancer is active only in specific cell types, particularly T cells.
Activity of TBLV enhancer mutations.
To determine the specific sequences required for T-cell enhancer activity, we prepared a reporter gene construct that replicated the TBLV LTR changes in the context of the C3H MMTV LTR. A 62-bp enhancer monomer from the TBLV LTR was synthesized, triplicated, and inserted into the StuI site of a C3H MMTV LTR that had been modified by deletion of the 443-bp sequence that includes the NRE and replacement with a StuI restriction site (pTBLV-WT-LUC) (Fig. 4A). Transient transfections comparing the transcriptional activities of pTBLV-WT-LUC and pTBLV-LUC (Fig. 2) revealed no significant differences (data not shown). Subsequently, 6- to 8-bp substitution mutations containing a BglII site were introduced across the length of the enhancer monomer, triplicated, and inserted into the StuI site as indicated for pTBLV-WT-LUC (Fig. 4A).
Transient transfection experiments were performed in two different T-cell lines (Jurkat and RL1) to compare the reporter gene activity of the wild-type construct to that of the mutants. In Jurkat cells, the 540, 564, and 572 mutations affected transcriptional activity less than twofold, whereas the mutations at positions 548, 578, and 594 suppressed enhancer activity approximately two- to three-fold (Fig. 4B). On the other hand, mutations at positions 556 and 586 affected reporter gene expression 20-fold or more. Interestingly, results from the CD4+ Jurkat line were not identical to those from RL1 cells (CD4+ CD8+). In RL1 cells, mutations at positions 540, 572, and 578 had wild-type activity, the 564, 586, and 594 mutations had less than a 2-fold effect on promoter function, and mutation at position 548 gave a 10- to 20-fold loss of reporter gene activity assayed in this cell type (Fig. 4B). Neither the 548 or 556 mutations compromised LTR reporter gene activity in HC11 mammary cells (data not shown), suggesting that the effect of these mutations is cell type specific. Since the mutation at 556 decreased reporter gene activity at least 20-fold in both CD4+ and CD4+ CD8+ T cells, these results suggested that the 556 mutation compromised one or more transcription factor binding sites that are critical for the T-cell enhancer activity of the TBLV LTR.
AML-1 binding to a crucial region of the TBLV enhancer.
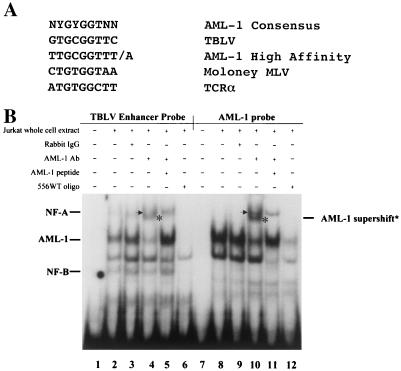
To further characterize the nature of the sequences at position 556, we used the TRANSFAC software program (64) to identify a putative AML-1 site spanning this mutation. This LTR sequence closely matched a consensus AML-1 site (39) as well as a high-affinity AML-1 site in the MuLV LTR described by Thornell et al. (63) (Fig. 5A). To determine if this TBLV region contains an AML-1 binding site, we synthesized a 26-bp oligonucleotide based on the wild-type TBLV LTR (556WT) sequence. The oligonucleotide was end labeled and used in a gel shift assay with whole-cell extracts from Jurkat T cells (Fig. 5B, lanes 1 to 6). As a control, we also used a labeled oligonucleotide containing a known AML-1 binding site (lanes 7 to 12) (6). Results of this experiment showed that the TBLV LTR probe bound at least two complexes with mobilities similar to those obtained with the AML-1 probe (compare lanes 2 and 8). Only the slower-migrating complex was specific, as judged by its ability to be competed with homologous oligomer; this complex contained AML-1 since it was supershifted with antibody specific for AML-1 (lane 4; supershifted band comigrates with NF-A) (41). This supershift was abolished by addition to the reaction of the peptide used to generate the antibody (lane 5). At least two other complexes that were not observed with the AML-1 probe (named NF-A and NF-B) also appeared to be specific, as judged by competition with homologous oligomer (lane 6).
FIG. 5.
AML-1 binding to the TBLV LTR enhancer region. (A) Comparison of the AML-1 consensus sequence to that from TBLV, MuLVs, and the T-cell receptor alpha chain (TCRα). The AML-1 high-affinity site was selected as described previously (63). Sites shown are as reported by Lewis et al. (34). (B) Supershift experiments show AML-specific binding to the TBLV enhancer monomer element. The TBLV enhancer probe (556WT) was used for EMSA with whole-cell Jurkat extracts in lanes 1 to 6, whereas a known AML-1 binding site probe was used in lanes 7 to 12. Sequences of probes are shown in Fig. 6A. The NF-A complex (indicated by an arrow) and the AML-1 supershifted complex (indicated by an asterisk) migrate similarly on the gel. The AML-1 antibody (Ab) specificity has been demonstrated (44). Normal rabbit immunoglobulin G (IgG) was used as a negative control in lanes 3 and 9.
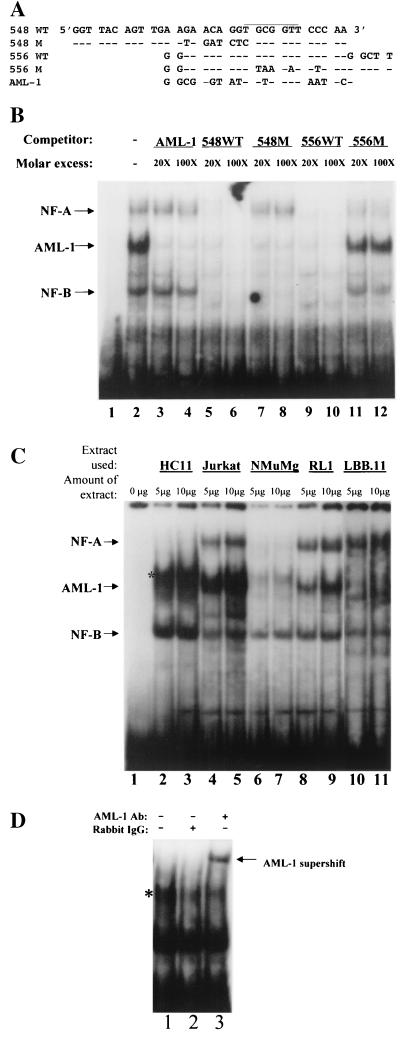
Transient transfection experiments in RL1 T cells showed that two adjacent mutations starting at positions 548 and 556 resulted in dramatic reductions in TBLV enhancer activity (Fig. 4B). Comparisons of the mutations to the consensus sequence indicated that the AML-1 binding site spanned these mutations. However, the effect of the 548 mutation was more dramatic in RL1 cells than in Jurkat T cells. To determine if the effect of both mutations in T cells was due to a decrease in AML-1 binding to the TBLV enhancer, we performed gel shift assays with Jurkat cell extracts to measure competition of various oligomers for AML-1 binding to the 556WT oligomer from the TBLV enhancer (Fig. 6A). Oligomers containing a known AML-1 binding site (Fig. 6B, lanes 3 and 4) or the TBLV LTR oligomers 548WT and 556WT (lanes 5, 6, 9, and 10) competed for AML-1 binding. The 548M oligomer also showed competition, suggesting that this mutation did not significantly affect AML-1 binding. As expected from previous results, the AML-1-specific oligonucleotide did not compete for binding of NF-A and -B (lanes 3 and 4, see arrows). The NF-A complex was competed with the 548WT and 556WT oligomers but showed poor competition with the 556M oligomer, whereas the 548M sequence did not compete for this complex. These results suggested that the NF-A binding site spans the 548 and 556 mutations. The NF-B complex was competed with 548WT, 556WT, and 548M sequences, but competition with the 556M oligomer was minimal (lanes 11 and 12). These assays suggested that the 556 mutation, but not the 548 mutation, affected binding of both AML-1 and NF-B binding. Together, these experiments suggest that there are at least three complexes (NF-A, AML-1, and NF-B) that bind to the TBLV LTR in close proximity (within 16 bp) to control enhancer activity. None of these complexes was competed by oligonucleotides containing consensus binding sites for Myb, Ets-1, or Ets family members (data not shown).
FIG. 6.
Cell-type specificity of factor binding to TBLV LTR probes. (A) Nucleotide sequences of probes and competitors used for gel shift assays. Dashes indicate sequence identities with the 548WT and 556WT probes; the AML-1 binding site is overlined. (B) Binding specificity of NF-A, AML-1, and NF-B complexes. The 556WT probe was labeled and used for EMSA with whole-cell Jurkat extracts in the presence of 20- to 100-fold molar excesses of the unlabeled competitor oligonucleotides. The positions of specific complexes are shown with arrows. Lane 1 had no added cell extract. (C) Cell type specificity of factor binding to the 556WT probe. Different amounts of whole-cell extracts from HC11 mammary cells (lanes 2 and 3), Jurkat T cells (lanes 4 and 5), NMuMG mammary cells (lanes 6 and 7), RL1 T cells (lanes 8 and 9), and LBB.11 B cells (lanes 10 and 11) were incubated with the TBLV enhancer probe. Lanes 10 and 11 were derived from a gel different from that shown for lanes 1 to 9. Lane 1 shows a reaction with no added cell extract. The positions of NF-A, AML-1, and NF-B complexes are shown with arrows on the left. Gel shifts using A20 B-cell extract were similar to those shown for LBB.11 (data not shown). (D) HC11 cells contain a small amount of AML-1. HC11 extract (5 μg) was incubated with the 556WT probe in the absence of added antibody (Ab; lane 1) or in the presence of rabbit immunoglobulin G (IgG; lane 2) or antibody specific for AML-1B (lane 3). Note that a complex that migrates slightly slower than AML-1 (larger amounts are seen in lanes 2 and 3 [asterisk in panel C]) is not AML-1, as judged by its failure to supershift with specific antisera. The small amount of AML-1 in HC11 cells is more apparent after the supershift.
The cell-type-specific distribution of these complexes also was determined using gel shift assays with the 556WT probe. As anticipated from previously published data (40, 47, 57), AML-1 complexes were abundant as measured using cellular extracts from Jurkat and RL1 cells (Fig. 6C, lanes 4, 5, 8, and 9). Small amounts of AML-1 also were detectable in mammary cell extracts, but a more abundant complex migrated slightly slower than those determined to contain AML-1 by supershift experiments (Fig. 6C, lanes 2 and 3) (Fig. 6D). LBB.11 B-cell extracts had no detectable AML-1 activity by gel shift assays or by Western blotting (data not shown). The NF-B complexes were particularly abundant in HC11 extracts but were easily detected in extracts from the NMuMG mouse mammary cell line, the Jurkat and RL1 T-cell lines, and the LBB.11 B-cell line. Complexes of NF-A were detected using Jurkat and RL1 cell extracts and B-cell extracts but were undetectable or in low amounts in mammary cell extracts.
Effect of the 586 mutation in Jurkat CD4+ cells.
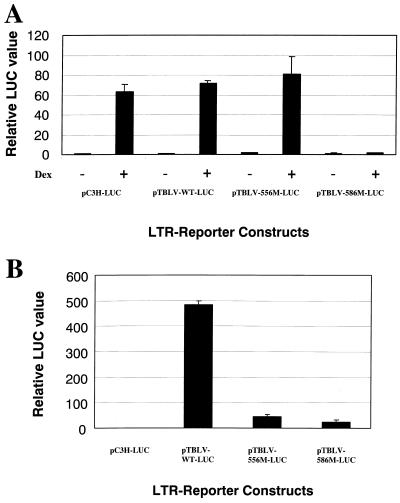
Transient transfection experiments showed that the 586 mutation dramatically reduced TBLV enhancer activity in Jurkat but not RL1 cells (Fig. 4B). Analysis of transcription factor binding sites in this region revealed the presence of a glucocorticoid receptor (GR) site. To determine if the 586 mutation eliminated the GR site, we performed transient transfection assays in XC rat cells in the presence or absence of dexamethasone (Fig. 7A). As expected, the wild-type C3H MMTV LTR showed a strong (over 60-fold) induction in the presence of glucocorticoids, as did the reconstructed TBLV-WT-LUC plasmid, confirming that the TBLV enhancer has a functional GR binding site. However, the 586 mutation, but not the 556 mutation, eliminated the glucocorticoid-induced stimulation of reporter gene expression.
FIG. 7.
The GR binding site mutation in the TBLV enhancer abolishes hormone-dependent transcriptional activation but not T-cell enhancer activity. (A) Activity of the GR binding site mutant 586M in transient transfections of XC rat cells. Hormones were added as indicated 24 h after transfection using the DMRIE-C method as specified by the manufacturer. After an additional 24 h in the presence or absence of 10−6 M dexamethasone, cell extracts were prepared for reporter gene assays. (B) Activity of the GR binding site mutant in transient transfection assays of Jurkat T cells grown in the absence of exogenous steroid hormones. Luciferase (LUC) activity was determined as described in the legend to Fig. 2 except that values are relative to that for pC3H-LUC in the absence of dexamethasone (assigned a value of 1). Means of triplicate assays with standard deviations are shown.
GR requires the presence of hormone to allow functional receptor to translocate into the nucleus and allow DNA binding (27). Jurkat cells appear to have low levels of functional GR, as measured by low-level enhancement of MMTV LTR reporter gene expression in the presence of hormones compared to that without added hormone (data not shown). To determine if the 586 mutation affects TBLV enhancer activity in the absence of steroid hormones, we used media supplemented with hormone-depleted serum to perform transient transfection assays in Jurkat cells grown without exogenous glucocorticoids (Fig. 7B). The TBLV enhancer-containing plasmid had approximately 500-fold greater expression than the C3H MMTV LTR, and this activity was greatly diminished by the 556 and 586 mutations. A similar result was obtained when the experiment was repeated with stripped serum and phenol red-free media (data not shown). Thus, the absence of glucocorticoid hormones did not affect TBLV transcriptional activity in Jurkat cells, suggesting that binding of GR is not critical for enhancer function.
AML-1 overexpression in non-T cells.
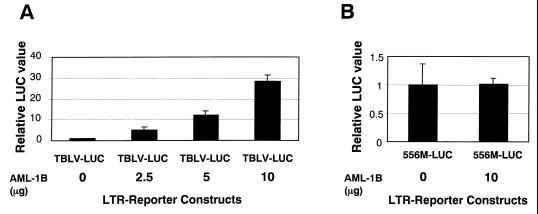
Transient transfection experiments identified a critical region of the TBLV enhancer for optimal transcriptional activity in both Jurkat and RL1 cells (Fig. 4); this region was shown to bind AML-1, a transcription factor that also binds the MuLV enhancers (69). Because the TBLV and MMTV LTRs have equivalent transcription levels in non-T-cell lines, we tested whether overexpression of the transcriptionally active splice variant of AML-1 (AML-1B) (43) would enhance reporter gene activity from the TBLV LTR. Plasmid pTBLV-LUC was transfected into HC11 mammary cells in the presence of increasing amounts of an AML-1B expression vector (43) (Fig. 8A). Cotransfection with the AML-1B expression plasmid gave approximately 30-fold enhancement of reporter gene activity relative to transfections containing a control plasmid lacking AML-1B. Activity of the TBLV LTR was dependent on the amount of AML-1B expression vector; doubling of the AML-1B vector amount resulted in twice as much reporter gene expression. This effect was specific for AML-1B since cotransfection of an AML-3 expression construct did not increase transcriptional activity from the TBLV LTR (data not shown). Overexpression of AML-1B failed to elevate expression of a TBLV LTR reporter construct that contained the 556 mutation (Fig. 8B).
FIG. 8.
Overexpression of AML-1B in transient transfection assays using HC11 cells. Luciferase (LUC) activity was determined as described in the legend to Fig. 2 except that values are relative to that for pTBLV-WT-LUC (A) or pTBLV-556M-LUC (B) without AML-B cotransfection (assigned a value of 1). Means of triplicate transfections with standard deviations are shown. The AML-1B vector contains the transcriptionally active splice variant of AML-1 with two additional exons, 7B and 8 (43). The amount of AML-1B expression vector used is indicated. All transfections contained 22.5 μg of total DNA.
DISCUSSION
Identification of a T-cell-specific enhancer in the TBLV LTR.
TBLV causes exclusively T-cell lymphomas in mice (2, 3). The major differences between TBLV and closely related MMTV strains that cause mammary carcinomas are a deletion of negative elements within the LTR and triplication of unique sequences flanking the deletion (4). In this study, we have shown that the TBLV LTR triplication constitutes a cell-type-specific enhancer element. In support of this idea, the LTR triplication increased MMTV promoter activity approximately 250-fold in transient reporter gene assays in Jurkat T cells (Fig. 2B). In addition, the triplication elevated reporter gene expression from the heterologous TK promoter 10- to 140-fold in T cells when inserted upstream or downstream in either orientation (Fig. 3B and C). Such properties are consistent with the action of transcriptional enhancer elements (7, 8, 31). Unlike some enhancers, however, the TBLV triplication enhances expression specifically in T cells (Fig. 2 and 3 and Table 1). Even a closely related lymphoid lineage, B cells, did not support TBLV enhancer function. Previous experiments by Paquette et al. (54) that use the TBLV LTR to drive CD4 or c-myc expression also are consistent with the T-cell-specific enhancer activity of the TBLV triplication. However, the latter experiments did not distinguish the transcriptional activity of the triplication from the effects of NRE deletion.
Recently members of our group reported that TBLV, similar to other retroviruses that induce leukemias, frequently integrates near the c-myc oncogene (55). In two of the tumors, the TBLV provirus inserted downstream up to 3 kb from the c-myc third exon in the same transcriptional orientation. Both tumors showed elevated levels of c-myc RNA compared to that obtained from normal murine thymus or thymomas lacking TBLV integrations. Since there was no alteration in the size of the c-myc RNA observed in TBLV-induced tumors, these results favor the idea that the TBLV LTR, like the MMTV LTR, activates oncogene expression primarily through enhancer, rather than promoter, insertion (12, 28, 52, 55). Preliminary experiments in which the TBLV LTR has been inserted upstream or downstream of a c-myc expression vector confirm that the LTR has enhancer activity in transient transfections of T cells (D. Broussard et al., unpublished data). Thus, it appears that TBLV enhancer elements can elevate transcription from MMTV, TK, and c-myc promoters.
AML-1 binding to a critical region of the TBLV enhancer.
To determine the sequences necessary for TBLV enhancer function, we engineered substitution mutations into a single copy of the 62-bp element that then were triplicated and inserted into a C3H MMTV LTR lacking the NREs. The activities of these mutant LTRs upstream of a luciferase reporter gene were measured in transient transfection assays in two different T-cell lines (Fig. 4B). A single substitution mutant (556M) showed dramatic loss of enhancer function in both cell lines, and this mutation overlapped a putative AML-1 binding site (GTGCGGTTC) (compare to consensus in Fig. 5A). Several pieces of evidence confirm that AML-1 (recently renamed RUNX1 [36, 38]) binding contributes to TBLV enhancer function. (i) Gel shift experiments showed that AML-1 DNA binding activity was detectable in whole-cell extracts from Jurkat cells using a wild-type probe that overlapped the 556 mutation within the TBLV LTR. This complex had a molecular mass similar to that detected with a known AML-1 binding site probe. (ii) The DNA binding activity for the TBLV LTR was confirmed to be AML-1, as judged by supershift experiments with specific antibody; the supershifted complex was not obtained with antibody against AML-2 or AML-3 (data not shown). (iii) The AML-1 supershift was greatly reduced by the addition of an excess of the AML-1 peptide used to produce the antibody. (iv) Overexpression of AML-1B, but not AML-3 (also known as Runx2 [17]), in mammary cells was sufficient to elevate TBLV LTR activity 30-fold compared to cells without AML-1B overexpression (Fig. 8 and data not shown). Therefore, enhancer mutations, gel shift experiments, and overexpression assays indicated that AML-1 DNA binding activity contributes to the cell-type-specific activity of the TBLV enhancer.
MMTV strains that induce leukemias (other than TBLV) also have been described (18, 32, 45, 67). Invariably these strains have an LTR deletion spanning the NREs, and in some cases, the sequences flanking the deletion are duplicated. The duplication encompasses sequences in the LTRs of several mammotropic MMTV proviruses (RGTGGT) that match five of six bases within a consensus AML-1 binding site (YGYGGT) (4, 32, 45, 67). Lee et al. showed that the altered LTR from the DBA/2 ML T-cell tumor was more transcriptionally active in NIH 3T3 cells than mammotropic MMTV LTRs (32), whereas another altered LTR from the DL-8 tumor showed enhanced activity in mammary cells compared to LTRs derived from mammotropic MMTVs (67). However, we observed TBLV enhancer activity only in T-cell lines. Similarly, Yanagawa et al. (67) and Theunissen et al. (61) showed that altered MMTV enhancer elements from DBA/2 or GR-derived leukemias could stimulate transcription in T cells above that observed with mammotropic LTRs. As pointed out by Yanagawa et al. (67), the region of the LTR containing an AML-1 binding site contributes greatly to T-cell enhancer function. Thus, AML-1 DNA binding activity may be important for T-cell-specific enhancer activities of many leukemogenic MMTV strains.
Mechanism of retroviral enhancers active in T cells.
AML-1 binding activity is crucial for the activity of the MuLV family of enhancers, including those from the gibbon ape and feline leukemia viruses (69). Many experiments have shown MuLV LTRs carry viral determinants of leukemogenicity (10, 15, 25, 33, 56) in tandem repeats of 50- to 100-bp segments of the U3 region (21). Exchange of Friend and Moloney MuLV LTR repeat regions also switched the type of leukemia induced (11, 20, 26). Within the enhancer repeat region of MuLVs that cause T-cell tumors are binding sites for AML-1. Some MuLVs that induce rapidly appearing T-cell leukemias (e.g., SL3-3) have two binding sites for AML-1 (called cores I and II) in the 72-bp repeat element (68, 69), and therefore four AML-1 binding sites in the enhancer, whereas other MuLVs, such as Moloney, have a single AML-1 binding site in each repeat element (51). Core I of SL3 appears to have the strongest affinity for binding to AML-1 (68), and mutations within this binding site reduce leukemogenicity in mice and transcriptional activity in T-cell lines (34, 37, 68, 69). An MuLV strain (SAA) that has a 1-bp mutation in core I of each enhancer repeat (TGTGGTCAA) is weakly leukemogenic compared to SL3-3 (containing TGTGGTTAA), and most SAA-induced lymphomas had reversions or second-site suppressor mutations within the enhancer (37). Interestingly, there is a general correlation between increased affinity of AML-1 for the core I enhancer and both transcriptional activity in T cells and leukemogenicity. However, this correlation can be subtle. For example, the AML-1 DNA-binding (Runt) domain has an apparent Kd of 3.5 × 10−11 for core I of the weakly leukemogenic Akv virus and an apparent Kd of 2.4 × 10−11 for the highly leukemogenic SL3-3 core (34). This observation suggests that binding of factors in addition to AML-1 is important for MuLV leukemogenicity.
A number of other transcription factor complexes have been reported to bind to the MuLV enhancer repeats, including Ets-1, Myb, GR, NF1, and basic helix-loop-helix (HLH) proteins (13, 21, 50, 58). In the Moloney MuLV enhancer, the AML-1 binding site is flanked by Ets-1 binding sites (also called LVb and LVc) (58, 59). Intact binding sites for both Ets-1 and AML-1 are required for constitutive activity of the MuLV and T-cell receptor β-chain enhancers in T-cells (59). Recent evidence suggests that interactions between Ets-1 and AML-1 stimulate binding to DNA (65) and that the interaction counteracts autoinhibitory sequences in both proteins (19, 22). The Myb-binding site (like core II) is present only in the SL3 and Gross passage A virus (51). Mutations of the Myb site in the SL3-3 LTR had greater effects on enhancer activity in T cells than did mutations of the Ets site; however, other MuLVs (e.g., Moloney) that lack Myb sites also have high transcriptional activity in T cells (51). Interestingly, like the MuLV enhancers, we found a consensus GR binding site in the TBLV 62-bp repeat, and this site could stimulate transcription in XC fibroblasts in the presence of glucocorticoids (Fig. 7A). Our experiments also showed that the GR site was most important for function of the enhancer in Jurkat CD4+ T cells, and not in CD4+ CD8+ cells, one of the major cell targets for TBLV-induced leukemias (42, 48). Moreover, experiments using hormone-stripped serum suggested that the factor in Jurkat cells that binds to the GR element of the TBLV enhancer is not GR (Fig. 7B) and may be related to the basic HLH protein SEF2 or ALF1 described for the MuLV enhancers (51). Nevertheless, these data indicate that AML-1 binding in conjunction with several other proteins may provide potent transcriptional enhancement in T cells.
The region spanning the 548 and 556 mutations is crucial for the function of the TBLV LTR enhancer in T cells. The AML-1 binding site in the TBLV LTR overlapped with both the 548 and 556 mutations, yet only the 556 mutation abolished AML-1 binding activity for the viral enhancer (Fig. 6B). The 556 mutation probably eliminates AML-1 binding because it alters a G residue that universally appears in PCR-based selections for the AML-1-binding site (41). Mutation of the 556 region also affected binding of the NF-A and NF-B complexes to the TBLV enhancer. Because the failure of both NF-A and NF-B to bind the TBLV triplication is correlated with diminished enhancer activity in CD4+ CD8+ T cells, a target for TBLV-induced disease, our results suggest that these factors may be necessary for optimal function of the TBLV enhancer. The identities of NF-A and -B are unclear since a transcription factor consensus site spanning the LTR sequence affected by 548 and 556 mutations (other than AML-1) was not detected by our software analysis. In addition, oligonucleotides with consensus sites for either Myb or Ets family members did not compete for NF-A, AML-1, or NF-B binding (data not shown), suggesting that the TBLV enhancer is unique compared to those described for the MuLVs. A sequence (CAGGTG) related to an E-box (CACGTG) overlaps with the 5′ end of the AML-1 binding site in the TBLV enhancer. Since NF-A binding is affected by the 548 mutation and appears to be present in T-cell and B-cell lines, but is absent or low in mammary cells, it is possible that NF-A is a lymphoid-specific E-box binding protein. These experiments are consistent with experiments of Zaiman et al. (69), suggesting that AML-1 requires assistance from other transcription factors for MuLV enhancer function. The role of NF-A, NF-B, or other factors in TBLV transcription will await their identification. However, the evidence that AML-1 overexpression in HC11 causes a 30-fold elevation of transcription from the TBLV LTR strongly suggests that AML-1 binding contributes to the activity of the novel TBLV enhancer.
ACKNOWLEDGMENTS
We thank Susan Ross and members of the Dudley lab for helpful discussions and comments on the manuscript.
This work was supported by grants R01 CA34780 and P01 CA77760 from the National Institutes of Health. F.M. is a recipient of an NRSA award from the National Institutes of Health.
REFERENCES
- 1.Bae S C, Yamaguchi-Iwai Y, Ogawa E, Maruyama M, Inuzuka M, Kagoshima H, Shigesada K, Satake M, Ito Y. Isolation of PEBP2αB cDNA representing the mouse homolog of human acute myeloid leukemia gene, AML1. Oncogene. 1993;8:809–814. [PubMed] [Google Scholar]
- 2.Ball J K, Arthur L O, Dekaban G A. The involvement of a type-B retrovirus in the induction of thymic lymphomas. Virology. 1985;140:159–172. doi: 10.1016/0042-6822(85)90455-6. [DOI] [PubMed] [Google Scholar]
- 3.Ball J K, Dekaban G A, McCarter J A, Loosmore S M. Molecular biological characterization of a highly leukaemogenic virus isolated from the mouse. III. Identity with mouse mammary tumour virus. J Gen Virol. 1983;64:2177–2190. doi: 10.1099/0022-1317-64-10-2177. [DOI] [PubMed] [Google Scholar]
- 4.Ball J K, Diggelmann H, Dekaban G A, Grossi G F, Semmier R, Waight P A, Fletcher R F. Alterations in the U3 region of the long terminal repeat of an infectious thymotropic type B retrovirus. J Virol. 1988;62:2985–2993. doi: 10.1128/jvi.62.8.2985-2993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ball R K, Friis R R, Schoenenberger C A, Doppler W, Groner B. Prolactin regulation of β-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J. 1988;7:2089–2095. doi: 10.1002/j.1460-2075.1988.tb03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee C, Hiebert S W, Stein J L, Lian J B, Stein G S. An AML-1 consensus sequence binds an osteoblast-specific complex and transcriptionally activates the osteocalcin gene. Proc Natl Acad Sci USA. 1996;93:4968–4973. doi: 10.1073/pnas.93.10.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerji J, Rusconi S, Schaffner W. Expression of a β-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981;27:299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- 8.Blackwood E M, Kadonaga J T. Going the distance: a current view of enhancer action. Science. 1998;281:61–63. doi: 10.1126/science.281.5373.60. [DOI] [PubMed] [Google Scholar]
- 9.Bramblett D, Hsu C L, Lozano M, Earnest K, Fabritius C, Dudley J. A redundant nuclear protein binding site contributes to negative regulation of the mouse mammary tumor virus long terminal repeat. J Virol. 1995;69:7868–7876. doi: 10.1128/jvi.69.12.7868-7876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatis P A, Holland C A, Hartley J W, Rowe W P, Hopkins N. Role for the 3′ end of the genome in determining disease specificity of Friend and Moloney murine leukemia viruses. Proc Natl Acad Sci USA. 1983;80:4408–4411. doi: 10.1073/pnas.80.14.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatis P A, Holland C A, Silver J E, Frederickson T N, Hopkins N, Hartley J W. A 3′ end fragment encompassing the transcriptional enhancers of nondefective Friend virus confers erythroleukemogenicity on Moloney leukemia virus. J Virol. 1984;52:248–254. doi: 10.1128/jvi.52.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clausse N, Baines D, Moore R, Brookes S, Dickson C, Peters G. Activation of both Wnt-1 and Fgf-3 by insertion of mouse mammary tumor virus downstream in the reverse orientation: a reappraisal of the enhancer insertion model. Virology. 1993;194:157–165. doi: 10.1006/viro.1993.1245. [DOI] [PubMed] [Google Scholar]
- 13.Corneliussen B, Thornell A, Hallberg B, Grundstrom T. Helix-loop-helix transcriptional activators bind to a sequence in glucocorticoid response elements of retrovirus enhancers. J Virol. 1991;65:6084–6093. doi: 10.1128/jvi.65.11.6084-6093.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dekaban G A, Ball J K. Integration of type B retroviral DNA in virus-induced primary murine thymic lymphomas. J Virol. 1984;52:784–792. doi: 10.1128/jvi.52.3.784-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DesGroseillers L, Jolicoeur P. The tandem direct repeats within the long terminal repeat of murine leukemia viruses are the primary determinant of their leukemogenic potential. J Virol. 1984;52:945–952. doi: 10.1128/jvi.52.3.945-952.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DesGroseillers L, Rassart E, Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci USA. 1983;80:4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drissi H, Luc Q, Shakoori R, Chuva D S L, Choi J Y, Terry A, Hu M, Jones S, Neil J C, Lian J B, Stein J L, van Wijnen A J, Stein G S. Transcriptional autoregulation of the bone related CBFA1/RUNX2 gene. J Cell Physiol. 2000;184:341–350. doi: 10.1002/1097-4652(200009)184:3<341::AID-JCP8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 18.Dudley J, Risser R. Amplification and novel locations of endogenous mouse mammary tumor virus genomes in mouse T-cell lymphomas. J Virol. 1984;49:92–101. doi: 10.1128/jvi.49.1.92-101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetz T L, Gu T L, Speck N A, Graves B J. Auto-inhibition of Ets-1 is counteracted by DNA binding cooperativity with core-binding factor α2. Mol Cell Biol. 2000;20:81–90. doi: 10.1128/mcb.20.1.81-90.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golemis E, Li Y, Fredrickson T N, Hartley J W, Hopkins N. Distinct segments within the enhancer region collaborate to specify the type of leukemia induced by nondefective Friend and Moloney viruses. J Virol. 1989;63:328–337. doi: 10.1128/jvi.63.1.328-337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golemis E A, Speck N A, Hopkins N. Alignment of U3 region sequences of mammalian type C viruses: identification of highly conserved motifs and implications for enhancer design. J Virol. 1990;64:534–542. doi: 10.1128/jvi.64.2.534-542.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu T L, Goetz T L, Graves B J, Speck N A. Auto-inhibition and partner proteins, core-binding factor beta (CBFβ) and Ets-1, modulate DNA binding by CBFα2 (AML1) Mol Cell Biol. 2000;20:91–103. doi: 10.1128/mcb.20.1.91-103.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higuchi R. Recombinant PCR. In: Innis M A, Gelfand D H, Sninsky J J, White T J, editors. PCR protocols, a guide to methods and applications. San Diego, Calif: Academic Press, Inc.; 1990. pp. 177–183. [Google Scholar]
- 24.Hsu C L, Fabritius C, Dudley J. Mouse mammary tumor virus proviruses in T-cell lymphomas lack a negative regulatory element in the long terminal repeat. J Virol. 1988;62:4644–4652. doi: 10.1128/jvi.62.12.4644-4652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishimoto A, Adachi A, Sakai K, Matsuyama M. Long terminal repeat of Friend-MCF virus contains the sequence responsible for erythroid leukemia. Virology. 1985;141:30–42. doi: 10.1016/0042-6822(85)90180-1. [DOI] [PubMed] [Google Scholar]
- 26.Ishimoto A, Takimoto M, Adachi A, Kakuyama M, Kato S, Kakimi K, Fukuoka, Ogiu T, Matsuyama M. Sequences responsible for erythroid and lymphoid leukemia in the long terminal repeats of Friend-mink cell focus-forming and Moloney murine leukemia viruses. J Virol. 1987;61:1861–1866. doi: 10.1128/jvi.61.6.1861-1866.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen E V, Suzuki T, Kawashima T, Stumpf W E, Jungblut P W, DeSombre E R. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci USA. 1968;59:632–638. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapoun A M, Shackleford G M. Preferential activation of Fgf8 by proviral insertion in mammary tumors of Wnt1 transgenic mice. Oncogene. 1997;14:2985–2989. doi: 10.1038/sj.onc.1201146. [DOI] [PubMed] [Google Scholar]
- 29.Kim K J, Kanellopoulos-Langevin C, Merwin R M, Sachs D H, Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979;122:549–554. [PubMed] [Google Scholar]
- 30.Knutson J C, Yee D. Electroporation: parameters affecting transfer of DNA into mammalian cells. Anal Biochem. 1987;164:44–52. doi: 10.1016/0003-2697(87)90365-4. [DOI] [PubMed] [Google Scholar]
- 31.Laimins L A, Khoury G, Gorman C, Howard B, Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci USA. 1982;79:6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee W T, Prakash O, Klein D, Sarkar N H. Structural alterations in the long terminal repeat of an acquired mouse mammary tumor virus provirus in a T-cell leukemia of DBA/2 mice. Virology. 1987;159:39–48. doi: 10.1016/0042-6822(87)90345-x. [DOI] [PubMed] [Google Scholar]
- 33.Lenz J, Celander D, Crowther R L, Patarca R, Perkins D W, Haseltine W A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. Nature. 1984;308:467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- 34.Lewis A F, Stacy T, Green W R, Taddesse-Heath L, Hartley J W, Speck N A. Core-binding factor influences the disease specificity of Moloney murine leukemia virus. J Virol. 1999;73:5535–5547. doi: 10.1128/jvi.73.7.5535-5547.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Bramblett D, Zhu Q, Lozano M, Kobayashi R, Ross S R, Dudley J P. The matrix attachment region-binding protein SATB1 participates in negative regulation of tissue-specific gene expression. Mol Cell Biol. 1997;17:5275–5287. doi: 10.1128/mcb.17.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutterbach B, Westendorf J J, Linggi B, Isaac S, Seto E, Hiebert S W. A mechanism of repression by acute myeloid leukemia-1, the target of multiple chromosomal translocations in acute leukemia. J Biol Chem. 2000;275:651–656. doi: 10.1074/jbc.275.1.651. [DOI] [PubMed] [Google Scholar]
- 37.Martiney M J, Rulli K, Beaty R, Levy L S, Lenz J. Selection of reversions and suppressors of a mutation in the CBF binding site of a lymphomagenic retrovirus. J Virol. 1999;73:7599–7606. doi: 10.1128/jvi.73.9.7599-7606.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarthy T L, Ji C, Chen Y, Kim K K, Imagawa M, Ito Y, Centrella M. Runt domain factor (Runx)-dependent effects on CCAAT/ enhancer-binding protein delta expression and activity in osteoblasts. J Biol Chem. 2000;275:21746–21753. doi: 10.1074/jbc.M002291200. [DOI] [PubMed] [Google Scholar]
- 39.Melnikova I N, Crute B E, Wang S, Speck N A. Sequence specificity of the core-binding factor. J Virol. 1993;67:2408–2411. doi: 10.1128/jvi.67.4.2408-2411.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merriman H L, van Wijnen A J, Hiebert S, Bidwell J P, Fey E, Lian J, Stein J, Stein G S. The tissue-specific nuclear matrix protein, NMP-2, is a member of the AML/CBF/PEBP2/runt domain transcription factor family: interactions with the osteocalcin gene promoter. Biochemistry. 1995;34:13125–13132. doi: 10.1021/bi00040a025. [DOI] [PubMed] [Google Scholar]
- 41.Meyers S, Downing J R, Hiebert S W. Identification of AML-1 and the (8;21) translocation protein (AML-1/ETO) as sequence-specific DNA-binding proteins: the runt homology domain is required for DNA binding and protein-protein interactions. Mol Cell Biol. 1993;13:6336–6345. doi: 10.1128/mcb.13.10.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyers S, Gottlieb P D, Dudley J P. Lymphomas with acquired mouse mammary tumor virus proviruses resemble distinct prethymic and intrathymic phenotypes defined in vivo. J Immunol. 1989;142:3342–3350. [PubMed] [Google Scholar]
- 43.Meyers S, Lenny N, Hiebert S W. The t(8;21) fusion protein interferes with AML-1B-dependent transcriptional activation. Mol Cell Biol. 1995;15:1974–1982. doi: 10.1128/mcb.15.4.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyers S, Lenny N, Sun W, Hiebert S W. AML-2 is a potential target for transcriptional regulation by the t(8;21) and t(12;21) fusion proteins in acute leukemia. Oncogene. 1996;13:303–312. [PubMed] [Google Scholar]
- 45.Michalides R, Wagenaar E. Site-specific rearrangements in the long terminal repeat of extra mouse mammary tumor proviruses in murine T-cell leukemias. Virology. 1986;154:76–84. doi: 10.1016/0042-6822(86)90431-9. [DOI] [PubMed] [Google Scholar]
- 46.Michalides R, Wagenaar E, Hilkens J, Hilgers J, Groner B, Hynes N E. Acquisition of proviral DNA of mouse mammary tumor virus in thymic leukemia cells from GR mice. J Virol. 1982;43:819–829. doi: 10.1128/jvi.43.3.819-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyoshi H, Shimizu K, Kozu T, Maseki N, Kaneko Y, Ohki M. t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene, AML1. Proc Natl Acad Sci USA. 1991;88:10431–10434. doi: 10.1073/pnas.88.23.10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller R E, Ball J K, Chan F P. Characterization of cell markers in type B retroviral-induced thymic lymphomas. I. Surface antigen phenotype and karyotype in developing and primary lymphomas. Leuk Res. 1989;13:553–559. doi: 10.1016/0145-2126(89)90122-7. [DOI] [PubMed] [Google Scholar]
- 49.Nicolas J F, Wegmann D, Lebrun P, Kaiserlian D, Tovey J, Glasebrook A L. Relationship of B cell Fc receptors to T cell recognition of Mls antigen. Eur J Immunol. 1987;17:1561–1565. doi: 10.1002/eji.1830171106. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen A L, Pallisgaard N, Pedersen F S, Jorgensen P. Basic helix-loop-helix proteins in murine type C retrovirus transcriptional regulation. J Virol. 1994;68:5638–5647. doi: 10.1128/jvi.68.9.5638-5647.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nieves A, Levy L S, Lenz J. Importance of a c-Myb binding site for lymphomagenesis by the retrovirus SL3–3. J Virol. 1997;71:1213–1219. doi: 10.1128/jvi.71.2.1213-1219.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nusse R. Insertional mutagenesis in mouse mammary tumorigenesis. Curr Top Microbiol Immunol. 1991;171:43–65. doi: 10.1007/978-3-642-76524-7_3. [DOI] [PubMed] [Google Scholar]
- 53.Owens R B. Glandular epithelial cells from mice: a method for selective cultivation. J Natl Cancer Inst. 1974;52:1375–1378. doi: 10.1093/jnci/52.4.1375. [DOI] [PubMed] [Google Scholar]
- 54.Paquette Y, Doyon L, Laperriere A, Hanna Z, Ball J, Sekaly R P, Jolicoeur P. A viral long terminal repeat expressed in CD4+ CD8+ precursors is downregulated in mature peripheral CD4− CD8+ or CD4+ CD8− T cells. Mol Cell Biol. 1992;12:3522–3530. doi: 10.1128/mcb.12.8.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajan L, Broussard D, Lozano M, Lee C G, Kozak C A, Dudley J P. The c-myc locus is a common integration site in type B retrovirus-induced T-cell lymphomas. J Virol. 2000;74:2466–2471. doi: 10.1128/jvi.74.5.2466-2471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosen C A, Haseltine W A, Lenz J, Ruprecht R, Cloyd M W. Tissue selectivity of murine leukemia virus infection is determined by long terminal repeat sequences. J Virol. 1985;55:862–866. doi: 10.1128/jvi.55.3.862-866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Satake M, Nomura S, Yamaguchi-Iwai Y, Takahama Y, Hashimoto Y, Niki M, Kitamura Y, Ito Y. Expression of the Runt domain-encoding PEBP2 alpha genes in T cells during thymic development. Mol Cell Biol. 1995;15:1662–1670. doi: 10.1128/mcb.15.3.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Speck N A, Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1987;7:1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun W, Graves B J, Speck N A. Transactivation of the Moloney murine leukemia virus and T-cell receptor beta-chain enhancers by CBF and Ets requires intact binding sites for both proteins. J Virol. 1995;69:4941–4949. doi: 10.1128/jvi.69.8.4941-4949.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun W, O'Connell M, Speck N A. Characterization of a protein that binds multiple sequences in mammalian type C retrovirus enhancers. J Virol. 1993;67:1976–1986. doi: 10.1128/jvi.67.4.1976-1986.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Theunissen H J, Paardekooper M, Maduro L J, Michalides R J, Nusse R. Phorbol ester-inducible T-cell-specific expression of variant mouse mammary tumor virus long terminal repeats. J Virol. 1989;63:3466–3471. doi: 10.1128/jvi.63.8.3466-3471.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thornell A, Hallberg B, Grundstrom T. Differential protein binding in lymphocytes to a sequence in the enhancer of the mouse retrovirus SL3–3. Mol Cell Biol. 1988;8:1625–1637. doi: 10.1128/mcb.8.4.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thornell A, Hallberg B, Grundstrom T. Binding of SL3–3 enhancer factor 1 transcriptional activators to viral and chromosomal enhancer sequences. J Virol. 1991;65:42–50. doi: 10.1128/jvi.65.1.42-50.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wingender E, Chen X, Hehl R, Karas H, Liebich I, Matys V, Meinhardt T, Pruss M, Reuter I, Schacherer F. TRANSFAC: an integrated system for gene expression regulation. Nucleic Acids Res. 2000;28:316–319. doi: 10.1093/nar/28.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wotton D, Ghysdael J, Wang S, Speck N A, Owen M J. Cooperative binding of Ets-1 and core binding factor to DNA. Mol Cell Biol. 1994;14:840–850. doi: 10.1128/mcb.14.1.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yanagawa S-I, Kakimi K, Tanaka H, Murakami A, Nakagawa Y, Kubo Y, Yamada Y, Hiai H, Kuribayashi K, Masuda T, Ishimoto A. Mouse mammary tumor virus with rearranged long terminal repeats causes murine lymphomas. J Virol. 1993;67:112–118. doi: 10.1128/jvi.67.1.112-118.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yanagawa S, Murakami A, Tanaka H. Extra mouse mammary tumor proviruses in DBA/2 mouse lymphomas acquire a selective advantage in lymphocytes by alteration in the U3 region of the long terminal repeat. J Virol. 1990;64:2474–2483. doi: 10.1128/jvi.64.6.2474-2483.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zaiman A L, Lewis A F, Crute B E, Speck N A, Lenz J. Transcriptional activity of core binding factor-α (AML1) and β subunits on murine leukemia virus enhancer cores. J Virol. 1995;69:2898–2906. doi: 10.1128/jvi.69.5.2898-2906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zaiman A L, Nieves A, Lenz J. CBF, Myb, and Ets binding sites are important for activity of the core I element of the murine retrovirus SL3–3 in T lymphocytes. J Virol. 1998;72:3129–3137. doi: 10.1128/jvi.72.4.3129-3137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu Q, Gregg K, Lozano M, Liu J, Dudley J P. CDP is a repressor of mouse mammary tumor virus expression in the mammary gland. J Virol. 2000;74:6348–6357. doi: 10.1128/jvi.74.14.6348-6357.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]