Abstract
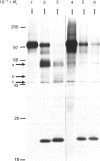
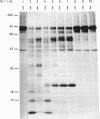
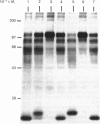
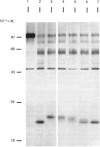
The nature and subcellular localization of the enzymic activities responsible for the production of the 20 kDa protein betagranin from its 100 kDa chromogranin-A-like precursor was investigated in transplantable insulinoma tissue. [35S]Methionine-labelled precursor was converted by lysed insulin-secretory granules into betagranin and one or more proteins of 47 kDa, via intermediates in the 60-65 kDa range. Lysosome-enriched fractions also processed the precursor, but not into the peptides found in vivo; other fractions, including those enriched in Golgi, were inactive. Conversion of the precursor by granules was quantitative and the products were stable. Inhibitor studies showed that processing occurred by initial endoproteolytic cleavage at sites marked by pairs of basic amino acids, followed by removal of these by carboxypeptidase H. The endopeptidase activity appeared to be a novel metalloenzyme, with a markedly acidic pH optimum (4.8-5). It was inhibited by alanyl-L-lysyl-L-arginyl chloromethane (K0.5 = 1.3 microM), but to a much lesser extent by inhibitor analogues of processing sites defined by single or unpaired basic amino acid residues, e.g. alanyl-L-norleucyl-L-arginylchloromethane (K0.5 greater than 100 microM), leupeptin (K0.5 = 150 microM) and antipain (K0.5 = 40 microM). p-Chloromercuribenzoate (K0.5 = 13 microM), Hg2+ (K0.5 = 16 microM), Zn2+ (K0.5 = 0.8 mM) and vanadate (K0.5 = 7 microM) also abolished activity, as did various anions (SCN- greater than I- greater than Cl- greater than SO4(2-). Group-specific inhibitors of serine, thiol and acidic endopeptidases were without effect. EDTA and CDTA (1,2-cyclohexanediaminetetra-acetic acid), but not 1,10-phenanthroline, abolished endoproteolytic activity. Several bivalent cations could restore activity after EDTA or CDTA inhibition, including Ca2+, Zn2+, Mn2+ and Sr2+; however, the ion of physiological importance appeared to be Ca2+ (K0.5 = 8 microM). The properties of the granule endopeptidase and its subcellular localization suggested that it is of importance in processing chromogranin A in the pancreatic beta-cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achstetter T., Wolf D. H. Hormone processing and membrane-bound proteinases in yeast. EMBO J. 1985 Jan;4(1):173–177. doi: 10.1002/j.1460-2075.1985.tb02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Human cathepsin B1. Purification and some properties of the enzyme. Biochem J. 1973 Apr;131(4):809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chick W. L., Warren S., Chute R. N., Like A. A., Lauris V., Kitchen K. C. A transplantable insulinoma in the rat. Proc Natl Acad Sci U S A. 1977 Feb;74(2):628–632. doi: 10.1073/pnas.74.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Carroll R. J., Steiner D. F. Conversion of proinsulin to insulin: involvement of a 31,500 molecular weight thiol protease. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4613–4617. doi: 10.1073/pnas.79.15.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Hutton J. C. Carboxypeptidase activity in the insulin secretory granule. FEBS Lett. 1983 Oct 3;162(1):137–141. doi: 10.1016/0014-5793(83)81065-5. [DOI] [PubMed] [Google Scholar]
- Fletcher D. J., Quigley J. P., Bauer G. E., Noe B. D. Characterization of proinsulin- and proglucagon-converting activities in isolated islet secretory granules. J Cell Biol. 1981 Aug;90(2):312–322. doi: 10.1083/jcb.90.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker L. D., Plummer T. H., Jr, Snyder S. H. Enkephalin convertase: potent, selective, and irreversible inhibitors. Biochem Biophys Res Commun. 1983 Mar 29;111(3):994–1000. doi: 10.1016/0006-291x(83)91398-0. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Chang H. T., Potts J. T., Jr Enzymic processing of proparathyroid hormone by cell-free extracts of parathyroid glands. Biochemistry. 1977 Aug 23;16(17):3910–3917. doi: 10.1021/bi00636a029. [DOI] [PubMed] [Google Scholar]
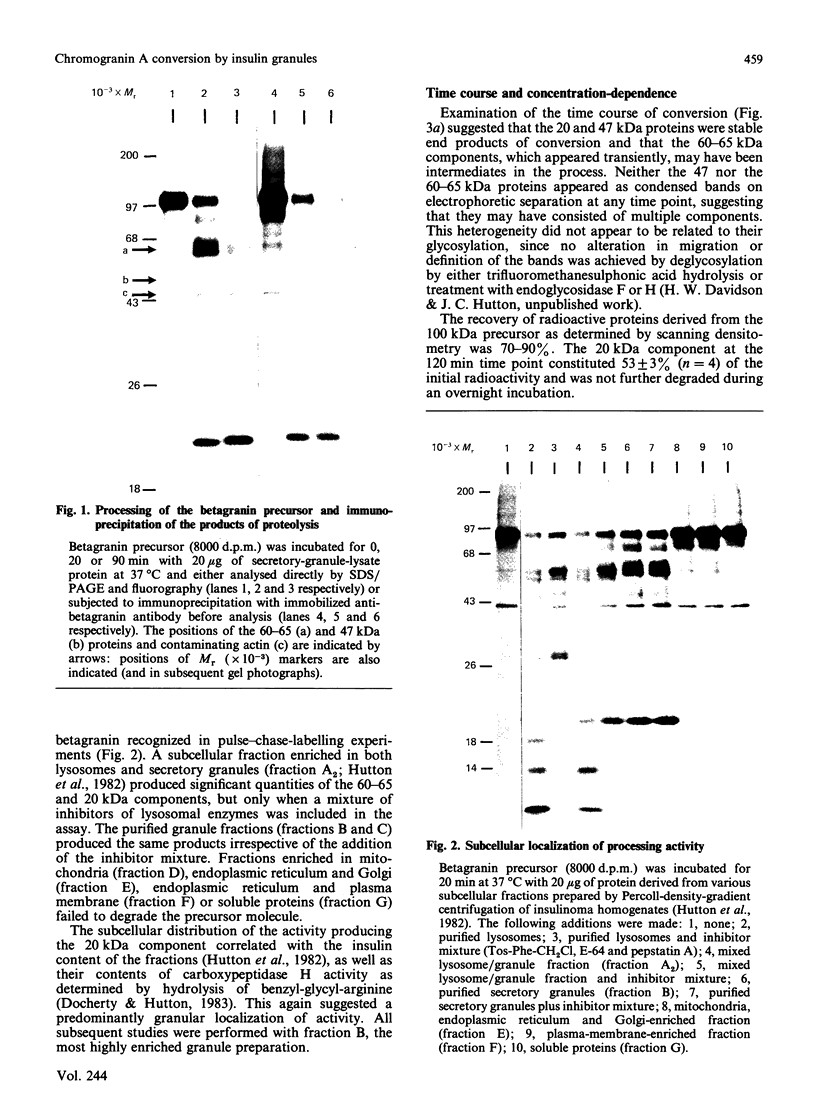
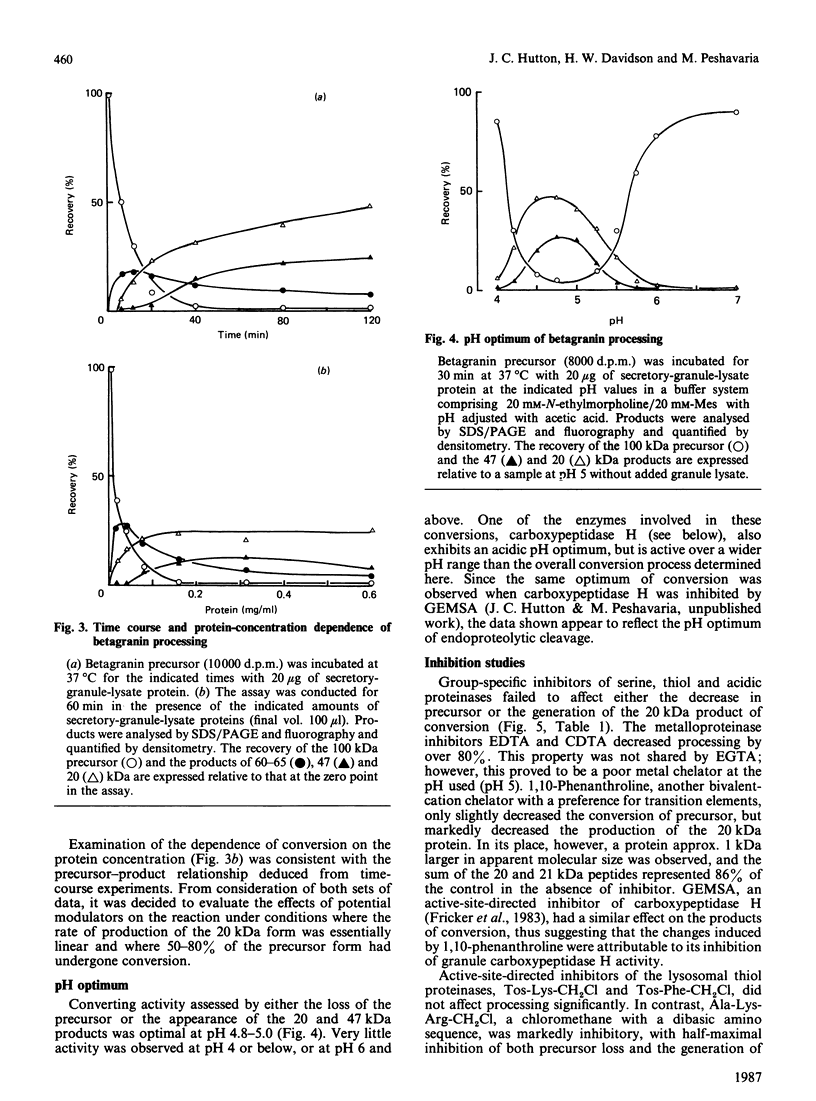
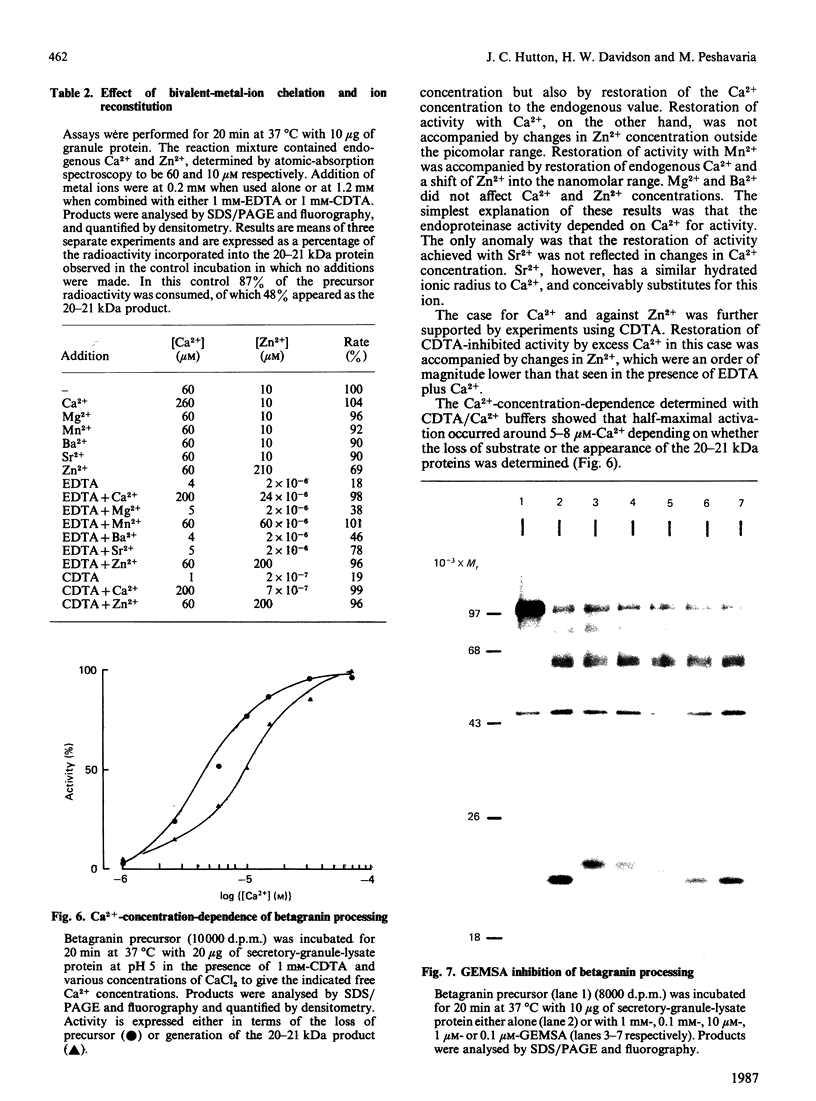
- Hutton J. C., Davidson H. W., Grimaldi K. A., Peshavaria M. Biosynthesis of betagranin in pancreatic beta-cells. Identification of a chromogranin A-like precursor and its parallel processing with proinsulin. Biochem J. 1987 Jun 1;244(2):449–456. doi: 10.1042/bj2440449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Hansen F., Peshavaria M. beta-Granins: 21 kDa co-secreted peptides of the insulin granule closely related to adrenal medullary chromogranin A. FEBS Lett. 1985 Sep 2;188(2):336–340. doi: 10.1016/0014-5793(85)80398-7. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Penn E. J., Peshavaria M. Isolation and characterisation of insulin secretory granules from a rat islet cell tumour. Diabetologia. 1982 Oct;23(4):365–373. doi: 10.1007/BF00253746. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Penn E. J., Peshavaria M. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J. 1983 Feb 15;210(2):297–305. doi: 10.1042/bj2100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C. Secretory granules. Experientia. 1984 Oct 15;40(10):1091–1098. doi: 10.1007/BF01971456. [DOI] [PubMed] [Google Scholar]
- Hutton J. C. The internal pH and membrane potential of the insulin-secretory granule. Biochem J. 1982 Apr 15;204(1):171–178. doi: 10.1042/bj2040171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., Brake A., Blair L., Kunisawa R., Thorner J. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell. 1984 Jul;37(3):1075–1089. doi: 10.1016/0092-8674(84)90442-2. [DOI] [PubMed] [Google Scholar]
- Kemmler W., Steiner D. F., Borg J. Studies on the conversion of proinsulin to insulin. 3. Studies in vitro with a crude secretion granule fraction isolated from rat islets of Langerhans. J Biol Chem. 1973 Jul 10;248(13):4544–4551. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loh Y. P., Brownstein M. J., Gainer H. Proteolysis in neuropeptide processing and other neural functions. Annu Rev Neurosci. 1984;7:189–222. doi: 10.1146/annurev.ne.07.030184.001201. [DOI] [PubMed] [Google Scholar]
- Loh Y. P., Parish D. C., Tuteja R. Purification and characterization of a paired basic residue-specific pro-opiomelanocortin converting enzyme from bovine pituitary intermediate lobe secretory vesicles. J Biol Chem. 1985 Jun 25;260(12):7194–7205. [PubMed] [Google Scholar]
- Mason R. W., Taylor M. A., Etherington D. J. The purification and properties of cathepsin L from rabbit liver. Biochem J. 1984 Jan 1;217(1):209–217. doi: 10.1042/bj2170209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L. The insulin factory: a tour of the plant surroundings and a visit to the assembly line. The Minkowski lecture 1973 revisited. Diabetologia. 1985 Aug;28(8):528–546. doi: 10.1007/BF00281987. [DOI] [PubMed] [Google Scholar]
- Pillai S., Zull J. E. Effects of ATP, vanadate, and molybdate on cathepsin D-catalyzed proteolysis. J Biol Chem. 1985 Jul 15;260(14):8384–8389. [PubMed] [Google Scholar]
- Sopwith A. M., Hales C. N., Hutton J. C. Pancreatic B-cells secrete a range of novel peptides besides insulin. Biochim Biophys Acta. 1984 Apr 16;803(4):342–345. doi: 10.1016/0167-4889(84)90127-7. [DOI] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe S., Kimura T. ATP-dependent protease in bovine adrenal cortex. Tissue specificity, subcellular localization, and partial characterization. J Biol Chem. 1985 May 10;260(9):5511–5517. [PubMed] [Google Scholar]