Abstract
Introduction
Osimertinib is used as the first-line treatment for EGFR mutation-positive NSCLC. Nevertheless, its efficacy and safety in clinical practice remain to be fully elucidated and the pattern of progression and the optimal subsequent treatment after osimertinib remains unclear.
Methods
This was a multicenter prospective observational study. EGFR mutation-positive patients with NSCLC who started first-line osimertinib from September 2018 to August 2020 were enrolled and followed up until August 2022.
Results
A total of 583 patients received osimertinib. The median progression-free and overall survival were 20.0 (95% confidence interval [CI]: 17.6–21.7) months and 41.0 (95% CI: 37.1–44.1) months, respectively. Grade 3 or worse adverse events were observed in 136 patients (23.3%). Progression patterns were categorized as central nervous system only, oligo-progression, and multiple organs on the basis of the Response Evaluation Criteria in Solid Tumors—progressive disease and occurred in 37 (10.8%), 156 (45.4%), and 151 patients (43.9%). The patient’s condition on progression was asymptomatic in 195 patients (56.7%). Osimertinib was continued in 163 patients (47.4%) after confirming progression. In clinically stable population with progressive disease (n = 247), survival after progression was 13.3 (95% CI: 10.9–16.4) months for those who continued osimertinib beyond progressive disease (n = 124), and 24.1 (95% CI: 17.7–34.0) months for those who discontinued osimertinib (n = 123) (hazard ratio = 2.01, 95% CI: 1.38–2.91, p = 0.0002). Platinum plus pemetrexed had the best overall survival benefits after osimertinib.
Conclusions
First-line osimertinib was found to have good effectiveness comparable to that reported in pivotal studies. Nevertheless, osimertinib should be discontinued among those who develop progression.
Trial registration number
UMIN000038683
Keywords: Osimertinib, EGFR, Non–small cell lung cancer, Progression patterns, Post-progression treatments
Introduction
Lung cancer has the highest mortality rate among all malignancies worldwide.1 Among cases of NSCLC, which account for around 70% of all lung cancers, 40% to 60% of Asian populations and 10% to 15% of Western populations have been reported to be positive for epidermal growth factor receptor (EGFR) mutation, the most frequently observed driver gene mutation in Asian populations.2,3 Approximately 80% to 90% of EGFR mutation-positive NSCLC cases exhibited exon 19 deletion or exon 21 L858R point mutations, with studies showing that EGFR tyrosine kinase inhibitors (TKIs) were effective against these mutations.4
Recently, the global phase 3 FLAURA trial reported that osimertinib, a third-generation EGFR TKI widely used in Europe, the United States, and Japan, prolonged progression-free survival (PFS) and overall survival (OS) among those with EGFR mutation-positive NSCLC with exon19 deletion and exon21 L858R point mutations compared with first-generation EGFR TKIs gefitinib and erlotinib.5,6 Nevertheless, subgroup analyses of OS reported a hazard ratio (HR) of 1.00 (95% confidence interval [CI]: 0.75–1.32) for Asians and 1.39 (95% CI: 0.83–2.34) for Japanese patients.6 Unfortunately, no study yet has fully investigated the real-world clinical effectiveness of osimertinib. In addition, although one study reported that pneumonitis was common in Japanese patients, osimertinib toxicities in daily practice remain to be thoroughly described.7 Furthermore, various mechanisms can cause osimertinib resistance, including on-target, off-target, and transformation,8 whereas a definitive posttreatment therapy has yet to be established. Diverse progression patterns after osimertinib have also been described, including brain-only relapse, oligoprogression, and systemic spread with or without clinical symptoms, similar to those after other TKIs.9,10 A detailed investigation of the exacerbation patterns of first-line osimertinib therapy and optimal posttreatment therapy according to exacerbation patterns is critical to the overall treatment strategy for EGFR mutation-positive NSCLC. Therefore, the current prospective observational study aimed to comprehensively investigate the circumstances surrounding first-line osimertinib therapy, including disease relapse and posttreatment.
Materials and Methods
Study Design
This multicenter prospective observational study used data collection methods detailed in our previous protocol article.11 Patients aged 20 years or older diagnosed with advanced or recurrent EGFR mutation-positive NSCLC and scheduled for EGFR TKI treatment at 30 Japanese centers from September 2018 to August 2020 were enrolled. The clinical efficacy, safety, exacerbation patterns, and subsequent posttreatment therapy of those receiving osimertinib monotherapy were followed using regular Case Report Forms every six months. This study was conducted in accordance with the Helsinki Declaration and the Japanese ethical guidelines for medical and biological research involving human subjects12 and was approved by the Ethical Review Committee of the Japanese Red Cross Medical Center (April 26, 2019, order number 976), and by the relevant committee of each site. Written informed consent was obtained from each patient.
End Points
The primary end points were PFS and progression pattern after osimertinib treatment. The progression patterns defined based on the Response Evaluation Criteria in Solid Tumors (RECIST)—progressive disease (PD) were classified into three mutually exclusive categories each for progression sites and clinical condition at the time of progression.9,10 For progression sites, patients were categorized as A1 (central nervous system [CNS] only, including brain metastasis and carcinomatous meningitis), A2 (oligometastasis; one to three lesions in one organ other than the brain), and A3 (progression in multiple organs). For the clinical condition at the time of progression, patients were categorized into B1 (asymptomatic without B3-defined clinical deterioration; i.e., judged as “PD” based on radiological findings only), B2 (symptomatic, but without B3-defined clinical deterioration), and B3 (clinical deterioration). Clinical deterioration defined in B3 included worsened performance status (PS) or major organ-threatening conditions, such as cancerous lymphangitis, bone marrow metastasis, carcinomatous meningitis, and hepatic metastasis with hepatic disorder. This categorization resulted in a total of nine (3 × 3) progression patterns.
The secondary end points included OS, Grade 3 or worse adverse event rate graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0, pneumonitis rate, and efficacy (response, PFS, and OS) of posttreatment therapy after osimertinib. PFS was defined as the period from the start of osimertinib treatment to the date of PD, whereas OS was defined as the period from the start of osimertinib treatment to death from any cause. The OS for those who did and did not use osimertinib after disease progression was defined as the time from osimertinib PD to death from any cause. The PFS and OS of the posttreatment therapies were calculated starting from the date at which postosimertinib therapy was started. Progression was determined by the investigator and not evaluated by the central committee. Patients who were lost to follow-up during the observation period were classified as censored cases on the date of discontinuation, whereas those who did not reveal progression during the observation period were classified as censored cases on the date of the final confirmation. The final follow-up was conducted in August 2022.
Statistical Analysis
All efficacy analyses were based on the full analysis set (and per protocol set for supportive analysis). All statistical analyses were performed using 95% CIs and two-sided p values. The PFS and OS of osimertinib treatment were summarized using the Kaplan-Meier method, and the 95% CIs of the median time were calculated using the Brookmeyer and Crowley method based on the log–log transformation of the survival function. Regarding the patients exhibiting the nine progression patterns as the primary end point, response rates, and those with various secondary end points, the percentages and 95% CIs were calculated using the Clopper-Pearson method. Multivariate analyses with Cox proportional hazard analysis were used to evaluate prognostic factors. A p value less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
From September 2018 to August 2020, 660 patients with EGFR mutation-positive NSCLC were enrolled after excluding one ineligible patient. Among the included patients, 583 (88.3%) were received osimertinib monotherapy (Supplementary Fig. 1). The median observation period was 28.5 (range: 0.1–48.0) months.
Patient Background
The patient background of the enrolled patients is summarized in Table 1. Notably, CNS metastases were observed in 169 (29.0%). EGFR mutation subtypes included exon 19 deletion in 285 (48.9%), L858R in 266 (45.6%), and others in 32 patients (5.5%).
Table 1.
Baseline Clinical Characteristics of the Patients
| Characteristics | N = 583 |
|---|---|
| Age, y | |
| Median (range) | 72 (30–95) |
| Sex, n (%) | |
| Male | 224 (38.4) |
| Female | 359 (61.6) |
| Smoking status, n (%) | |
| Never | 325 (55.8) |
| Current | 34 (5.8) |
| Former | 224 (38.4) |
| ECOG performance status, n (%) | |
| 0 | 216 (37.1) |
| 1 | 281 (48.2) |
| 2 | 60 (10.3) |
| 3 | 20 (3.4) |
| 4 | 2 (0.3) |
| Missing | 4 (0.7) |
| Histologic type, n (%) | |
| Adenocarcinoma | 571 (97.9) |
| Squamous | 9 (1.5) |
| NOS | 2 (0.3) |
| LCNEC | 1 (0.2) |
| Overall disease classification, n (%) | |
| Metastatic | 395 (67.8) |
| Recurrence | 169 (29.0) |
| Locally advanced | 19 (3.3) |
| CNS metastases, n (%) | |
| Yes | 169 (29.0) |
| No | 414 (71.0) |
| EGFR mutation type, n (%) | |
| Exon 19 deletion | 285 (48.9) |
| L858R | 266 (45.6) |
| Others | 32 (5.5) |
CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; LCNEC, large cell neuroendocrine carcinoma; NOS, not otherwise specified.
Clinical Response and Survival
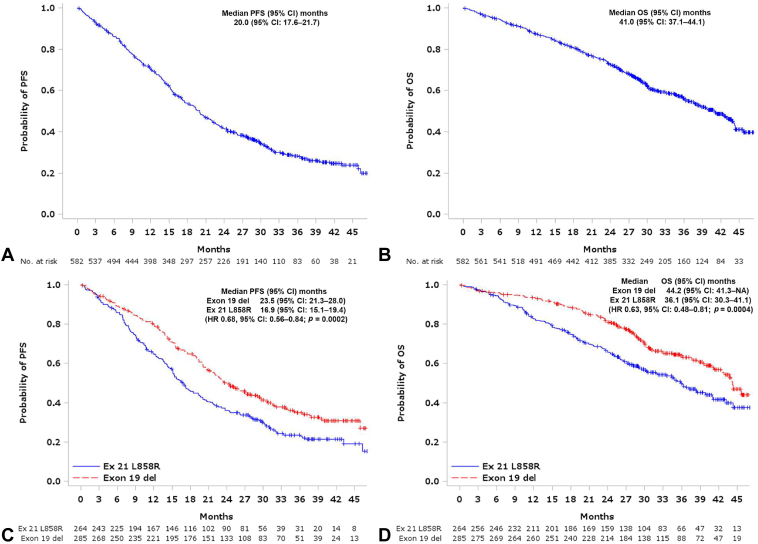
The overall response rate was 68.3% (95% CI: 54.3%–72.0%), whereas the disease control rate was 87.0% (95% CI: 84.0%–89.6%) (Supplementary Table 1). Response rates and disease control rates were similar between those with exon 19 deletions and those with exon 21 L858R point mutations. The median PFS and OS for all patients were 20.0 (95% CI: 17.6–21.7) and 41.0 (95% CI: 37.1–44.1) months, respectively (Fig. 1A and B). Both PFS and OS were significantly better in patients with exon 19 deletion than in those with exon 21 L858R point mutations (PFS = 23.5 mo, 95% CI: 21.3–28.0 versus PFS = 16.9 mo, 95% CI: 15.1–19.4; HR = 0.68, 95% CI: 0.56–0.84, p = 0.0002; OS = 44.2 mo, 95% CI: 41.3–not applicable, versus HR = 36.1 mo, 95% CI: 30.3–41.1; HR = 0.63, 95% CI: 0.48–0.81, p = 0.0004; respectively) (Fig. 1C and D). The median PFS according to a PS of zero to one and two to four was 20.2 (95% CI: 18.9–22.6) months and 12.9 (95% CI: 8.0–19.1) months, respectively, whereas the median OS according to a PS of zero to one and two to four was 43.2 (95% CI: 39.8–45.6) and 18.7 (95% CI: 14.0–28.7) months, respectively (Supplementary Fig. 2).
Figure 1.
PFS and OS in all 583 patients. Kaplan-Meier curves estimating the PFS (A), OS (B), and PFS according to EGFR mutation type (C), and OS according to EGFR mutation type (D). PFS and OS was determined starting from the date of osimertinib treatment. CI, confidence interval; Ex 21, exon 21; Exon 19 del, Exon 19 deletion; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Adverse Events
Among the included patients, 136 (23.3%) exhibited grade 3 or worse adverse events, whereas 95 (16.3%) required discontinuation owing to toxicities (Supplementary Table 2). Pneumonitis of any grade occurred in 75 patients (12.9%), with grade 1 in 21 (3.6%), grade 2 in 36 (6.2%), grade 3 in 12 (2.0%), grade 4 in six (1.0%) and no grade 5 (Supplementary Table 3). Moreover, 109 (21.9%) and 27 (32.9%) of the patients with a PS of zero to one and two to four had grade 3 or worse adverse events, whereas 14 (2.8%) and four (4.9%) had pneumonitis, respectively, indicating that adverse events were more common in patients with poor PS (Supplementary Table 2).
Progression Patterns Based on RECIST-PD and Osimertinib Continuation After Disease Progression
Progressive disease was observed in 344 patients (59.0%) during the observation period. Notably, (A1) CNS only, (A2) oligoprogression, and (A3) multiple-organ progression patterns were observed in 37 (10.8%), 156 (45.4%), and 151 patients (43.9%), respectively (Table 2). With regard to the patients’ conditions at the time of progression, (B1) asymptomatic, (B2) symptomatic without clinical exacerbation, and (B3) symptomatic with clinical exacerbation were observed in 195 (56.7%), 73 (21.2%), and 76 patients (22.1%), respectively (Table 2).
Table 2.
Exacerbation Pattern on Progression With Osimertinib
| Progression Pattern | Asymptomatic and No Clinical Exacerbation | Symptomatic and No Clinical Exacerbation | Clinical Exacerbationa | Total |
|---|---|---|---|---|
| CNS metastasis only | 16 | 4 | 17 | 37 |
| (4.7) | (1.2) | (4.9) | (10.8) | |
| Oligoprogressionb | 109 | 30 | 17 | 156 |
| (31.7) | (8.7) | (4.9) | (45.4) | |
| Multiple organs | 70 | 39 | 42 | 151 |
| (20.4) | (11.3) | (12.2) | (43.9) | |
| Total | 195 | 73 | 76 | 344 |
| (56.7) | (21.2) | (22.1) | (100) |
Note: All values are n (%).
CNS, central nervous system; PS, performance status.
Clinical exacerbation was defined as a decline in the PS and or or exacerbation threatening major organs (carcinomatous lymphangiosis, bone marrow metastasis, carcinomatous meningitis, liver metastasis with liver damage, etc.).
Single organ other than the CNS (up to three per organ).
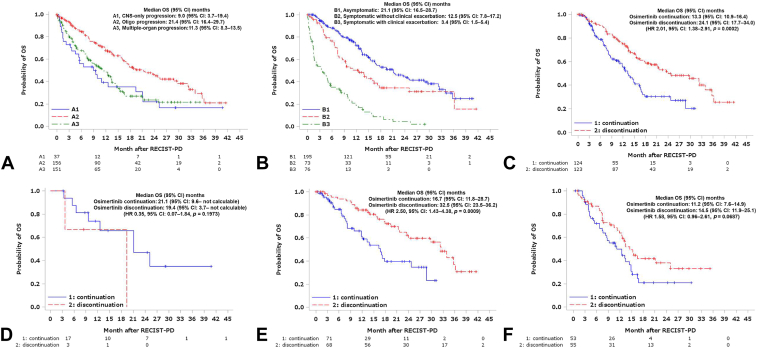
Among the 344 patients with PD, 163 (47.4%) continued osimertinib. Table 3 shows the progression patterns and conditions of patients who continued with osimertinib. Survival data after disease progression with osimertinib are presented in Figure 2A and B. Notably, the A2 (oligoprogression) and B1 (asymptomatic) groups had the longest post-PD survival.
Table 3.
Percentage of Patients Who Continued With Osimertinib After Progression
| Progression Pattern | Asymptomatic and No Clinical Exacerbation | Symptomatic and No Clinical Exacerbation | Clinical Exacerbationa | Total |
|---|---|---|---|---|
| CNS metastasis only | 87.5 | 75.0 | 47.1 | 67.6 |
| (14/16) | (3/4) | (8/17) | (25/37) | |
| Oligoprogressionb | 49.5 | 56.7 | 35.3 | 49.4 |
| (54/109) | (17/30) | (6/17) | (77/156) | |
| Multiple organs | 55.7 | 35.9 | 19.0 | 40.4 |
| (39/70) | (14/39) | (8/42) | (61/151) | |
| Total | 54.9 | 46.6 | 28.9 | 47.4 |
| (107/195) | (34/73) | (22/76) | (163/344) |
Note: All values are % (n/N).
CNS, central nervous system; PS, performance status.
Clinical exacerbation was defined as a decline in the PS and or or exacerbation threatening major organs (carcinomatous lymphangiosis, bone marrow metastasis, carcinomatous meningitis, liver metastasis with liver damage, etc.).
Single organ other than the CNS (up to three per organ).
Figure 2.
OS after progression with osimertinib. Kaplan-Meier curves estimating the OS according to progression patterns (A), patients’ conditions (B), continuation or discontinuation of osimertinib in clinically stable patients (C), continuation or discontinuation of osimertinib in clinically stable patients with CNS metastasis only progression (D), continuation or discontinuation of osimertinib in clinically stable patients with oligoprogression (E) and continuation or discontinuation of osimertinib in clinically stable patients with multiple organs progression (F). OS was determined starting from the date of progression with osimertinib. CI, confidence interval; CNS, central nervous system; HR, hazard ratio; OS, overall survival; PD, progressive disease. RECIST, Response Evaluation Criteria in Solid Tumors.
Among clinically stable patients at the time of PD, that is, those categorized as (A2) or (A3) and (B1) or (B2) (n = 247), those who continued osimertinib after disease progression (n = 124) had a post-PD survival of 13.3 (95% CI: 10.9–16.4) months, whereas those who discontinued osimertinib (n = 123) reported a post-PD survival of 24.1 (95% CI: 17.7–34.0) months (HR = 2.01, 95% CI: 1.38–2.91, p = 0.0002) (Fig. 2C).
A multivariate analysis was performed to confirm that continuing osimertinib treatment after osimertinib RECIST-PD decreases OS after osimertinib PD. After adjusting for age (as a continuous variable), sex, mutation subtype, smoking history, baseline PS, presence of distant metastases, presence of brain metastases, progression pattern (A2: oligo-metastasis versus A3: multiple organs metastasis), patient status at progression (B1: asymptomatic versus B2: symptomatic), and time to progression (PFS of osimertinib, as a continuous variable), OS after osimertinib RECIST-PD remained significantly different, favoring discontinuation group (continuation versus discontinuation: adjusted HR = 2.173, 95% CI: 1.460–3.233, p = 0.0001). No other factors were significantly associated with post-PD OS, except for the progression pattern (A2 better than A3), with an adjusted HR of 0.553, p value equal to 0.0036.
In addition, the above results were analyzed separately for three progression patterns: (A1) CNS-only progression, (A2) oligoprogression, and (A3) multiple-organ progression, and in each case (Fig. 2D–F). Post-PD OS was worse in the osimertinib continuation group than in the osimertinib discontinuation group, especially in patients with A2 oligoprogression pattern (HR = 2.50), but also in those with A3 multiple-organ progression pattern (HR = 1.58). The number of patients with an A1 pattern was too small for valid analysis.
Second-line cytotoxic chemotherapy was provided to 63 of the 163 patients (38.7%) in the osimertinib continuation group and 114 of the 180 patients (63.3%) in the osimertinib discontinuation group. The median time intervals between disease progression on osimertinib and the start of the second-line therapies were 63 days (1Q: 40 d to 3Q: 130 d) for the continuation group and 20 days (1Q: 9 d to 3Q: 28 d) for the discontinuation group, respectively. The osimertinib continuation and discontinuation groups had a median PFS of 5.5 (95% CI: 2.8–7.7) and 7.4 (95% CI: 5.8–9.1) months, respectively (Supplementary Fig. 3). In the osimertinib continuation group, the overall objective response rate and disease control rate were 18.8% (95% CI: 10.9–30.1) and 46.9% (95% CI: 35.2–58.9), respectively. In the osimertinib discontinuation group, the overall objective response rate and disease control rate were 27.6% (95% CI: 20.5–36.2) and 67.5% (95% CI: 58.8–75.1), respectively. Among patients treated with second-line chemotherapy, including cytotoxic agents, both PFS and response rate tended to be better in the osimertinib discontinuation group than in the osimertinib continuation group.
Post-Treatment Therapy
Second-line therapy after osimertinib was administered in 244 patients (41.9%). Platinum (carboplatin or cisplatin) plus pemetrexed (PP) was the most frequently administered therapy (65 patients, 26.6%), followed by carboplatin, paclitaxel, bevacizumab plus atezolizumab (ABCP) (36 patients, 14.8%) (Supplementary Table 4). The PP group and ABCP group had response rates of 29.2% (95% CI: 19.5–41.3) and 36.1% (95% CI: 22.4–52.5) and disease control rates of 66.2% (95% CI: 54.0–76.5) and 77.8% (95% CI: 61.7–88.5), respectively (Supplementary Table 5). Moreover, the PP group and ABCP group had a median PFS of 6.2 (95% CI: 5.1–8.7) and 10.0 (95% CI: 5.8–16.6) months and a median OS of 28.8 (95% CI: 14.7–not reported) and 18.1 (95% CI: 11.8–not reported) months, respectively (Supplementary Fig. 4). Second-line therapy regimens after osimertinib were similar in the osimertinib continuation groups (n = 73) and osimertinib discontinuation groups (n = 131). The proportion of patients treated with platinum combination-based therapy was almost the same, 50 (68.5%) and 88 (67.2%), and the proportion of patients treated with PP and ABCP was also almost the same, 27 (37.0%) and 51 (38.9%), respectively (Supplement Table 4).
Discussion
This study had been the largest real-world investigation on first-line osimertinib for EGFR mutation-positive NSCLC. Given that clinical trials for drug approval are conducted in patients with generally good health who satisfy eligibility and exclusion criteria at the time of enrollment, real-world data on the efficacy and safety of osimertinib among patients with various complications and comorbidities in clinical practice are of considerable significance, highlighting the need for studies that addressing this matter. Considering the limited number of Japanese patients who have been included in global trials, the safety of osimertinib in Japanese patients, who are thought to develop more adverse events, such as pneumonitis, than do Western patients, can only be confirmed through real-world data.
The FLAURA study reported that the median PFS and OS in the osimertinib group were 18.9 and 38.6 months, respectively. After stratifying patients according to EGFR subtype, the same study found that those with EGFR exon19 deletion and L858R had a median PFS of 21.4 and 14.4 months, respectively.5,6 In the current study, those with EGFR exon 19 deletion and L858R had a median PFS of 23.5 and 16.9 months and a median OS of 44.2 and 36.1 months, respectively, confirming the difference in efficacy according to EGFR subtype. This trend is consistent with the results of a meta-analysis of other EGFR TKIs.13 In this study, the overall response rate for those with exon 19 deletions was similar to those with exon 21 L858R point mutations, but both PFS and OS were significantly better in patients with exon 19 deletion than in those with exon 21 L858R point mutations. These clinical results may guide future treatment strategies by genetic subtype. No new safety signals for adverse events have been observed in clinical practice. Nevertheless, unlike the FLAURA study, our study reported that the frequency of pneumonitis was slightly higher in clinical practice, with 12.9% of the patients having pneumonitis of any grade and 3.1% having grade 3 or worse pneumonitis, which requires caution.
Management of the CNS, including brain metastases, is important in EGFR mutation-positive lung cancer. Median CNS PFS in the FLAURA trial was not reached for osimertinib (95% CI: 16.5 mo–not calculable) and 13.9 months for standard EGFR TKIs (95% CI: 8.3 months–not calculable). The CNS objective response rates were 91% and 68% for osimertinib and standard EGFR TKIs, respectively (OR = 4.6, 95% CI: 0.9–34.9, p = 0.066), and the rate of CNS exacerbations was reported to be lower with osimertinib (9.3%) than with other EGFR TKIs (15.9%).14 Although CNS PFS and CNS objective response rate were not evaluated in this study, the rate of CNS metastasis-only recurrence with osimertinib was also low at 10.8%. The efficacy of osimertinib in CNS was reconfirmed.
Although studies have reported some mechanisms for the development of resistance to first-line osimertinib, including on-target, off-target, and histologic transformation, several unknown mechanisms still exist.15,16 Moreover, the optimal posttreatment therapy after osimertinib PD has yet to be established, although cytotoxic anticancer agents have been the mainstay. Given that clinical practice data have also noted the efficacy of local therapy in CNS-only exacerbations or exacerbations with oligo-metastases,17,18 confirming the recurrence pattern after osimertinib is extremely important. In the current study, around 10% of the patients had CNS metastasis-only recurrence, which had a poor prognosis. Among those who did not have CNS metastasis-only recurrence, several were clinically stable at the time of recurrence, with 47.4% of them continuing with osimertinib after disease progression. Nevertheless, those who continued with osimertinib had a worse prognosis than those who discontinued, even after adjusting for possible confounders.
This could be primarily attributed to the delay in the treatment with cytotoxic anticancer agents. In this study, the median difference in time to second-line treatment was approximately 40 days (20 d versus 63 d), even though there was no difference in chemotherapy regimen between the two groups. Although several reports on first-generation EGFR TKIs have recognized the significance of EGFR TKI continuation after disease progression, the current study found contradictory results, perhaps because the main treatment after disease progression with third-generation EGFR TKI should be cytotoxic anticancer agents and EGFR TKIs, with emphasis placed on the timing of their administration.10 In the current study, the response rate and PFS of cytotoxic anticancer agents after osimertinib PD were both higher in the osimertinib discontinuation group than in the osimertinib continuation group.
PP and ABCP have been often used after disease progression with osimertinib. Although ABCP was found to have better response rates and PFS than PP, no difference in OS was observed, consistent with the results of the Korean ATTLAS trial.19 The KEYNOTE-789 and CheckMate-722 trials did not reveal significant results for immune checkpoint inhibitor (ICI)-containing therapies after disease progression with EGFR TKIs, suggesting the need to elucidate the yet unclear significance of ICI in addition to cytotoxic anticancer agents.20,21 Several ongoing trials have sought to uncover future treatment options, including the ORCHARD trial, an ongoing platform-based design to examine the effect of posttreatment according to resistance mechanisms after first-line osimertinib22; the HERTHENA-Lung02 trial, an ongoing phase 3 trial of patritumab deruxtecan in EGFR-mutated NSCLC after progression on EGFR TKI23; and the MARIPOSA-2 study, an ongoing phase 3 trial of amivantamab plus chemotherapy with and without lazertinib in EGFR-mutated NSCLC after progression on osimertinib.24 The data obtained from this study will certainly become a benchmark for determining treatment outcomes after osimertinib PD in the future.
One strength of the current prospective, observational study is that all of the results reflect clinical practice. In addition, several limitations need to be addressed. First, given the observational nature of this study, no stipulations were provided regarding computed tomography for determining treatment efficacy. Therefore, PFS may have been overestimated. Second, the proportion of CNS metastases may have been underestimated given that brain metastasis examination was not mandatory at the time of progression with osimertinib. Third, the background factors of patients who received post-treatment therapy after osimertinib could not be compared. Therefore, our findings must be considered exploratory because of patient selection bias.
Conclusions
This study reported that the efficacy of first-line osimertinib in patients with EGFR mutation-positive NSCLC in clinical practice was consistent with those reported in previous trials and that the safety of osimertinib was tolerable. Continuing osimertinib after progression was associated with shorter survival than discontinuing osimertinib. Cytotoxic chemotherapy plays a major role in posttreatment therapy after osimertinib, with the role of ICI still remaining unclear.
CRediT Authorship Contribution Statement
Kageaki Watanabe: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing - original draft, Writing - review & editing.
Yukio Hosomi: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing - review & editing.
Katsuhiko Naoki: Data curation, Investigation, Resources, Writing - review & editing.
Yoshiro Nakahara: Data curation, Investigation, Resources, Writing - review & editing.
Yoko Tsukita: Data curation, Investigation, Resources, Writing - review & editing.
Hirotaka Matsumoto: Data curation, Investigation, Resources, Writing - review & editing.
Kiyotaka Yoh: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing - review & editing.
Yasuhito Fujisaka: Data curation, Investigation, Resources, Writing - review & editing.
Satoshi Takahashi: Data curation, Investigation, Resources, Writing - review & editing.
Saori Takata: Data curation, Investigation, Resources, Writing - review & editing.
Kazuhiro Usui: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing - review & editing.
Kazuma Kishi: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing - review & editing.
Go Naka: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing - review & editing.
Shu Tamano: Data curation, Formal analysis, Investigation, Writing - review & editing.
Kohei Uemura: Data curation, Formal analysis, Investigation, Writing - review & editing.
Hideo Kunitoh: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing - original draft, Writing - review & editing.
Disclosure
Dr. Watanabe reports personal fees from AstraZeneca, Chugai Pharmaceutical, Merck Biopharma Co., Ltd., Amgen K.K., Novartis Pharma, Bristol-Myers Squibb, Merck Sharp & Dohme, Nippon Boehringer Ingelheim, Ono Pharmaceutical Co., Ltd., Riken Genesis Co., Ltd., Sysmex Corporation, and Takeda Pharmaceutical Company, Limited outside of the submitted work. Dr. Hosomi reports personal fees from AstraZeneca, Eli Lilly Japan, Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, Kyowa Kirin, Nippon Kayaku, Takeda, Eisai, Novartis, and Pfizer outside the submitted work. Dr. Naoki reports personal fees from AstraZeneca, Chugai Pharmaceutical, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim outside of the submitted work. Dr. Nakahara reports personal fees from Ono, Takeda, Eli Lilly, Kyowa Kirin, Boehringer Ingelheim, AstraZeneca, and Bristol-Myers Squibb outside of the submitted work. Dr. Tsukita reports personal fees from AstraZeneca, Chugai Pharmaceutical, Taiho Pharmaceutical, Daiichi Sankyo, Eli Lilly, Bristol-Myers Squibb, Merck Sharp & Dohme, Nippon Boehringer Ingelheim, and Eisai outside of the submitted work. Dr. Matsumoto reports personal fees from AstraZeneca, Boehringer Ingelheim, Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical, Takeda Pharmaceutical Co., Ltd., Merck Sharp & Dohme, Nippon Kayaku Co., Ltd., and Kyowa Kirin Co., Ltd. outside of the submitted work. Dr. Yoh reports personal fees from Abbvie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Janssen, Kyowa Kirin, Lilly, Merck Serono, Novartis, Ono, Otsuka, Taiho and Takeda and grants from Abbvie, Amgen, AstraZeneca, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Lilly, Merck Sharp & Dohme, Pfizer, Taiho, and Takeda outside of the submitted work. Dr. Fujisaka reports personal fees from AstraZeneca, Lilly, Boehringer Ingelheim, Takeda, Chugai Pharma, Ono Pharmaceutical, Pfizer, and Novartis, and grants from Merck, Bristol-Myers Squibb, Regeneron, Taiho Pharmaceutical, Ono Pharmaceutical outside of the submitted work. Dr. Takahashi reports personal fees from AstraZeneca, Bristol-Myers Squibb Japan, Chugai Pharma, Kyowa Kirin, Lilly Japan, Merck Sharp & Dohme, Nippon Kayaku, Taiho Pharmaceutical, and Takeda outside of the submitted work. Dr. Kishi reports personal fees from AstraZeneca outside of the submitted work. Dr. Naka reports personal fees from AstraZeneca, Chugai, Ono, and Merck Sharp & Dohme outside of the submitted work. Dr. Kunitoh reports personal fees from Taiho Pharmaceutical, Daiichi Sankyo, Merck Sharp & Dohme, Johnson and Johnson, and AstraZeneca outside of the submitted work. The remaining authors declare no conflict of interest.
Acknowledgments
This work was supported by AstraZeneca. The Comprehensive Support Project for Oncology Research (CSPOR) of the Public Health Research Foundation conducted this study. The CSPOR received funding support from an AstraZeneca-investigator-sponsored study. AstraZeneca had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation or approval of the manuscript but reviewed it before submission. We thank all patients, their families, and the investigators who participated in the study, and Akira Yamao and Miho Akita of the CSPOR Secretariat for their overall management of this study and the Clinical Research Center of the Global Center for Medical Research for their assistance with data management and support. No one received compensation from study funds for the stated contributions. We thank Sandra E, Enago (https://www.enago.jp/) for editing the draft of this manuscript.
Footnotes
Cite this article as: Watanabe K, Hosomi Y, Naoki K, et al. The whole picture of first-line osimertinib for EGFR mutation-positive advanced NSCLC: real-world efficacy, safety, progression pattern, and posttreatment therapy (Reiwa study). JTO Clin Res Rep. 2024;5:100720.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2024.100720.
Supplementary Data
References
- 1.Thandra K.C., Barsouk A., Saginala K., Aluru J.S., Barsouk A. Epidemiology of lung cancer. Contemp Oncol (Pozn) 2021;25:45–52. doi: 10.5114/wo.2021.103829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shigematsu H., Lin L., Takahashi T., et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 3.Tan D.S.W., Mok T.S.K., Rebbeck T.R. Cancer genomics: diversity and disparity across ethnicity and geography. J Clin Oncol. 2016;34:91–101. doi: 10.1200/JCO.2015.62.0096. [DOI] [PubMed] [Google Scholar]
- 4.Gazdar A.F. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(suppl 1):S24–S31. doi: 10.1038/onc.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soria J.C., Ohe Y., Vansteenkiste J., et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 6.Ramalingam S.S., Vansteenkiste J., Planchard D., et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 7.Ohe Y., Imamura F., Nogami N., et al. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J Clin Oncol. 2019;49:29–36. doi: 10.1093/jjco/hyy179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonetti A., Sharma S., Minari R., Perego P., Giovannetti E., Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121:725–737. doi: 10.1038/s41416-019-0573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandara D.R., Li T., Lara P.N., et al. Acquired resistance to targeted therapies against oncogene-driven non-small-cell lung cancer: approach to subtyping progressive disease and clinical implications. Clin Lung Cancer. 2014;15:1–6. doi: 10.1016/j.cllc.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goto Y., Tanai C., Yoh K., et al. Continuing EGFR-TKI beyond radiological progression in patients with advanced or recurrent, EGFR mutation-positive non-small-cell lung cancer: an observational study. ESMO Open. 2017;2 doi: 10.1136/esmoopen-2017-000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe K., Yoh K., Hosomi Y., et al. Efficacy and safety of first-line osimertinib treatment and postprogression patterns of care in patients with epidermal growth factor receptor activating mutation-positive advanced non-small cell lung cancer (Reiwa study): study protocol of a multicentre, real-world observational study. BMJ (Open) 2022;12 doi: 10.1136/bmjopen-2020-046451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eba J., Nakamura K. Overview of the ethical guidelines for medical and biological research involving human subjects in Japan. Jpn J Clin Oncol. 2022;52:539–544. doi: 10.1093/jjco/hyac034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y., Sheng J., Kang S., et al. Patients with exon 19 deletion were associated with longer progression-free survival compared to those with L858R mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: a meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer [e-pub ahead of print]. J Clin Oncol. https://doi.org/10.1200/JCO.2018.78.3118, accessed August 1, 2024. [DOI] [PubMed]
- 15.Leonetti A., Verzè M., Minari R., et al. Resistance to osimertinib in advanced EGFR-mutated NSCLC: a prospective study of molecular genotyping on tissue and liquid biopsies. Br J Cancer. 2024;130:135–142. doi: 10.1038/s41416-023-02475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenfeld A.J., Chan J.M., Kubota D., et al. Tumor analyses reveal squamous transformation and off-target alterations as early resistance mechanisms to first-line osimertinib in EGFR-Mutant Lung Cancer. Clin Cancer Res. 2020;26:2654–2663. doi: 10.1158/1078-0432.CCR-19-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez D.R., Tang C., Zhang J., et al. Local Consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37:1558–1565. doi: 10.1200/JCO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyengar P., Wardak Z., Gerber D.E., et al. Consolidative Radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S., Kim T.M., Han J.Y., et al. A Phase III, randomized study of atezolizumab plus bevacizumab and chemotherapy in patients with EGFR or ALK mutated in non-small cell lung cancer (ATTLAS, KCSG-LU19-04) J Clin Oncol. 2024;42:1241–1251. doi: 10.1200/JCO.23.01891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J.C.H., Lee D.H., Lee J.S., et al. Pemetrexed and platinum with or without pembrolizumab for tyrosine kinase inhibitor (TKI)-resistant, EGFR-mutant, metastatic nonsquamous NSCLC: phase 3 KEYNOTE-789 study. J Clin Oncol. 2023;41(suppl 17) doi: 10.1200/JCO.23.02747. LBA9000–LBA9000. [DOI] [PubMed] [Google Scholar]
- 21.Lee A.T.M., Nagasaka M. CheckMate-722: the rise and fall of nivolumab with chemotherapy in TKI-refractory EGFR-mutant NSCLC. Lung Cancer (Auckl) 2023;14:41–46. doi: 10.2147/LCTT.S408886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu H.A., Goldberg S.B., Le X., et al. Biomarker-directed Phase II platform study in patients with EGFR sensitizing mutation-positive advanced/metastatic non-small cell lung cancer whose disease has progressed on first-line osimertinib therapy (ORCHARD) Clin Lung Cancer. 2021;22:601–606. doi: 10.1016/j.cllc.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mok T., Jänne P.A., Nishio M., et al. HERTHENA-Lung02: phase III study of patritumab deruxtecan in advanced EGFR-mutated NSCLC after a third-generation EGFR TKI. Future Oncol. 2024;20:969–980. doi: 10.2217/fon-2023-0602. [DOI] [PubMed] [Google Scholar]
- 24.Passaro A., Wang J., Wang Y., et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: primary results from the phase III MARIPOSA-2 study. Ann Oncol. 2024;35:77–90. doi: 10.1016/j.annonc.2023.10.117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.